Rybelsus prior authorization resources
Most recent state uniform prior authorization forms
Brand Resources
Rybelsus patient education
Patient toolkit
Dosage & administration
DOSAGE AND ADMINISTRATION
- There are two RYBELSUS formulations (i.e., formulation R1 and formulation R2) with different recommended dosages. (2.1 )
- These formulations are not substitutable on a mg per mg basis
- Use either formulation but do not use both formulations at the same time.
- Take RYBELSUS on an empty stomach in the morning with water (up to 4 ounces of water); do not take with other liquids besides water. (2.2 )
- After taking RYBELSUS, wait at least 30 minutes before eating food, drinking beverages or taking other oral medications. (2.2 )
- Swallow tablets whole. Do not split, crush or chew tablets. (2.2 )
- See the Full Prescribing Information for instructions on switching from OZEMPIC to RYBELSUS and switching between the two different RYBELSUS formulations. (2.4 )
Recommended Dosage of Starting, Escalation and Maintenance Dosage of RYBELSUS Formulations R1 and R2 (2.3 )
RYBELSUS (formulation R1) (2.3 )
- Day 1 to 30: Recommended starting dosage is 3 mg orally once daily for 30 days (this dosage is not effective for glycemic control).
- Days 31 to 60: Increase the dosage to 7 mg orally once daily.
- On Day 61 or thereafter, if: (2.3 )
- No additional glycemic control is needed, maintain the dosage at 7 mg orally once daily.
- Additional glycemic control is needed, increase the dosage to 14 mg orally once daily.
RYBELSUS (formulation R2) (2.3 )
- Day 1 to 30: Recommended starting dosage is 1.5 mg orally once daily for 30 days (this dosage is not effective for glycemic control).
- Days 31 to 60: Increase the dosage to 4 mg orally once daily.
- On Day 61 or thereafter, if: (2.3 )
- No additional glycemic control is needed, maintain the dosage at 4 mg orally once daily.
- Additional glycemic control is needed, increase the dosage to 9 mg orally once daily.
Overview of RYBELSUS Formulations
- There are two RYBELSUS formulations (i.e., formulation R1 and formulation R2) with different recommended dosages. Refer to recommendations on how to switch from one formulation to another formulation [see Dosage and Administration (2.3 , 2.4 )] .
- RYBELSUS (formulation R1) includes strengths 3 mg, 7 mg and 14 mg.
- RYBELSUS (formulation R2) includes strengths 1.5 mg, 4 mg and 9 mg.
- These formulations are not substitutable on a mg per mg basis.
- Use either RYBELSUS formulation R1 or formulation R2; do not use both formulations at the same time.
- Do not take more than one tablet per day.
Important Administration Instructions
- Take RYBELSUS on an empty stomach in the morning with water (up to 4 ounces of water). Do not take RYBELSUS with other liquids besides water.
- After taking RYBELSUS, wait at least 30 minutes before eating food, drinking beverages or taking other oral medications [see Clinical Pharmacology (12.3 )] .
- Swallow tablets whole. Do not split crush or chew.
- If a dose is missed, skip the missed dose and take the next dose the following day.
Recommended Starting, Escalation and Maintenance Dosage of RYBELSUS Formulations R1 and R2
RYBELSUS (formulation R1)
RYBELSUS (formulation R1) includes the following strengths: 3 mg, 7 mg and 14 mg.
Recommend the following RYBELSUS (formulation R1) dosage to reduce the risk of gastrointestinal adverse reactions [see Warnings and Precautions (5.6 ), Adverse Reactions (6.1 )]:
- Starting Dosage (Initiation Phase) (Days 1 to 30) : The recommended starting dosage is 3 mg orally once daily (this dosage is not effective for glycemic control).
- Escalation and Maintenance Dosage (Days 31 and beyond) :
- Days 31 to 60: Increase the dosage to 7 mg orally once daily.
- On Day 61 or thereafter, if:
- No additional glycemic control is needed, maintain the dosage at 7 mg orally once daily.
- Additional glycemic control is needed, increase the dosage to 14 mg orally once daily.
RYBELSUS (formulation R2)
RYBELSUS (formulation R2) includes the following strengths: 1.5 mg, 4 mg and 9 mg.
Recommend the following RYBELSUS (formulation R2) dosage to reduce the risk of gastrointestinal adverse reactions [see Warnings and Precautions (5.6 ), Adverse Reactions (6.1 )] :
- Starting Dosage (Initiation Phase) (Days 1 through 30) : The recommended starting dosage is 1.5 mg orally once daily (this dosage is not effective for glycemic control).
- Escalation and Maintenance Dosage (Days 31 and beyond) :
- Days 31 to 60: Increase the dosage to 4 mg orally once daily.
- On Day 61 or thereafter, if:
- No additional glycemic control is needed maintain the dosage at 4 mg orally once daily.
- Additional glycemic control is needed increase the dosage to 9 mg orally once daily.
Switching Between RYBELSUS (Formulations R1 or R2) or from OZEMPIC to RYBELSUS
Switching Between RYBELSUS Formulations
- Do not switch between RYBELSUS formulations during the initiation phase (Days 1-30) [see Dosage and Administration (2.3 )] .
- After 30 days of RYBELSUS treatment (after the initiation phase) [see Dosage and Administration (2.3 )] , patients may switch between RYBELSUS formulations (see Table 1 ).
- When switching between the formulations, initiate the other RYBELSUS formulation the day after discontinuing the previous RYBELSUS formulation.
Table 1. Switching Between Escalation or Maintenance Dosage of RYBELSUS Formulations
RYBELSUS (formulation R1) • | RYBELSUS (formulation R2) • |
7 mg orally once daily | 4 mg orally once daily |
14 mg orally once daily | 9 mg orally once daily |
- •Discontinue this formulation and initiate the alternate formulation the day after
Switching from OZEMPIC to RYBELSUS (formulation R1) or RYBELSUS (formulation R2)
Switching from OZEMPIC to RYBELSUS (formulation R1)
- One week after discontinuing 0.5 mg of subcutaneous OZEMPIC, start 7 mg or 14 mg of RYBELSUS (formulation R1) orally once daily.
- Switching recommendations for patients taking OZEMPIC 0.25 mg, 1 mg or 2 mg subcutaneously once weekly to RYBELSUS (formulation R1) are not available.
Switching from OZEMPIC to RYBELSUS (formulation R2)
- One week after discontinuing 0.5 mg of subcutaneous OZEMPIC, start 4 mg or 9 mg of RYBELSUS (formulation R2) orally once daily.
- Switching recommendations for patients taking OZEMPIC 0.25 mg, 1 mg or 2 mg subcutaneously once weekly to RYBELSUS (formulation R2) are not available.

By using PrescriberAI, you agree to the AI Terms of Use.
Rybelsus prescribing information
WARNING: RISK OF THYROID C-CELL TUMORS
- In rodents, semaglutide causes dose-dependent and treatment-duration-dependent thyroid C-cell tumors at clinically relevant exposures. It is unknown whether RYBELSUS causes thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans as human relevance of semaglutide-induced rodent thyroid C-cell tumors has not been determined [see Warnings and Precautions (5.1 ), Nonclinical Toxicology (13.1 )] .
- RYBELSUS is contraindicated in patients with a personal or family history of MTC or in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2) [see Contraindications (4 )] . Counsel patients regarding the potential risk for MTC with the use of RYBELSUS and inform them of symptoms of thyroid tumors (e.g., a mass in the neck, dysphagia, dyspnea, persistent hoarseness). Routine monitoring of serum calcitonin or using thyroid ultrasound is of uncertain value for early detection of MTC in patients treated with RYBELSUS [see Contraindications (4 ), Warnings and Precautions (5.1 )] .
Indications and Usage (1 ) ………………………………………...10/2025
Dosage and Administration, Overview of RYBELSUS formulations (2.1 ), Recommended Dosage (2.3 ), Switching Patients between OZEMPIC and RYBELSUS (2.4 ) ………………………………………………...12/2024
Warning and Precautions
- Acute Pancreatitis (5.2 )………………………………..……….10/2025
- Acute Kidney Injury Due to Volume Depletion (5.5 )………….10/2025
- Severe Gastrointestinal Adverse Reactions (5.6 )….……..…….10/2025
- Acute Gallbladder Disease (5.8 )………………..………..……..10/2025
- Pulmonary Aspiration During General Anesthesia or Deep Sedation (5.9 ) ……………………………………………………..……...11/2024
INDICATIONS AND USAGE
RYBELSUS is indicated:
- as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus.
- to reduce the risk of major adverse cardiovascular (CV) events (CV death, non-fatal myocardial infarction or non-fatal stroke) in adults with type 2 diabetes mellitus who are at high risk for these events.
DOSAGE AND ADMINISTRATION
- There are two RYBELSUS formulations (i.e., formulation R1 and formulation R2) with different recommended dosages. (2.1 )
- These formulations are not substitutable on a mg per mg basis
- Use either formulation but do not use both formulations at the same time.
- Take RYBELSUS on an empty stomach in the morning with water (up to 4 ounces of water); do not take with other liquids besides water. (2.2 )
- After taking RYBELSUS, wait at least 30 minutes before eating food, drinking beverages or taking other oral medications. (2.2 )
- Swallow tablets whole. Do not split, crush or chew tablets. (2.2 )
- See the Full Prescribing Information for instructions on switching from OZEMPIC to RYBELSUS and switching between the two different RYBELSUS formulations. (2.4 )
Recommended Dosage of Starting, Escalation and Maintenance Dosage of RYBELSUS Formulations R1 and R2 (2.3 )
RYBELSUS (formulation R1) (2.3 )
- Day 1 to 30: Recommended starting dosage is 3 mg orally once daily for 30 days (this dosage is not effective for glycemic control).
- Days 31 to 60: Increase the dosage to 7 mg orally once daily.
- On Day 61 or thereafter, if: (2.3 )
- No additional glycemic control is needed, maintain the dosage at 7 mg orally once daily.
- Additional glycemic control is needed, increase the dosage to 14 mg orally once daily.
RYBELSUS (formulation R2) (2.3 )
- Day 1 to 30: Recommended starting dosage is 1.5 mg orally once daily for 30 days (this dosage is not effective for glycemic control).
- Days 31 to 60: Increase the dosage to 4 mg orally once daily.
- On Day 61 or thereafter, if: (2.3 )
- No additional glycemic control is needed, maintain the dosage at 4 mg orally once daily.
- Additional glycemic control is needed, increase the dosage to 9 mg orally once daily.
Overview of RYBELSUS Formulations
- There are two RYBELSUS formulations (i.e., formulation R1 and formulation R2) with different recommended dosages. Refer to recommendations on how to switch from one formulation to another formulation [see Dosage and Administration (2.3 , 2.4 )] .
- RYBELSUS (formulation R1) includes strengths 3 mg, 7 mg and 14 mg.
- RYBELSUS (formulation R2) includes strengths 1.5 mg, 4 mg and 9 mg.
- These formulations are not substitutable on a mg per mg basis.
- Use either RYBELSUS formulation R1 or formulation R2; do not use both formulations at the same time.
- Do not take more than one tablet per day.
Important Administration Instructions
- Take RYBELSUS on an empty stomach in the morning with water (up to 4 ounces of water). Do not take RYBELSUS with other liquids besides water.
- After taking RYBELSUS, wait at least 30 minutes before eating food, drinking beverages or taking other oral medications [see Clinical Pharmacology (12.3 )] .
- Swallow tablets whole. Do not split crush or chew.
- If a dose is missed, skip the missed dose and take the next dose the following day.
Recommended Starting, Escalation and Maintenance Dosage of RYBELSUS Formulations R1 and R2
RYBELSUS (formulation R1)
RYBELSUS (formulation R1) includes the following strengths: 3 mg, 7 mg and 14 mg.
Recommend the following RYBELSUS (formulation R1) dosage to reduce the risk of gastrointestinal adverse reactions [see Warnings and Precautions (5.6 ), Adverse Reactions (6.1 )]:
- Starting Dosage (Initiation Phase) (Days 1 to 30) : The recommended starting dosage is 3 mg orally once daily (this dosage is not effective for glycemic control).
- Escalation and Maintenance Dosage (Days 31 and beyond) :
- Days 31 to 60: Increase the dosage to 7 mg orally once daily.
- On Day 61 or thereafter, if:
- No additional glycemic control is needed, maintain the dosage at 7 mg orally once daily.
- Additional glycemic control is needed, increase the dosage to 14 mg orally once daily.
RYBELSUS (formulation R2)
RYBELSUS (formulation R2) includes the following strengths: 1.5 mg, 4 mg and 9 mg.
Recommend the following RYBELSUS (formulation R2) dosage to reduce the risk of gastrointestinal adverse reactions [see Warnings and Precautions (5.6 ), Adverse Reactions (6.1 )] :
- Starting Dosage (Initiation Phase) (Days 1 through 30) : The recommended starting dosage is 1.5 mg orally once daily (this dosage is not effective for glycemic control).
- Escalation and Maintenance Dosage (Days 31 and beyond) :
- Days 31 to 60: Increase the dosage to 4 mg orally once daily.
- On Day 61 or thereafter, if:
- No additional glycemic control is needed maintain the dosage at 4 mg orally once daily.
- Additional glycemic control is needed increase the dosage to 9 mg orally once daily.
Switching Between RYBELSUS (Formulations R1 or R2) or from OZEMPIC to RYBELSUS
Switching Between RYBELSUS Formulations
- Do not switch between RYBELSUS formulations during the initiation phase (Days 1-30) [see Dosage and Administration (2.3 )] .
- After 30 days of RYBELSUS treatment (after the initiation phase) [see Dosage and Administration (2.3 )] , patients may switch between RYBELSUS formulations (see Table 1 ).
- When switching between the formulations, initiate the other RYBELSUS formulation the day after discontinuing the previous RYBELSUS formulation.
Table 1. Switching Between Escalation or Maintenance Dosage of RYBELSUS Formulations
RYBELSUS (formulation R1) • | RYBELSUS (formulation R2) • |
7 mg orally once daily | 4 mg orally once daily |
14 mg orally once daily | 9 mg orally once daily |
- •Discontinue this formulation and initiate the alternate formulation the day after
Switching from OZEMPIC to RYBELSUS (formulation R1) or RYBELSUS (formulation R2)
Switching from OZEMPIC to RYBELSUS (formulation R1)
- One week after discontinuing 0.5 mg of subcutaneous OZEMPIC, start 7 mg or 14 mg of RYBELSUS (formulation R1) orally once daily.
- Switching recommendations for patients taking OZEMPIC 0.25 mg, 1 mg or 2 mg subcutaneously once weekly to RYBELSUS (formulation R1) are not available.
Switching from OZEMPIC to RYBELSUS (formulation R2)
- One week after discontinuing 0.5 mg of subcutaneous OZEMPIC, start 4 mg or 9 mg of RYBELSUS (formulation R2) orally once daily.
- Switching recommendations for patients taking OZEMPIC 0.25 mg, 1 mg or 2 mg subcutaneously once weekly to RYBELSUS (formulation R2) are not available.
DOSAGE FORMS AND STRENGTHS
RYBELSUS (semaglutide) tablets (formulation R1) are available as:
- 3 mg: white to light yellow, oval shaped debossed with “3” on one side and “novo” on the other side.
- 7 mg: white to light yellow, oval shaped debossed with “7” on one side and “novo” on the other side.
- 14 mg: white to light yellow, oval shaped debossed with “14” on one side and “novo” on the other side.
RYBELSUS (semaglutide) tablets (formulation R2) are available as:
- 1.5 mg: white to light yellow, round shaped debossed with “1.5” on one side and “novo” on the other side.
- 4 mg: white to light yellow, round shaped debossed with “4” on one side and “novo” on the other side.
- 9 mg: white to light yellow, round shaped debossed with “9” on one side and “novo” on the other side.
USE IN SPECIFIC POPULATIONS
Pregnancy
Risk Summary
Available data with RYBELSUS use in pregnant women are insufficient to evaluate for a drug-associated risk of major birth defects, miscarriage or other adverse maternal or fetal outcomes. There are clinical considerations regarding the risks of poorly controlled diabetes in pregnancy (see Clinical Considerations) . Based on animal reproduction studies, there may be potential risks to the fetus from exposure to RYBELSUS during pregnancy. RYBELSUS should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
In pregnant rats administered semaglutide during organogenesis, embryofetal mortality, structural abnormalities and alterations to growth occurred at maternal exposures below the maximum recommended human dose (MRHD) based on AUC. In rabbits and cynomolgus monkeys administered semaglutide during organogenesis, early pregnancy losses and structural abnormalities were observed at exposure below the MRHD (rabbit) and ≥10-fold the MRHD (monkey). These findings coincided with a marked maternal body weight loss in both animal species (see Data) .
The estimated background risk of major birth defects is 6 to 10% in women with pre-gestational diabetes with an HbA 1c >7 and has been reported to be as high as 20 to 25% in women with a HbA 1c >10. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Clinical Considerations
Disease Associated Maternal and Fetal Risk: Poorly controlled diabetes during pregnancy increases the maternal risk for diabetic ketoacidosis, pre-eclampsia, spontaneous abortions, preterm delivery and delivery complications. Poorly controlled diabetes increases the fetal risk for major birth defects, stillbirth and macrosomia related morbidity.
Data
Animal Data: In a combined fertility and embryofetal development study in rats, subcutaneous doses of 0.01, 0.03 and 0.09 mg/kg/day (0.2-, 0.7- and 2.1-fold the MRHD) were administered to males for 4 weeks prior to and throughout mating and to females for 2 weeks prior to mating, and throughout organogenesis to Gestation Day 17. In parental animals, pharmacologically mediated reductions in body weight gain and food consumption were observed at all dose levels. In the offspring, reduced growth and fetuses with visceral (heart blood vessels) and skeletal (cranial bones, vertebra, ribs) abnormalities were observed at the human exposure.
In an embryofetal development study in pregnant rabbits, subcutaneous doses of 0.0010, 0.0025 or 0.0075 mg/kg/day (0.06-, 0.6- and 4.4-fold the MRHD) were administered throughout organogenesis from Gestation Day 6 to 19. Pharmacologically mediated reductions in maternal body weight gain and food consumption were observed at all dose levels. Early pregnancy losses and increased incidences of minor visceral (kidney, liver) and skeletal (sternebra) fetal abnormalities were observed at ≥0.0025 mg/kg/day, at clinically relevant exposures.
In an embryofetal development study in pregnant cynomolgus monkeys, subcutaneous doses of 0.015, 0.075 and 0.15 mg/kg twice weekly (1.9-, 9.9- and 29-fold the MRHD) were administered throughout organogenesis, from Gestation Day 16 to 50. Pharmacologically mediated, marked initial maternal body weight loss and reductions in body weight gain and food consumption coincided with the occurrence of sporadic abnormalities (vertebra, sternebra, ribs) at ≥0.075 mg/kg twice weekly (≥9X human exposure).
In a pre- and postnatal development study in pregnant cynomolgus monkeys, subcutaneous doses of 0.015, 0.075 and 0.15 mg/kg twice weekly (1.3-, 6.4- and 14-fold the MRHD) were administered from Gestation Day 16 to 140. Pharmacologically mediated marked initial maternal body weight loss and reductions in body weight gain and food consumption coincided with an increase in early pregnancy losses and led to delivery of slightly smaller offspring at ≥0.075 mg/kg twice weekly (≥6X human exposure).
Salcaprozate sodium (SNAC), an absorption enhancer in RYBELSUS, crosses the placenta and reaches fetal tissues in rats. In a pre- and postnatal development study in pregnant Sprague Dawley rats, SNAC was administered orally at 1,000 mg/kg/day (exposure levels were not measured) on Gestation Day 7 through lactation day 20. An increase in gestation length, an increase in the number of stillbirths and a decrease in pup viability were observed.
Lactation
Risk Summary
A clinical lactation study reported semaglutide concentrations below the lower limit of quantification in human breast milk. However, salcaprozate sodium (SNAC) and/or its metabolites are present in human milk. Since the activity of enzymes involved in SNAC clearance may be lower in infants compared to adults, higher SNAC plasma levels may occur in neonates and infants. Because of the unknown potential for serious adverse reactions in the breastfed infant due to the possible accumulation of SNAC, and because there are alternative formulations of semaglutide that do not contain SNAC that can be used during lactation, advise patients that breastfeeding is not recommended during treatment with RYBELSUS.
Females and Males of Reproductive Potential
Discontinue RYBELSUS in women at least 2 months before a planned pregnancy due to the long washout period for semaglutide [see Use in Specific Populations (8.1 )] .
Pediatric Use
The safety and effectiveness of RYBELSUS have not been established in pediatric patients.
Geriatric Use
In the pool of glycemic control trials, 1,229 (30%) RYBELSUS-treated patients were 65 years of age and over and 199 (5%) RYBELSUS-treated patients were 75 years of age and over [see Clinical Studies (14 )] . In Trial 8, one of the CV outcomes trials, 891 (56%) RYBELSUS-treated patients were 65 years of age and over and 200 (13%) RYBELSUS-treated patients were 75 years of age and over.
No overall differences in safety or effectiveness for RYBELSUS have been observed between patients 65 years of age and older and younger adult patients.
Renal Impairment
The recommended dosage of RYBELSUS in patients with renal impairment is the same as those with normal renal function.
The safety and effectiveness of RYBELSUS was evaluated in a 26-week clinical study that included 324 patients with moderate renal impairment (eGFR 30 to 59 mL/min/1.73 m 2 ) [see Clinical Studies (14.1 )] . In patients with renal impairment including end-stage renal disease (ESRD), no clinically relevant change in semaglutide pharmacokinetics was observed [see Clinical Pharmacology (12.3 )] .
Hepatic Impairment
The recommended dosage in patients with hepatic impairment is the same as those with normal hepatic function.
In a study in subjects with different degrees of hepatic impairment, no clinically relevant change in semaglutide pharmacokinetics was observed [see Clinical Pharmacology (12.3 )] .
CONTRAINDICATIONS
RYBELSUS is contraindicated in patients with:
- A personal or family history of medullary thyroid carcinoma (MTC) or in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2) [see Warnings and Precautions (5.1 )] .
- A prior serious hypersensitivity reaction to semaglutide or to any of the excipients in RYBELSUS. Serious hypersensitivity reactions including anaphylaxis and angioedema have been reported with RYBELSUS [see Warnings and Precautions (5.7 )] .
WARNINGS AND PRECAUTIONS
- Acute Pancreatitis : Has been observed in patients treated with GLP-1 receptor agonists, including RYBELSUS. Discontinue promptly if pancreatitis is suspected. (5.2 )
- Diabetic Retinopathy Complications : Has been reported in a cardiovascular outcomes trial with semaglutide injection. Patients with a history of diabetic retinopathy should be monitored. (5.3 )
- Hypoglycemia with Concomitant Use of Insulin Secretagogues or Insulin : May increase the risk of hypoglycemia, including severe hypoglycemia. Reducing the dosage of insulin secretagogue or insulin may be necessary. (5.4 )
- Acute Kidney Injury Due to Volume Depletion : Monitor renal function in patients with renal impairment reporting adverse reactions that could lead to volume depletion. (5.5 )
- Severe Gastrointestinal Adverse Reactions : Use of RYBELSUS has been associated with gastrointestinal adverse reactions, sometimes severe. RYBELSUS is not recommended in patients with severe gastroparesis. (5.6 )
- Hypersensitivity Reactions : Serious hypersensitivity reactions (e.g., anaphylaxis and angioedema) have been reported. Discontinue RYBELSUS if hypersensitivity reactions occur and monitor until signs and symptoms resolve. (5.7 )
- Acute Gallbladder Disease : If cholelithiasis or cholecystitis are suspected, gallbladder studies are indicated. (5.8 )
- Pulmonary Aspiration During General Anesthesia or Deep Sedation : Has been reported in patients receiving GLP-1 receptor agonists undergoing elective surgeries or procedures. Instruct patients to inform healthcare providers of any planned surgeries or procedures. (5.9 )
Risk of Thyroid C-Cell Tumors
In mice and rats, semaglutide caused a dose-dependent and treatment-duration-dependent increase in the incidence of thyroid C-cell tumors (adenomas and carcinomas) after lifetime exposure at clinically relevant plasma exposures [see Nonclinical Toxicology (13.1 )] . It is unknown whether RYBELSUS causes thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans as human relevance of semaglutide-induced rodent thyroid C-cell tumors has not been determined.
Cases of MTC in patients treated with liraglutide, another GLP-1 receptor agonist, have been reported in the postmarketing period; the data in these reports are insufficient to establish or exclude a causal relationship between MTC and GLP-1 receptor agonist use in humans.
RYBELSUS is contraindicated in patients with a personal or family history of MTC or in patients with MEN 2. Counsel patients regarding the potential risk for MTC with the use of RYBELSUS and inform them of symptoms of thyroid tumors (e.g., a mass in the neck, dysphagia, dyspnea, persistent hoarseness).
Routine monitoring of serum calcitonin or using thyroid ultrasound is of uncertain value for early detection of MTC in patients treated with RYBELSUS. Such monitoring may increase the risk of unnecessary procedures, due to the low-test specificity for serum calcitonin and a high background incidence of thyroid disease. Significantly elevated serum calcitonin value may indicate MTC and patients with MTC usually have calcitonin values >50 ng/L. If serum calcitonin is measured and found to be elevated, the patient should be further evaluated. Patients with thyroid nodules noted on physical examination or neck imaging should also be further evaluated.
Acute Pancreatitis
Acute pancreatitis, including fatal and non-fatal hemorrhagic or necrotizing pancreatitis, has been observed in patients treated with GLP-1 receptor agonists, including RYBELSUS [see Adverse Reactions (6 )] .
After initiation of RYBELSUS, observe patients carefully for signs and symptoms of pancreatitis, which may include persistent or severe abdominal pain (sometimes radiating to the back), and which may or may not be accompanied by nausea or vomiting. If pancreatitis is suspected, discontinue RYBELSUS and initiate appropriate management.
Diabetic Retinopathy Complications
In a pooled analysis of glycemic control trials with RYBELSUS, patients reported diabetic retinopathy related adverse reactions during the trial (4.2% with RYBELSUS and 3.8% with comparator) [see Adverse Reactions (6.1 )] .
In a 2-year CV outcomes trial with semaglutide injection involving patients with type 2 diabetes mellitus and high CV risk, diabetic retinopathy complications (which was a 4-component adjudicated endpoint) occurred in patients treated with semaglutide injection (3%) compared to placebo (1.8%). The absolute risk increase for diabetic retinopathy complications was larger among patients with a history of diabetic retinopathy at baseline (semaglutide injection 8.2%, placebo 5.2%) than among patients without a known history of diabetic retinopathy (semaglutide injection 0.7%, placebo 0.4%).
Rapid improvement in glucose control has been associated with a temporary worsening of diabetic retinopathy. The effect of long-term glycemic control with semaglutide on diabetic retinopathy complications has not been studied. Patients with a history of diabetic retinopathy should be monitored for progression of diabetic retinopathy.
Hypoglycemia with Concomitant Use of Insulin Secretagogues or Insulin
Patients receiving RYBELSUS in combination with an insulin secretagogue (e.g., sulfonylurea) or insulin may have an increased risk of hypoglycemia, including severe hypoglycemia [see Adverse Reactions (6.1 ), Drug Interactions (7 )] .
The risk of hypoglycemia may be lowered by a reduction in the dosage of sulfonylurea (or other concomitantly administered insulin secretagogue) or insulin. Inform patients using these concomitant medications of the risk of hypoglycemia and educate them on the signs and symptoms of hypoglycemia.
Acute Kidney Injury Due to Volume Depletion
There have been postmarketing reports of acute kidney injury, in some cases requiring hemodialysis, in patients treated with semaglutide. The majority of the reported events occurred in patients who experienced gastrointestinal reactions leading to dehydration such as nausea, vomiting, or diarrhea [see Adverse Reactions (6 )].
Monitor renal function in patients reporting adverse reactions to RYBELSUS that could lead to volume depletion, especially during dosage initiation and escalation of RYBELSUS.
Severe Gastrointestinal Adverse Reactions
Use of RYBELSUS has been associated with gastrointestinal adverse reactions, sometimes severe [see Adverse Reactions (6 )] . In RYBELSUS clinical trials, severe gastrointestinal adverse reactions were reported more frequently among patients receiving RYBELSUS (7 mg 0.6%, 14 mg 2%) than placebo (0.3%). Severe gastrointestinal adverse reactions have also been reported postmarketing with GLP-1 receptor agonists.
RYBELSUS is not recommended in patients with severe gastroparesis.
Hypersensitivity Reactions
Serious hypersensitivity reactions (e.g., anaphylaxis, angioedema) have been reported in patients treated with RYBELSUS. If hypersensitivity reactions occur, discontinue use of RYBELSUS; treat promptly per standard of care and monitor until signs and symptoms resolve. RYBELSUS is contraindicated in patients with a prior serious hypersensitivity reaction to semaglutide or to any of the excipients in RYBELSUS [see Adverse Reactions (6.2 )] .
Anaphylaxis and angioedema have been reported with GLP-1 receptor agonists. Use caution in a patient with a history of angioedema or anaphylaxis with another GLP-1 receptor agonist because it is unknown whether such patients will be predisposed to anaphylaxis with RYBELSUS.
Acute Gallbladder Disease
Acute events of gallbladder disease such as cholelithiasis or cholecystitis have been reported in GLP-1 receptor agonist trials and postmarketing. In placebo-controlled trials to improve glycemic control, cholelithiasis was reported in 1% of patients treated with RYBELSUS 7 mg. In a 4-year CV outcomes trial (Trial 7), cholelithiasis was reported in 1.1% of patients treated with RYBELSUS 14 mg and in 0.9% of placebo-treated patients. In Trial 7, cholecystitis was reported in 1.1% of patients treated with RYBELSUS 14 mg and in 0.7% of placebo-treated patients [see Adverse Reactions (6.1 )] . If cholelithiasis or cholecystitis is suspected, gallbladder studies and appropriate clinical follow-up are indicated [see Adverse Reactions (6.2 ) ] .
Pulmonary Aspiration During General Anesthesia or Deep Sedation
RYBELSUS delays gastric emptying [see Clinical Pharmacology (12.2 )]. There have been rare postmarketing reports of pulmonary aspiration in patients receiving GLP-1 receptor agonists undergoing elective surgeries or procedures requiring general anesthesia or deep sedation who had residual gastric contents despite reported adherence to preoperative fasting recommendations.
Available data are insufficient to inform recommendations to mitigate the risk of pulmonary aspiration during general anesthesia or deep sedation in patients taking RYBELSUS, including whether modifying preoperative fasting recommendations or temporarily discontinuing RYBELSUS could reduce the incidence of retained gastric contents. Instruct patients to inform healthcare providers prior to any planned surgeries or procedures if they are taking RYBELSUS.
ADVERSE REACTIONS
The following serious adverse reactions are described below or elsewhere in the prescribing information:
- Risk of Thyroid C-cell Tumors [see Warnings and Precautions (5.1 )]
- Acute Pancreatitis [see Warnings and Precautions (5.2 )]
- Diabetic Retinopathy Complications [see Warnings and Precautions (5.3 )]
- Hypoglycemia with Concomitant Use of Insulin Secretagogues or Insulin [see Warnings and Precautions (5.4 )]
- Acute Kidney Injury Due to Volume Depletion [see Warnings and Precautions (5.5 )]
- Severe Gastrointestinal Adverse Reactions [see Warnings and Precautions (5.6 )]
- Hypersensitivity Reactions [see Warnings and Precautions (5.7 )]
- Acute Gallbladder Disease [see Warnings and Precautions (5.8 )]
- Pulmonary Aspiration During General Anesthesia or Deep Sedation [see Warnings and Precautions (5.9 )]
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of RYBELSUS (formulation R2 - 1.5 mg, 4 mg and 9 mg strengths) [see Dosage and Administration (2.2 )] and RYBELSUS (formulation R1 - 3 mg, 7, mg and 14 mg strengths) [see Dosage and Administration (2.3 )] has been established as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus based on adequate and well-controlled studies of RYBELSUS (formulation R1) in adult patients with type 2 diabetes mellitus [see Clinical Pharmacology (12.3 ), Clinical Studies (14 )] . Below is a display of the safety results of the adequate and well-controlled studies of RYBELSUS (formulation R1) in adult patients with type 2 diabetes mellitus.
Pool of Placebo-Controlled Trials
The data in Table 2 are derived from 2 placebo-controlled trials in adult patients with type 2 diabetes mellitus [see Clinical Studies (14 )] . These data reflect exposure of 1,071 patients to RYBELSUS with a mean duration of exposure of 41.8 weeks. The mean age of patients was 58 years, 3.9% were 75 years or older and 52% were male. In these trials, 63% were White, 6% were Black or African American and 27% were Asian; 19% identified as Hispanic or Latino ethnicity. At baseline, patients had type 2 mellitus diabetes for an average of 9.4 years and had a mean HbA 1c of 8.1%. At baseline, 20.1% of the population reported retinopathy. Baseline estimated renal function was normal (eGFR ≥90 mL/min/1.73 m 2 ) in 66.2%, mildly impaired (eGFR 60 to 90 mL/min/1.73 m 2 ) in 32.4% and moderately impaired (eGFR 30 to 60 mL/min/1.73 m 2 ) in 1.4% of patients.
Pool of Placebo- and Active-Controlled Trials
The occurrence of adverse reactions was also evaluated in a larger pool of adult patients with type 2 diabetes mellitus participating in 9 placebo- and active-controlled trials [see Clinical Studies (14 )] . In this pool, 4,116 patients with type 2 diabetes mellitus were treated with RYBELSUS for a mean duration of 59.8 weeks. The mean age of patients was 58 years, 5% were 75 years or older and 55% were male. In these trials, 65% were White, 6% were Black or African American and 24% were Asian; 15% identified as Hispanic or Latino ethnicity. At baseline, patients had type 2 diabetes mellitus for an average of 8.8 years and had a mean HbA 1c of 8.2%. At baseline, 16.6% of the population reported retinopathy. Baseline estimated renal function was normal (eGFR ≥90 mL/min/1.73 m 2 ) in 65.9%, mildly impaired (eGFR 60 to 90 mL/min/1.73 m 2 ) in 28.5% and moderately impaired (eGFR 30 to 60 mL/min/1.73 m 2 ) in 5.4% of the patients.
Common Adverse Reactions
Table 2 shows common adverse reactions, excluding hypoglycemia, associated with the use of RYBELSUS in adult patients with type 2 diabetes mellitus in the pool of placebo-controlled trials. These adverse reactions occurred more commonly on RYBELSUS than on placebo and occurred in at least 5% of patients treated with RYBELSUS.
Table 2. Adverse Reactions in Placebo-Controlled Trials Reported in ≥5% of RYBELSUS-Treated Patients with Type 2 Diabetes Mellitus
Adverse Reaction | Placebo (N=362) % | RYBELSUS 7 mg (N=356) % | RYBELSUS 14 mg (N=356) % |
Nausea | 6 | 11 | 20 |
Abdominal Pain | 4 | 10 | 11 |
Diarrhea | 4 | 9 | 10 |
Decreased appetite | 1 | 6 | 9 |
Vomiting | 3 | 6 | 8 |
Constipation | 2 | 6 | 5 |
In the pool of placebo- and active-controlled trials, the types and frequency of common adverse reactions, excluding hypoglycemia, were similar to those listed in Table 2 .
In a 4-year CV outcomes trial (Trial 7), 4,825 patients were randomized to RYBELSUS for a median follow-up of 49.6 months and 4,825 patients were randomized to placebo for a median follow-up of 49.4 months [see Clinical Studies (14.4 )]. Safety data collection was limited to serious adverse events (including death), adverse events leading to discontinuation, and adverse events of special interest. Study drug was permanently discontinued due to an adverse event in 15.5% of RYBELSUS-treated patients and 11.6% of placebo-treated patients.
Additional information from this trial is included in subsequent sections below, when relevant.
Gastrointestinal Adverse Reactions
In the pool of placebo-controlled trials, gastrointestinal adverse reactions occurred more frequently among patients who received RYBELSUS than placebo: RYBELSUS 14 mg once daily (41%), RYBELSUS 7 mg once daily (32%) and placebo (21%), including severe reactions (RYBELSUS 14 mg 2.0%, RYBELSUS 7 mg 0.6%, placebo 0.3%). The majority of reports of nausea, vomiting and/or diarrhea occurred during dose escalation. A greater percentage of patients who received RYBELSUS 14 mg once daily (8%) and RYBELSUS 7 mg once daily (4%) discontinued treatment due to gastrointestinal adverse reactions than patients who received placebo (1%).
In addition to the reactions in Table 2 , the following gastrointestinal adverse reactions with a frequency of <5% occurred in RYBELSUS-treated patients (frequencies listed, respectively, as 14 mg once daily, 7 mg once daily and placebo): abdominal distension (3%, 2% and 1%), dyspepsia (0.6%, 3%, 0.6%), eructation (2%, 0.6%, 0%,), flatulence (1%, 2%, 0%), gastroesophageal reflux disease (2%, 2%, 0.3%) and gastritis (2%, 2%, 0.8%).
Other Adverse Reactions
Pancreatitis: In the pool of placebo- and active-controlled trials with RYBELSUS, pancreatitis was reported as a serious adverse event in 6 RYBELSUS-treated patients (0.1 events per 100 patient years) versus 1 in comparator-treated patients (<0.1 events per 100 patient years).
Diabetic Retinopathy Complications: In the pool of placebo- and active-controlled trials with RYBELSUS, patients reported diabetic retinopathy related adverse reactions during the trial (4.2% with RYBELSUS and 3.8% with comparator).
Hypoglycemia: Table 3 summarizes the incidence of hypoglycemia by various definitions in the placebo-controlled trials.
Table 3. Hypoglycemia Adverse Reactions in Placebo-Controlled Trials in Patients with Type 2 Diabetes Mellitus
Placebo | RYBELSUS 7 mg | RYBELSUS 14 mg | |
Monotherapy | |||
(26 weeks) | N=178 | N=175 | N=175 |
Severe• | 0% | 1% | 0% |
| 1% | 0% | 0% |
Add-on to metformin and/or sulfonylurea, basal insulin alone or metformin in combination with basal insulin in patients with moderate renal impairment | |||
| N=161 | N=163 | |
Severe• | 0% | 0% | |
| 3% | 6% | |
Add-on to insulin with or without metformin | |||
| N=184 | N=181 | N=181 |
| 1% | 0% | 1% |
| 32% | 26% | 30% |
• “Severe” hypoglycemia adverse reactions are episodes requiring the assistance of another person.
Hypoglycemia was more frequent when RYBELSUS was used in combination with insulin secretagogues (e.g., sulfonylureas) or insulin .
Increases in Amylase and Lipase: In placebo-controlled trials, patients exposed to RYBELSUS 7 mg and 14 mg had a mean increase from baseline in amylase of 10% and 13%, respectively and lipase of 30% and 34%, respectively. These changes were not observed in placebo-treated patients.
Cholelithiasis: In placebo-controlled trials to improve glycemic control, cholelithiasis was reported in 1% of patients treated with RYBELSUS 7 mg.
In a 4-year CV outcomes trial (Trial 7), cholelithiasis was reported in 1.1% of patients treated with RYBELSUS 14 mg and in 0.9% of placebo-treated patients. In Trial 7, cholecystitis was reported in 1.1% of patients treated with RYBELSUS 14 mg and in 0.7% of placebo-treated patients.
Increases in Heart Rate: In placebo-controlled trials, RYBELSUS 7 mg and 14 mg resulted in a mean increase in heart rate of 1 to 3 beats per minute. There was no change in heart rate in placebo-treated patients.
Postmarketing Experience
The following adverse reactions have been reported during post-approval use of semaglutide, the active ingredient of RYBELSUS. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Gastrointestinal: acute pancreatitis and necrotizing pancreatitis, sometimes resulting in death; ileus, intestinal obstruction, severe constipation including fecal impaction
- Hypersensitivity: anaphylaxis, angioedema, rash, urticaria
- Hepatobiliary: cholecystitis, cholelithiasis requiring cholecystectomy
- Nervous system disorders: dizziness, dysesthesia, dysgeusia, headache
- Pulmonary: Pulmonary aspiration has occurred in patients receiving GLP-1 receptor agonists undergoing elective surgeries or procedures requiring general anesthesia or deep sedation
- Renal: acute kidney injury
- Skin and Subcutaneous Tissue: alopecia
DRUG INTERACTIONS
Other Oral Drugs: RYBELSUS delays gastric emptying. Consider increased clinical or laboratory monitoring when co-administered with other oral medications that have a narrow therapeutic index or that require clinical monitoring. (7.2 )
Concomitant Use with an Insulin Secretagogue (e.g., Sulfonylurea) or with Insulin
RYBELSUS stimulates insulin release in the presence of elevated blood glucose concentrations. Patients receiving RYBELSUS in combination with an insulin secretagogue (e.g., sulfonylurea) or insulin may have an increased risk of hypoglycemia, including severe hypoglycemia.
When initiating RYBELSUS, consider reducing the dose of concomitantly administered insulin secretagogue (such as sulfonylureas) or insulin to reduce the risk of hypoglycemia [see Warnings and Precautions (5.4 ), Adverse Reactions (6.1 )] .
Other Oral Drugs
RYBELSUS causes a delay of gastric emptying and thereby has the potential to impact the absorption of other oral drugs. Levothyroxine exposure was increased 33% (90% CI: 1.25-1.42) when administered with RYBELSUS in a drug interaction study [see Clinical Pharmacology (12.3 )] .
When coadministering RYBELSUS with other oral drugs that have a narrow therapeutic index or that require clinical monitoring, consider increased clinical or laboratory monitoring [see Dosage and Administration (2 )] .
DESCRIPTION
RYBELSUS tablets, for oral use, contain semaglutide, a GLP-1 receptor agonist. The peptide backbone is produced by yeast fermentation. The main protraction mechanism of semaglutide is albumin binding, facilitated by modification of position 26 lysine with a hydrophilic spacer and a C18 fatty di-acid. Furthermore, semaglutide is modified in position 8 to provide stabilization against degradation by the enzyme dipeptidyl-peptidase 4 (DPP-4). A minor modification was made in position 34 to ensure the attachment of only one fatty di-acid. The molecular formula is C 187 H 291 N 45 O 59 and the molecular weight is 4113.58 g/mol.
Structural formula:
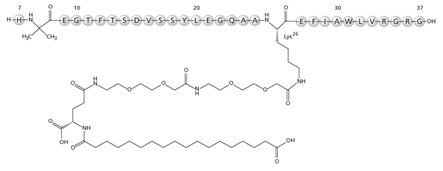
Semaglutide is a white to almost white hygroscopic powder.
Each tablet of:
- RYBELSUS (formulation R1) contains 3 mg, 7 mg or 14 mg of semaglutide and the following inactive ingredients: magnesium stearate, microcrystalline cellulose, povidone and salcaprozate sodium (SNAC).
- RYBELSUS (formulation R2) contains 1.5 mg, 4 mg or 9 mg of semaglutide and the following inactive ingredients: SNAC and magnesium stearate.
CLINICAL PHARMACOLOGY
Mechanism of Action
Semaglutide is a GLP-1 analogue with 94% sequence homology to human GLP-1. Semaglutide acts as a GLP-1 receptor agonist that selectively binds to and activates the GLP-1 receptor, the target for native GLP-1.
GLP-1 is a physiological hormone that has multiple actions on glucose, mediated by the GLP-1 receptors.
The principal mechanism of protraction resulting in the long half-life of semaglutide is albumin binding, which results in decreased renal clearance and protection from metabolic degradation. Furthermore, semaglutide is stabilized against degradation by the DPP-4 enzyme.
Semaglutide reduces blood glucose through a mechanism where it stimulates insulin secretion and lowers glucagon secretion, both in a glucose-dependent manner. Thus, when blood glucose is high, insulin secretion is stimulated and glucagon secretion is inhibited. The mechanism of blood glucose lowering also involves a minor delay in gastric emptying in the early postprandial phase.
Pharmacodynamics
The pharmacodynamic evaluations described below were in patients with type 2 diabetes mellitus who received once weekly subcutaneous injections of placebo or semaglutide injection over 12 weeks (semaglutide injection-treated patients were started on lower dosages and then titrated up to 1 mg once weekly). RYBELSUS is not approved for subcutaneous use.
Fasting and Postprandial Glucose
Semaglutide reduces fasting and postprandial glucose concentrations. In patients with type 2 diabetes mellitus, treatment with semaglutide injection 1 mg resulted in reductions in glucose in terms of absolute change from baseline and relative reduction compared to placebo of 29 mg/dL (22%) for fasting glucose, 74 mg/dL (36%) for 2-hour postprandial glucose and 30 mg/dL (22%) for mean 24 hour glucose concentration.
Insulin Secretion
Both first- and second-phase insulin secretion are increased in patients with type 2 diabetes mellitus treated with semaglutide compared with placebo.
Glucagon Secretion
Semaglutide lowers the fasting and postprandial glucagon concentrations.
Glucose dependent insulin and glucagon secretion
Semaglutide lowers high blood glucose concentrations by stimulating insulin secretion and lowering glucagon secretion in a glucose-dependent manner.
During induced hypoglycemia, semaglutide did not alter the counter regulatory responses of increased glucagon compared to placebo and did not impair the decrease of C-peptide in patients with type 2 diabetes mellitus.
Gastric emptying
Semaglutide causes a delay of early postprandial gastric emptying, thereby reducing the rate at which glucose appears in the circulation postprandially.
Cardiac Electrophysiology
The effect of subcutaneously administered semaglutide on cardiac repolarization was tested in a thorough QTc trial. At an average exposure level 4-fold higher than that of the maximum recommended dose of RYBELSUS, semaglutide does not prolong QTc intervals to any clinically relevant extent.
Pharmacokinetics
Semaglutide estimated mean steady-state concentration was 6.7 nmol/L and 14.6 nmol/L following once daily oral administration of 7 mg and 14 mg of RYBELSUS (formulation R1), respectively, in patients with type 2 diabetes mellitus.
Semaglutide exposures increased in a dose-proportional manner. Steady-state exposure was achieved following 4-5 weeks of RYBELSUS oral administration.
Absorption
Semaglutide is co-formulated with salcaprozate sodium which facilitates the absorption of semaglutide after oral RYBELSUS administration. The absorption of semaglutide predominantly occurs in the stomach.
Semaglutide estimated absolute bioavailability was approximately:
- 0.4 to 1% after oral administration of 3 mg, 7 mg and 14 mg of RYBELSUS (formulation R1).
- 1 to 2% after oral administration of 1.5 mg, 4 mg and 9 mg of RYBELSUS (formulation R2).
Semaglutide maximum concentration was reached approximately 1-hour after oral RYBELSUS administration.
Effect of RYBELSUS Formulation: There were no clinically significant differences observed in the mean steady state AUC 0-24h,SS and C max,SS between the 3 mg, 7 mg and 14 mg doses of RYBELSUS (formulation R1) and the 1.5 mg, 4 mg and 9 mg doses of RYBELSUS (formulation R2), respectively, in a clinical study conducted in healthy subjects.
Effect of Volume and Timing of Water Consumption: Single 10 mg doses of oral semaglutide (formulation R1) were administered with 50 mL or 240 mL of water after an 8-hour overnight fast and a continued fast of 4 hours post-dose in healthy subjects. Semaglutide absorption (i.e., area under the curve (AUC) and peak concentrations (Cmax)) were higher following dosing with 50 mL water compared to that of 240 mL water.
For 10 days, healthy subjects received 10 mg of oral semaglutide (formulation R1) once daily doses with 50 mL or 120 mL of water under fasting conditions with post-dose fasting period of 15, 30, 60 or 120 minutes. In this study, semaglutide absorption (i.e., AUC and C max ) was higher after a longer post-dose fasting period. There were no clinically significant differences in semaglutide absorption with administration of 50 mL or 120 mL of water.
Distribution
Semaglutide absolute volume of distribution is approximately 8 L in patients with type 2 diabetes. Semaglutide is >99% bound to plasma albumin.
Elimination
Semaglutide elimination half-life is approximately one week with an absolute clearance of approximately 0.04 L/hour in patients with type 2 diabetes. Semaglutide is present in the circulation for about five weeks after the last RYBELSUS dose.
Metabolism: The primary route of elimination for semaglutide is metabolism following proteolytic cleavage of the peptide backbone and sequential beta-oxidation of the fatty acid side chain.
Excretion: The primary excretion routes of semaglutide-related material are via the urine and feces. Approximately 3% of the absorbed dose is excreted in the urine as intact semaglutide.
Specific Populations
No clinically significant differences in semaglutide pharmacokinetics were observed based on age (≥18 years old), sex, race (White, Asian or Black or African American), ethnicity, body weight (40 to 188 kg), upper GI disease (i.e., chronic gastritis and/or gastroesophageal reflux disease), hepatic impairment (i.e., mild, moderate, severe based on the Child-Pugh system) and renal impairment (i.e., mild, moderate, severe, end staged renal disease).
Drug Interaction Studies
Clinical Studies and Model-Informed Approaches: The delay of gastric emptying with semaglutide may influence the absorption of concomitantly administered oral drugs. Trials were conducted to study the potential effect of semaglutide on the absorption of oral drugs taken with semaglutide administered orally at steady-state exposure.
- Levothyroxine : Total thyroxine (i.e., adjusted for endogenous levels) AUC of was increased by 33% following administration of a single dose of levothyroxine 600 mcg concomitantly administered with oral semaglutide. Maximum exposure (C max ) was unchanged.
- Other Drugs : No clinically significant differences in semaglutide pharmacokinetics were observed when used concomitantly with omeprazole. No clinically significant differences in the pharmacokinetics of the following drugs were observed when used concomitantly with semaglutide: lisinopril, S-warfarin, R-warfarin, metformin, digoxin, ethinyl estradiol, levonorgestrel, furosemide or rosuvastatin.
In Vitro Studies: Semaglutide has very low potential to inhibit or induce CYP enzymes, and to inhibit drug transporters.
Immunogenicity
The observed incidence of anti-drug antibodies is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of anti-drug antibodies in the studies described below with the incidence of anti-drug antibodies in other studies, including those of semaglutide or of other semaglutide products.
During the 26- to 78-week treatment periods in 5 clinical trials in adults with type 2 diabetes mellitus [see Clinical Studies (14.2 , 14.3 )] and 1 clinical trial in Japanese adults with type 2 diabetes mellitus , 14/2924 (0.5%) of RYBELSUS-treated patients developed anti-semaglutide antibodies. Of these 14 RYBELSUS-treated patients, 7 patients (0.2% of the total RYBELSUS-treated study population) developed antibodies that cross-reacted with native GLP-1. No identified clinically significant effect of anti-semaglutide antibodies on pharmacokinetics of RYBELSUS was observed. There is insufficient information to characterize the effects of anti-semaglutide antibodies on pharmacodynamics, safety or effectiveness of semaglutide.
NONCLINICAL TOXICOLOGY
Carcinogenesis, Mutagenesis, Impairment of Fertility
In a 2-year carcinogenicity study in CD-1 mice, subcutaneous doses of 0.3, 1 and 3 mg/kg/day [9-, 33- and 113- fold the maximum recommended human dose (MRHD) of RYBELSUS 14 mg, based on AUC] were administered to the males, and 0.1, 0.3 and 1 mg/kg/day (3-, 9- and 33-fold MRHD) were administered to the females. A statistically significant increase in thyroid C-cell adenomas and a numerical increase in C-cell carcinomas were observed in males and females at all dose levels (>3X human exposure).
In a 2-year carcinogenicity study in Sprague Dawley rats, subcutaneous doses of 0.0025, 0.01, 0.025 and 0.1 mg/kg/day were administered (below quantification, 0.8-, 1.8- and 11-fold the exposure at the MRHD). A statistically significant increase in thyroid C-cell adenomas was observed in males and females at all dose levels, and a statistically significant increase in thyroid C-cell carcinomas was observed in males at ≥0.01 mg/kg/day, at clinically relevant exposures.
Human relevance of thyroid C-cell tumors in rats is unknown and could not be determined by clinical studies or nonclinical studies [see Boxed Warning, Warnings and Precautions (5.1 )] .
Semaglutide was not mutagenic or clastogenic in a standard battery of genotoxicity tests (bacterial mutagenicity (Ames), human lymphocyte chromosome aberration, rat bone marrow micronucleus).
In a combined fertility and embryo-fetal development study in rats, subcutaneous doses of 0.01, 0.03 and 0.09 mg/kg/day (0.2-, 0.7- and 2.1-fold the MRHD) were administered to male and female rats. Males were dosed for 4 weeks prior to mating, and females were dosed for 2 weeks prior to mating and throughout organogenesis until Gestation Day 17. No effects were observed on male fertility. In females, an increase in estrus cycle length was observed at all dose levels, together with a small reduction in numbers of corpora lutea at ≥0.03 mg/kg/day. These effects were likely an adaptive response secondary to the pharmacological effect of semaglutide on food consumption and body weight.
Animal Toxicology and/or Pharmacology
Increase in lactate levels and decrease in glucose levels in the plasma and cerebrospinal fluid (CSF) were observed in mechanistic studies with SNAC in rats. Small but statistically significant increases in lactate levels (up to 2-fold) were observed in a few animals at approximately the clinical exposure. At higher exposures these findings were associated with moderate to marked adverse clinical signs (lethargy, abnormal respiration, ataxia, reduced activity, body tone and reflexes) and marked decreases in plasma and CSF glucose levels. These findings are consistent with inhibition of cellular respiration and lead to mortality at SNAC concentrations ≥100-times the clinical C max .
CLINICAL STUDIES
Overview of Clinical Studies
RYBELSUS has been studied as monotherapy and in combination with metformin, sulfonylureas, sodium-glucose co-transporter-2 (SGLT-2) inhibitors, insulins, and thiazolidinediones in patients with type 2 diabetes mellitus. The efficacy of RYBELSUS was compared with placebo, empagliflozin, sitagliptin and liraglutide. RYBELSUS has also been studied in patients with type 2 diabetes mellitus with mild and moderate renal impairment.
In patients with type 2 diabetes mellitus, RYBELSUS produced clinically significant reduction from baseline in HbA 1c compared with placebo.
The effectiveness of RYBELSUS (formulation R2 - 1.5 mg, 4 mg and 9 mg strengths) [see Dosage and Administration (2.2 )] and RYBELSUS (formulation R1 - 3 mg, 7, mg and 14 mg strengths) [see Dosage and Administration (2.3 )] has been established as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus based on adequate and well-controlled studies of RYBELSUS (formulation R1) in adult patients with type 2 diabetes mellitus [see Clinical Pharmacology (12.3 )] . Below is a display of the efficacy results of the adequate and well-controlled studies of RYBELSUS (formulation R1) in adult patients with type 2 diabetes mellitus.
The efficacy of RYBELSUS was not impacted by baseline age, sex, race, ethnicity, BMI, body weight, duration of diabetes and degree of renal impairment.
Monotherapy Use of RYBELSUS in Patients with Type 2 Diabetes Mellitus
In a 26-week double-blind trial (Trial 1, NCT02906930), 703 adult patients with type 2 diabetes mellitus inadequately controlled with diet and exercise were randomized to RYBELSUS 3 mg, RYBELSUS 7 mg or RYBELSUS 14 mg once daily or placebo. Patients had a mean age of 55 years and 51% were men. The mean duration of type 2 diabetes mellitus was 3.5 years and the mean BMI was 32 kg/m 2 . Overall, 75% were White, 5% were Black or African American and 17% were Asian; 26% identified as Hispanic or Latino ethnicity.
Monotherapy with RYBELSUS 7 mg and RYBELSUS 14 mg once daily for 26 weeks resulted in a statistically significant reduction in HbA 1c compared with placebo (see Table 4 ).
Table 4. Trial 1 Results at Week 26 in a Trial of RYBELSUS as Monotherapy in Adult Patients with Type 2 Diabetes Mellitus Inadequately Controlled with Diet and Exercise
Placebo | RYBELSUS 7 mg | RYBELSUS 14 mg | |
Intent-to-Treat (ITT) Population (N) a | 178 | 175 | 175 |
HbA 1c (%) | |||
| 7.9 | 8 | 8 |
| -0.3 | -1.2 | -1.4 |
| -0.9 [-1.1; -0.6] c | -1.1 [-1.3; -0.9] c | |
Patients (%) achieving HbA 1c <7% | 31 | 69 | 77 |
FPG (mg/dL) | |||
| 160 | 162 | 158 |
| -3 | -28 | -33 |
a The intent-to-treat population includes all randomized patients. At week 26, the primary HbA 1c endpoint was missing for 5.6%, 8.6% and 8.6% of patients randomized to placebo, RYBELSUS 7 mg and RYBELSUS 14 mg, respectively. Missing data were imputed by a pattern mixture model using multiple imputation (MI). Pattern was defined by randomized treatment and treatment status at week 26. During the trial, additional anti-diabetic medication was initiated as an add on to randomized treatment by 15%, 2% and 1% of patients randomized to placebo, RYBELSUS 7 mg and RYBELSUS 14 mg, respectively.
b Estimated using an ANCOVA model based on data irrespectively of discontinuation of trial product or initiation of rescue medication adjusted for baseline value and region.
c p<0.001 (unadjusted 2-sided) for superiority, controlled for multiplicity.
The mean baseline body weight was 88.6 kg, 89.0 kg and 88.1 kg in the placebo, RYBELSUS 7 mg, and RYBELSUS 14 mg arms, respectively. The mean changes from baseline to week 26 were -1.4 kg, -2.3 kg and
-3.7 kg in the placebo, RYBELSUS 7 mg and RYBELSUS 14 mg arms, respectively. The difference from placebo (95% CI) for RYBELSUS 7 mg was -0.9 kg (-1.9, 0.1) and for RYBELSUS 14 mg was -2.3 kg (-3.1, -1.5).
Combination Therapy Use of RYBELSUS in Patients with Type 2 Diabetes Mellitus
Combination with Metformin
In a 26-week trial (Trial 2, NCT02863328), 822 adult patients with type 2 mellitus diabetes were randomized to RYBELSUS 14 mg once daily or empagliflozin 25 mg once daily, all in combination with metformin. Patients had a mean age of 58 years and 50% were men. The mean duration of type 2 diabetes mellitus was 7.4 years and the mean BMI was 33 kg/m 2 . Overall, 86% were White, 7% were Black or African American and 6% were Asian; 24% identified as Hispanic or Latino ethnicity.
Treatment with RYBELSUS 14 mg once daily for 26 weeks resulted in a statistically significant reduction in HbA 1c compared to empagliflozin 25 mg once daily (see Table 5 ).
Table 5. Trial 2 Results at Week 26 in a Trial of RYBELSUS Compared to Empagliflozin in Adult Patients with Type 2 Diabetes Mellitus in Combination with Metformin
RYBELSUS 14 mg | Empagliflozin 25 mg | |
Intent-to-Treat (ITT) Population (N) a | 411 | 410 |
HbA 1c (%) | ||
| 8.1 | 8.1 |
| -1.3 | -0.9 |
| -0.4 [-0.6, -0.3] c | |
Patients (%) achieving HbA 1c <7% | 67 | 40 |
FPG (mg/dL) | ||
| 172 | 174 |
| -36 | -36 |
a The intent-to-treat population includes all randomized patients. At week 26, the primary HbA 1c endpoint was missing for 4.6% and 3.7% of patients randomized to RYBELSUS 14 mg and empagliflozin 25 mg, respectively. Missing data were imputed by a pattern mixture model using multiple imputation (MI). Pattern was defined by randomized treatment and treatment status at week 26. During the trial, additional anti-diabetic medication was initiated as an add on to randomized treatment by 1.9% and 1.2% of patients randomized to RYBELSUS 14 mg and empagliflozin 25 mg, respectively.
b Estimated using an ANCOVA based on data irrespectively of discontinuation of trial product or initiation of rescue medication adjusted for baseline value and region.
c p <0.001 (unadjusted 2-sided) for superiority, controlled for multiplicity.
The mean baseline body weight was 91.9 kg and 91.3 kg in the RYBELSUS 14 mg and empagliflozin 25 mg arms, respectively. The mean changes from baseline to week 26 were -3.8 kg and -3.7 kg in the RYBELSUS 14 mg and empagliflozin 25 mg arms, respectively. The difference from empagliflozin (95% CI) for RYBELSUS 14 mg was -0.1 kg (-0.7, 0.5).
Combination with Metformin or Metformin with Sulfonylurea
In a 26-week, double-blind trial (Trial 3, NCT02607865), 1864 adult patients with type 2 mellitus diabetes on metformin alone or metformin with sulfonylurea were randomized to RYBELSUS 3 mg, RYBELSUS 7 mg, RYBELSUS 14 mg or sitagliptin 100 mg once daily. Patients had a mean age of 58 years and 53% were men. The mean duration of type 2 diabetes mellitus was 8.6 years, and the mean BMI was 32 kg/m 2 . Overall, 71% were White, 9% were Black or African American and 13% were Asian; 17% identified as Hispanic or Latino ethnicity.
Treatment with RYBELSUS 7 mg and RYBELSUS 14 mg once daily for 26 weeks resulted in a statistically significant reduction in HbA 1c compared to sitagliptin 100 mg once daily (see Table 6 ).
Table 6. Trial 3 Results at Week 26 in a Trial of RYBELSUS Compared to Sitagliptin 100 mg Once Daily in Adult Patients with Type 2 Diabetes Mellitus in Combination with Metformin or Metformin with Sulfonylurea
RYBELSUS 7 mg | RYBELSUS 14 mg | Sitagliptin 100 mg | |
Intent-to-Treat (ITT) Population (N) a | 465 | 465 | 467 |
HbA 1c (%) | |||
| 8.4 | 8.3 | 8.3 |
| -1 | -1.3 | -0.8 |
| -0.3 [-0.4; -0.1] c | -0.5 [-0.6; -0.4] c | |
Patients (%) achieving HbA 1c <7% | 44 | 56 | 32 |
FPG (mg/dL) | |||
| 170 | 168 | 172 |
| -21 | -31 | -15 |
a The intent-to-treat population includes all randomized patients. At week 26, the primary HbA 1c endpoint was missing for 5.8%, 6.2% and 4.5% of patients randomized to RYBELSUS 7 mg, RYBELSUS 14 mg and sitagliptin 100 mg, respectively. Missing values were imputed by a pattern mixture model using multiple imputation (MI). Pattern was defined by randomized treatment and treatment status at week 26. During the trial, additional anti-diabetic medication was initiated as an add on to randomized treatment by 2.4%, 1.1% and 2.8% of patients randomized to RYBELSUS 7 mg, RYBELSUS 14 mg and sitagliptin 100 mg, respectively.
b Estimated using an ANCOVA based on data irrespectively of discontinuation of trial product or initiation of rescue medication adjusted for baseline value, background medication and region.
c p <0.001 (unadjusted 2-sided) for superiority, controlled for multiplicity.
The mean baseline body weight was 91.3 kg, 91.2 kg and 90.9 kg in the RYBELSUS 7 mg, RYBELSUS 14 mg and sitagliptin 100 mg arms, respectively. The mean changes from baseline to week 26 were -2.2 kg, -3.1 kg and -0.6 kg in the RYBELSUS 7 mg, RYBELSUS 14 mg and sitagliptin 100 mg arms, respectively. The difference from sitagliptin (95% CI) for RYBELSUS 7 mg was -1.6 kg (-2.0, -1.1) and RYBELSUS 14 mg was -2.5 kg (-3.0, -2.0).
Combination with Metformin or Metformin with SGLT-2 Inhibitors
In a 26-week, double-blind, double-dummy trial (Trial 4, NCT02863419), 711 adult patients with type 2 diabetes mellitus on metformin alone or metformin with SGLT-2 inhibitors were randomized to RYBELSUS 14 mg once daily, liraglutide 1.8 mg subcutaneous injection once daily or placebo. Patients had a mean age of 56 years and 52% were men. The mean duration of type 2 diabetes mellitus was 7.6 years and the mean BMI was 33 kg/m 2 . Overall, 73% were White, 4% were Black or African American and 13% were Asian; 6% identified as Hispanic or Latino ethnicity.
Treatment with RYBELSUS 14 mg once daily for 26 weeks resulted in statistically significant reductions in HbA 1c compared to placebo. Treatment with RYBELSUS 14 mg once daily for 26 weeks resulted in non-inferior reductions in HbA 1c compared to liraglutide 1.8 mg (see Table 7 ).
Table 7. Trial 4 Results at Week 26 in a Trial of RYBELSUS Compared to Liraglutide and Placebo in Adult Patients with Type 2 Diabetes Mellitus in Combination with Metformin or Metformin with SGLT-2i
Placebo | RYBELSUS 14 mg | Liraglutide 1.8 mg | |
Intent-to-Treat (ITT) Population (N) a | 142 | 285 | 284 |
HbA 1c (%) | |||
| 7.9 | 8 | 8 |
| -0.2 | -1.2 | -1.1 |
| -1.1 [-1.2; -0.9] c | ||
| -0.1 [-0.3; 0] | ||
Patients (%) achieving HbA 1c <7% | 14 | 68 | 62 |
FPG (mg/dL) | |||
| 167 | 167 | 168 |
| -7 | -36 | -34 |
a The intent-to-treat population includes all randomized patients. At week 26, the primary HbA 1c endpoint was missing for 5.6%, 4.2% and 2.5% of patients randomized to placebo, liraglutide 1.8 mg and RYBELSUS 14 mg, respectively. Missing values were imputed by a pattern mixture model using multiple imputation (MI). Pattern was defined by randomized treatment and treatment status at week 26. During the trial, additional anti-diabetic medication was initiated as an add on to randomized treatment by 7.7%, 3.2% and 3.5% of patients randomized to placebo, liraglutide 1.8 mg and RYBELSUS 14 mg respectively.
b Estimated using an ANCOVA based on data irrespectively of discontinuation of trial product or initiation of rescue medication adjusted for baseline value, background medication and region.
c p <0.001 (unadjusted 2-sided) for superiority, controlled for multiplicity.
The mean baseline body weight was 93.2 kg, 95.5 kg and 92.9 kg in the placebo, liraglutide 1.8 mg and RYBELSUS 14 mg arms, respectively. The mean changes from baseline to week 26 were -0.5 kg, -3.1 kg and
-4.4 kg in the placebo, liraglutide 1.8 mg and RYBELSUS 14 mg arms, respectively. The difference from placebo (95% CI) for RYBELSUS 14 mg was -3.8 kg (-4.7, -3.0). The difference from liraglutide 1.8 mg for RYBELSUS 14 mg was -1.2 (-1.9, -0.6).
Combination in patients with Type 2 Diabetes Mellitus and Moderate Renal Impairment with Metformin alone, Sulfonylurea alone, Basal Insulin alone or Metformin in Combination with either Sulfonylurea or Basal Insulin
In a 26-week, double-blind trial (Trial 5, NCT02827708), 324 adult patients with moderate renal impairment (eGFR CKD-EPI 30-59 mL/min/1.73 m 2 ) were randomized to RYBELSUS 14 mg or placebo once daily. RYBELSUS was added to the patient’s stable pre-trial antidiabetic regimen. The insulin dose was reduced by 20% at randomization for patients on basal insulin. Dose reduction of insulin and sulfonylurea was allowed in case of hypoglycemia; up titration of insulin was allowed but not beyond the pre-trial dose.
Patients had a mean age of 70 years and 48% were men. The mean duration of type 2 diabetes mellitus was 14 years and the mean BMI was 32 kg/m 2 . Overall, 96% were White, 4% were Black or African American and 0.3% were Asian; 6.5% identified as Hispanic or Latino ethnicity. 39.5% of patients had an eGFR value of 30 to 44 mL/min/1.73 m 2 .
Treatment with RYBELSUS 14 mg once daily for 26 weeks resulted in a statistically significant reduction in HbA 1c from baseline compared to placebo (see Table 8 ).
Table 8. Trial 5 Results at Week 26 in a Trial of RYBELSUS Compared to Placebo in Patients with Moderate Renal Impairment
Placebo | RYBELSUS 14 mg | |
Intent-to-Treat (ITT) Population (N) a | 161 | 163 |
HbA 1c (%) | ||
| 7.9 | 8 |
| -0.2 | -1 |
| -0.8 [-1.0; -0.6] c | |
Patients (%) achieving HbA 1c <7% | 23 | 58 |
FPG (mg/dL) | ||
| 164 | 164 |
| -7 | -28 |
a The intent-to-treat population includes all randomized patients including patients on rescue medication. At week 26, the primary HbA 1c endpoint was missing for 3.7% and 5.5% of patients randomized to placebo and RYBELSUS 14 mg, respectively. Missing values were imputed by a pattern mixture model using multiple imputation (MI). Pattern was defined by randomized treatment and treatment status at week 26. During the trial, additional anti-diabetic medication was initiated as an add on to randomized treatment by 10% and 4.3% of patients randomized to placebo and RYBELSUS 14 mg, respectively.
b Estimated using an ANCOVA based on data irrespectively of discontinuation of trial product or initiation of rescue medication adjusted for baseline value, background medication, renal status and region.
c p <0.001 (unadjusted 2-sided) for superiority, controlled for multiplicity.
The mean baseline body weight was 90.4 kg and 91.3 kg in the placebo and RYBELSUS 14 mg arms, respectively. The mean changes from baseline to week 26 were -0.9 kg and -3.4 kg in the placebo and RYBELSUS 14 mg arms, respectively. The difference from placebo (95% CI) for RYBELSUS 14 mg was -2.5 kg (-3.2, -1.8).
Combination with Insulin with or without Metformin
In a 26-week double blind trial (Trial 6, NCT03021187), 731 adult patients with type 2 diabetes mellitus inadequately controlled on insulin (basal, basal/bolus or premixed) with or without metformin, were randomized to RYBELSUS 3 mg, 7 mg and 14 mg once daily or placebo once daily. All patients reduced their insulin dose by 20% at randomization to reduce the risk of hypoglycemia. Patients were allowed to increase the insulin dose only up to the starting insulin dose prior to randomization.
Patients had a mean age of 61 years and 54% were men. The mean duration of type 2 diabetes mellitus was 15 years and the mean BMI was 31 kg/m 2 . Overall, 51% were White, 7% were Black or African American and 36% were Asian; 13% identified as Hispanic or Latino ethnicity.
Treatment with RYBELSUS 7 mg and 14 mg once daily for 26 weeks resulted in a statistically significant reduction in HbA 1c from baseline compared to placebo once daily (see Table 9 ).
Table 9. Trial 6 Results at Week 26 in a Trial of RYBELSUS Compared to Placebo in Adult Patients with Type 2 Diabetes Mellitus in Combination with Insulin alone or with Metformin
Placebo | RYBELSUS 7 mg | RYBELSUS 14 mg | |
Intent-to-Treat (ITT) Population (N) a | 184 | 182 | 181 |
HbA 1c (%) | |||
| 8.2 | 8.2 | 8.2 |
| -0.1 | -0.9 | -1.3 |
| -0.9 [-1.1; -0.7] c | -1.2 [-1.4; -1.0] c | |
Patients (%) achieving HbA 1c <7% | 7 | 43 | 58 |
FPG (mg/dL) | |||
| 150 | 153 | 150 |
| 5 | -20 | -24 |
a The intent-to-treat population includes all randomized patients. At week 26, the primary HbA 1c endpoint was missing for 4.3%, 4.4% and 4.4% of patients randomized to placebo, RYBELSUS 7 mg and RYBELSUS 14 mg, respectively. Missing values were imputed by a pattern mixture model using multiple imputation (MI). Pattern was defined by randomized treatment and treatment status at week 26. During the trial, additional anti-diabetic medication was initiated as an add on to randomized treatment by 4.9%, 1.1 % and 2.2% of patients randomized to placebo, RYBELSUS 7 mg and RYBELSUS 14 mg, respectively.
b Estimated using an ANCOVA based on data irrespectively of discontinuation of trial product or initiation of rescue medication adjusted for baseline value, background medication and region.
c p <0.001 (unadjusted 2-sided) for superiority, controlled for multiplicity.
The mean baseline body weight was 86.0 kg, 87.1 kg and 84.6 kg in the placebo, RYBELSUS 7 mg and RYBELSUS 14 mg arms, respectively. The mean changes from baseline to week 26 were -0.4 kg, -2.4 kg and -3.7 kg in the placebo, RYBELSUS 7 mg and RYBELSUS 14 mg arms, respectively. The difference from placebo (95% CI) for RYBELSUS 7 mg was -2.0 kg (-3.0, -1.0) and for RYBELSUS 14 mg was -3.3 kg (-4.2, -2.3).
Cardiovascular Outcomes Trial of RYBELSUS in Adult Patients with Type 2 Diabetes Mellitus and High Risk of Having a Cardiovascular Event
Trial 7 [Semaglutide Cardiovascular Outcomes Trial (SOUL), NCT03914326] was a randomized, double-blind, parallel-group, placebo-controlled trial. In this trial, 9,650 patients with type 2 diabetes mellitus and established cardiovascular (CV) disease and/or chronic kidney disease (CKD) (defined as eGFR <60 mL/min/1.73 m 2 ), were randomized to either once daily oral RYBELSUS 14 mg or placebo, in addition to standard of care. The primary endpoint, major adverse cardiovascular events (MACE), was the time to first occurrence of a three-part composite outcome which included CV death, non-fatal myocardial infarction (MI) and non-fatal stroke.
Patients eligible to enter the trial were: 50 years of age or older and with established CV disease or CKD. In total, 5,468 patients (56.7%) had established CV disease without CKD, 1,241 (13%) had CKD only, and 2,620 (27%) had both CV disease and CKD. Most patients (97%) were treated with one or more glucose lowering medications at baseline: metformin (76%), insulin (51%), SGLT-2 inhibitor (27%), sulfonylurea (29%), and DPP-4 inhibitor (23%). At baseline, CV disease and risk factors were managed with lipid-lowering medications (89%), platelet-aggregation inhibitors including aspirin (77%), angiotensin converting enzyme inhibitors or angiotensin II receptor blockers (79%), and beta blockers (64%).
The mean age at baseline was 66.1 years, and 71% of the patients were men. Overall, 69% were White, 3% were Black or African American, and 23% were Asian; 14% identified as Hispanic or Latino ethnicity.
The mean duration of diabetes was 15.4 years, the mean BMI was 31.1 kg/m 2 , the mean HbA 1c was 8%, and the mean eGFR was 73.8 mL/min/1.73 m 2 . Concomitant diseases included heart failure (23%), hypertension (91%), previous ischemic stroke (12%), previous MI (40%), and peripheral artery disease (16%). In total, 98.4% patients completed the trial, and the vital status was known at the end of the trial for 99.5%.
RYBELSUS significantly reduced the occurrence of MACE. The estimated hazard ratio for time to first MACE was 0.86 (95% CI: 0.77, 0.96) over the median follow-up duration of 49.6 months and 49.4 months for patients
randomized to RYBELSUS and placebo, respectively. Refer to Table 10 and Figure 1 . The treatment effect for the primary composite endpoint and its components in Trial 7 are shown in Table 10.
Table 10. Trial 7 Treatment Effect for MACE and its Components
PLACEBO N=4,825 | RYBELSUS 14 mg N=4,825 | Hazard ratio vs Placebo (95% CI) | |
Primary composite endpoint | |||
MACE: Composite of CV death, non-fatal MI, non-fatal stroke (time to first occurrence) | 668 (13.8%) | 579 (12.0%) | 0.86 (0.77, 0.96) |
CV Death | 320 (6.6%) | 301 (6.2%) | 0.93 (0.80, 1.09) |
Non-fatal MI | 253 (5.2%) | 191 (4.0%) | 0.74 (0.61, 0.89) |
Non-fatal Stroke | 161 (3.3%) | 144 (3.0%) | 0.88 (0.70, 1.11) |
Note: Data from the in-trial period based on full analysis set defined as all randomized patients.
Time from randomization to each endpoint was analyzed using a Cox proportional hazards model with treatment as categorical fixed factor. Subjects without events of interest were censored at the end of their in-trial period. For the primary endpoint the hazard ratio and CI were adjusted for the group sequential design using likelihood ratio ordering. CV death, non-fatal MI and non-fatal stroke are listed descriptively for supportive purpose. CV death includes both CV death and undetermined cause of death.
N=number of patients; %: percentage of participants in the full analysis set with at least one event; CI: confidence interval
Figure 1: Time to First Occurrence of a MACE in Trial 7

Data from the in-trial period based on full analysis set defined as all randomized patients. Cumulative incidence estimates are based on time from randomization to first EAC-confirmed MACE with non-CV death modeled as competing risk using the Aalen-Johansen estimator. Patients without events of interest were censored at the end of their in-trial observation period. Time from randomization to first MACE was analyzed using a Cox proportional hazards model with treatment as categorical fixed factor. The hazard ratio and confidence interval are adjusted for the group sequential design using the likelihood ratio ordering. HR: Hazard ratio; CI: confidence interval.
Cardiovascular Outcomes Trial in Patients with Type 2 Diabetes Mellitus and Cardiovascular Disease
Trial 8 [Peptide Innovation for Early Diabetes Treatment (PIONEER 6), NCT02692716] was a multi-center, multi-national, placebo-controlled, double-blind trial. In this trial, 3,183 adult patients with inadequately controlled type 2 diabetes mellitus and atherosclerotic CV disease were randomized to RYBELSUS 14 mg once daily or placebo for a median observation time of 16 months. The trial compared the risk of a MACE between RYBELSUS 14 mg and placebo when added to current standard of care treatments for diabetes and CV disease. The primary endpoint, MACE, was the time to first occurrence of a three-part composite outcome which included CV death, non-fatal MI and non-fatal stroke.
Patients eligible to enter the trial were 50 years of age or older and had established, stable, CV, cerebrovascular, peripheral artery disease, CKD or NYHA class II and III heart failure or were 60 years of age or older and had other specified risk factors for CV disease. In total, 1,797 patients (56.5%) had established CV disease without CKD, 354 patients (11.1%) had CKD only, and 544 patients (17.1%) had both CV disease and CKD; 488 patients (15.3%) had CV risk factors without established CV disease or CKD. The mean age at baseline was 66 years and 68% were men. The mean duration of diabetes was 14.9 years and mean BMI was 32 kg/m 2 . Overall, 72% were White, 6% were Black or African American and 20% were Asian; 16% identified as Hispanic or Latino ethnicity. Concomitant diseases of patients in this trial included, but were not limited to, heart failure (12%), history of ischemic stroke (8%) and history of a MI (36%). In total, 99.7% of the patients completed the trial and the vital status was known at the end of the trial for 100%.
For the primary analysis, a Cox proportional hazards model was used to test for non-inferiority of RYBELSUS 14 mg to placebo for time to first MACE using a risk margin of 1.3. Type-1 error was controlled across multiple tests using a hierarchical testing strategy. Non‑inferiority to placebo was established, with a hazard ratio equal to 0.79 (95% CI: 0.57, 1.11) over the median observation time of 16-months. The proportion of patients who experienced at least one MACE was 3.8% (61/1591) for RYBELSUS 14 mg and 4.8% (76/1592) for placebo.
HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
RYBELSUS (formulation R1) strengths are available as follows:
Tablet Strength | Description | Package Configuration | NDC Number |
3 mg | White to light yellow, oval shaped debossed with “3” on one side and “novo” on the other side | Bottle of 30 tablets | 0169-4303-30 |
7 mg | White to light yellow, oval shaped debossed with “7” on one side and “novo” on the other side | Bottle of 30 tablets | 0169-4307-30 |
14 mg | White to light yellow, oval shaped debossed with “14” on one side and “novo” on the other side | Bottle of 30 tablets | 0169-4314-30 |
RYBELSUS (formulation R2) strengths are available as follows:
Tablet Strength | Description | Package Configuration | NDC Number |
1.5 mg | White to light yellow, round shaped debossed with “1.5” on one side and “novo” on the other side | Bottle of 30 tablets | 0169-4815-30 |
4 mg | White to light yellow, round shaped debossed with “4” on one side and “novo” on the other side | Bottle of 30 tablets | 0169-4804-30 |
9 mg | White to light yellow, round shaped debossed with “9” on one side and “novo” on the other side | Bottle of 30 tablets | 0169-4809-30 |
Storage and Handling
Store at 68°F to 77°F (20°C to 25°C); excursions permitted to 59°F to 86°F (15°C to 30°C) [see USP Controlled Room Temperature]. Store and dispense in the original bottle.
Store tablet in the original bottle until use to protect tablets from moisture. Store product in a dry place away from moisture.
Mechanism of Action
Semaglutide is a GLP-1 analogue with 94% sequence homology to human GLP-1. Semaglutide acts as a GLP-1 receptor agonist that selectively binds to and activates the GLP-1 receptor, the target for native GLP-1.
GLP-1 is a physiological hormone that has multiple actions on glucose, mediated by the GLP-1 receptors.
The principal mechanism of protraction resulting in the long half-life of semaglutide is albumin binding, which results in decreased renal clearance and protection from metabolic degradation. Furthermore, semaglutide is stabilized against degradation by the DPP-4 enzyme.
Semaglutide reduces blood glucose through a mechanism where it stimulates insulin secretion and lowers glucagon secretion, both in a glucose-dependent manner. Thus, when blood glucose is high, insulin secretion is stimulated and glucagon secretion is inhibited. The mechanism of blood glucose lowering also involves a minor delay in gastric emptying in the early postprandial phase.