Metronidazole - Metronidazole lotion
(Metronidazole)Metronidazole - Metronidazole lotion Prescribing Information
Metronidazole topical lotion is indicated for topical application in the treatment of inflammatory papules and pustules of rosacea.
Apply a thin layer to entire affected areas after washing. Use morning and evening or as directed by physician. Avoid application close to the eyes. Patients may use cosmetics after waiting for the metronidazole topical lotion to dry (not less than 5 minutes).
Metronidazole topical lotion is contraindicated in individuals with a history of hypersensitivity to metronidazole or to other ingredients of the formulation.
In a controlled clinical trial, safety data from 141 patients who used metronidazole topical lotion (n=71), or the lotion vehicle (n=70), twice daily and experienced a local cutaneous adverse event which may or may not have been related to the treatments include: local allergic reaction, metronidazole topical lotion 2 (3%), lotion vehicle 0; contact dermatitis, metronidazole topical lotion 2 (3%), lotion vehicle 1 (1%); pruritus, metronidazole topical lotion 1 (1%), lotion vehicle 0; skin discomfort (burning and stinging), metronidazole topical lotion 1 (1%), lotion vehicle 2 (3%); erythema, metronidazole topical lotion 4 (6%), lotion vehicle 0; dry skin, metronidazole topical lotion 0, lotion vehicle 1 (1%); and worsening of rosacea, metronidazole topical lotion 1 (1%), and lotion vehicle 7 (10%).
The following additional adverse experiences have been reported with the topical use of metronidazole: skin irritation, transient redness, metallic taste, tingling or numbness of extremities, and nausea.
Oral metronidazole has been reported to potentiate the anticoagulant effect of warfarin and coumarin anticoagulants, resulting in a prolongation of prothrombin time. The effect of topical metronidazole on prothrombin time is not known.
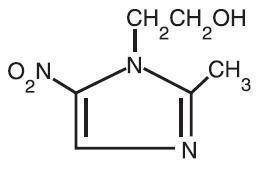
Metronidazole Topical Lotion, 0.75% contains metronidazole, USP, at a concentration of 7.5 mg per gram (0.75% w/w) in a lotion consisting of benzyl alcohol, carbomer 941, cyclomethicone, glycerin, glyceryl stearate, light mineral oil, PEG-100 stearate, polyethylene glycol 400, potassium sorbate, purified water, steareth-21, stearyl alcohol, and sodium hydroxide and/or lactic acid to adjust pH. Metronidazole is an imidazole and is classified therapeutically as an antiprotozoal and antibacterial agent. Chemically, metronidazole is 2-methyl-5-nitro-1