Get your patient on Ameluz (Aminolevulinic Acid Hydrochloride)
Ameluz prior authorization resources
Most recent state uniform prior authorization forms
Brand Resources
Ameluz patient education
Patient toolkit
Dosage & administration
DOSAGE AND ADMINISTRATION
- Administer AMELUZ only by a health care provider (2.1 ).
- AMELUZ is for topical use only (2.1 ).
- Use AMELUZ in combination with red light photodynamic therapy (PDT). See BF-RhodoLED or RhodoLED XL user manual for detailed lamp safety and operating instructions (2.1 ).
- Photodynamic therapy with AMELUZ involves preparation of lesions, application of the product, occlusion and illumination with BF-RhodoLED or RhodoLED XL lamp (2.3 ).
- Apply an approximately 1 mm thick layer of AMELUZ to skin lesion(s). Cover individual lesions or the entire AK-field with AMELUZ. Include approximately 5 mm of the surrounding skin. Do not exceed an application area of 60 cm 2 . Do not use more than 6 grams of AMELUZ (3 tubes) at one time (2.2 ).
- Retreat lesions that have not completely resolved 3 months after the initial treatment (2.2 ).
Important Administration Information
AMELUZ, in conjunction with lesion preparation, is only to be administered by a health care provider.
AMELUZ is for topical use only. Not for ophthalmic, oral, or intravaginal use.
Treat single lesions or an entire field affected by multiple lesions with AMELUZ, in combination with red light photodynamic therapy (PDT). PDT requires administration of both AMELUZ and BF-RhodoLED or RhodoLED XL light.
Refer to BF-RhodoLED or RhodoLED XL user manual for detailed lamp safety and operating instructions. Adhere to all safety instructions for both patient and medical personnel while conducting the PDT.
Recommended Dosage
Apply an approximately 1-mm thick layer of AMELUZ to skin lesion(s). Cover individual lesions or the entire AK-field with AMELUZ. Include approximately 5 mm of the surrounding skin. Do not exceed an application area of 60 cm 2 . Do not use more than 6 grams of AMELUZ (3 tubes) at one time.
Retreat lesions that have not completely resolved after 3 months after the initial treatment.
Administration Instructions
PDT is a multi-stage process:
Step 1. Preparation of Lesions
Before applying AMELUZ, carefully wipe all lesions with an ethanol or isopropanol-soaked cotton pad to ensure degreasing of the skin.
 Figure 1A: Degreasing the skin
Figure 1A: Degreasing the skin
Thereafter, remove any scaling and crusts and gently roughen all lesion surfaces, taking care to avoid bleeding.
 Figure 1B: Removal of scales and crusts
Figure 1B: Removal of scales and crusts
Step 2. Application of AMELUZ
Apply AMELUZ using glove protected fingertips or a spatula. Use sufficient amount of gel to cover individual lesions or the entire field:
- Lesion-directed treatment: Apply gel approximately 1 mm thick to one or more individual AK lesions and include approximately 5 mm of the surrounding healthy skin.
- Field-directed treatment: Apply gel approximately 1 mm thick to the treatment field. Apply gel to the lesions and the skin in-between the lesions. Additionally, cover approximately 5 mm of the surrounding healthy area.
Do not exceed an application area of 60 cm 2 and do not use more than 6 grams of AMELUZ (3 tubes) at one time. The gel can be applied to healthy skin around the lesions. Avoid application near mucous membranes such as the eyes, nostrils, mouth, and ears (keep a distance of 1 cm from these areas). In case of accidental contact with mucous membranes, thoroughly rinse with water [ see Warnings and Precautions (5.7) ].
Allow the gel to dry for approximately 10 minutes before applying occlusive dressing.
 Figure 2: Drug application
Figure 2: Drug application
Step 3. Occlusion for 3 Hours
Cover the area where the gel has been applied with a light-blocking, occlusive dressing. Following 3 hours of occlusion, remove the dressing and wipe off any remaining gel.
 Figure 3: Occlusion
Figure 3: Occlusion
Step 4. Illumination with Red Light
For patient and medical personnel, wear suitable protective eyewear during illumination. Avoid staring directly into the light source [ see Warnings and Precautions (5.3) ].
Illuminate the treatment area with the BF-RhodoLED or RhodoLED XL lamp immediately after removing occlusion and any remaining gel. BF-RhodoLED and RhodoLED XL lamps are red light sources with a narrow spectrum around 635 nm that deliver a light dose of approximately 37 J/cm 2 . Calibration by the operator is not needed; the illumination time is calculated automatically. Physical measures such as cooling with an air stream or nebulized water may help reduce pain during illumination.
Either the BF-RhodoLED or RhodoLED XL lamp can be used:
- BF-RhodoLED has an effective treatment area of 6 x 16 cm when an area of 8 x 18 cm is illuminated. Position the lamp head 5-8 cm from the skin’s surface. Larger areas can be illuminated in several steps.
- RhodoLED XL has a curved configuration with an effective treatment area up to 23 x 29 cm. Position the lamp head of the RhodoLED XL 11-14 cm from the skin’s surface. This usually allows a full-face illumination with the use of 5 panels. The smallest recommended number of panels to be used are 3 adjacent panels (see chapter 8.4.6 of RhodoLED XL user manual).
Healthy untreated skin surrounding the AK lesions does not need protection during illumination.
 Figure 4A: Illumination with BF-RhodoLED
Figure 4A: Illumination with BF-RhodoLED
 Figure 4B: Illumination with RhodoLED XL
Figure 4B: Illumination with RhodoLED XL
If for any reason, the lesions cannot be illuminated within 3 hours after AMELUZ application, rinse off the gel with saline and water. For 2 days, protect the lesion sites and surrounding skin from sunlight or prolonged or intense light (e.g., tanning beds, sun lamps).

By using PrescriberAI, you agree to the AI Terms of Use.
Ameluz prescribing information
INDICATIONS AND USAGE
AMELUZ, in combination with photodynamic therapy (PDT) using BF-RhodoLED ® or RhodoLED ® XL lamp, a narrowband, red light illumination source, is indicated for lesion-directed and field-directed treatment of actinic keratoses (AKs) of mild-to-moderate severity on the face and scalp.
DOSAGE AND ADMINISTRATION
- Administer AMELUZ only by a health care provider (2.1 ).
- AMELUZ is for topical use only (2.1 ).
- Use AMELUZ in combination with red light photodynamic therapy (PDT). See BF-RhodoLED or RhodoLED XL user manual for detailed lamp safety and operating instructions (2.1 ).
- Photodynamic therapy with AMELUZ involves preparation of lesions, application of the product, occlusion and illumination with BF-RhodoLED or RhodoLED XL lamp (2.3 ).
- Apply an approximately 1 mm thick layer of AMELUZ to skin lesion(s). Cover individual lesions or the entire AK-field with AMELUZ. Include approximately 5 mm of the surrounding skin. Do not exceed an application area of 60 cm 2 . Do not use more than 6 grams of AMELUZ (3 tubes) at one time (2.2 ).
- Retreat lesions that have not completely resolved 3 months after the initial treatment (2.2 ).
Important Administration Information
AMELUZ, in conjunction with lesion preparation, is only to be administered by a health care provider.
AMELUZ is for topical use only. Not for ophthalmic, oral, or intravaginal use.
Treat single lesions or an entire field affected by multiple lesions with AMELUZ, in combination with red light photodynamic therapy (PDT). PDT requires administration of both AMELUZ and BF-RhodoLED or RhodoLED XL light.
Refer to BF-RhodoLED or RhodoLED XL user manual for detailed lamp safety and operating instructions. Adhere to all safety instructions for both patient and medical personnel while conducting the PDT.
Recommended Dosage
Apply an approximately 1-mm thick layer of AMELUZ to skin lesion(s). Cover individual lesions or the entire AK-field with AMELUZ. Include approximately 5 mm of the surrounding skin. Do not exceed an application area of 60 cm 2 . Do not use more than 6 grams of AMELUZ (3 tubes) at one time. Retreat lesions that have not completely resolved after 3 months after the initial treatment.
Administration Instructions
PDT is a multi-stage process:
Step 1. Preparation of Lesions
Before applying AMELUZ, carefully wipe all lesions with an ethanol or isopropanol-soaked cotton pad to ensure degreasing of the skin.
 Figure 1A: Degreasing the skin
Figure 1A: Degreasing the skin
Thereafter, remove any scaling and crusts and gently roughen all lesion surfaces, taking care to avoid bleeding.
 Figure 1B: Removal of scales and crusts
Figure 1B: Removal of scales and crusts
Step 2. Application of AMELUZ
Apply AMELUZ using glove protected fingertips or a spatula. Use sufficient amount of gel to cover individual lesions or the entire field:
- Lesion-directed treatment: Apply gel approximately 1 mm thick to one or more individual AK lesions and include approximately 5 mm of the surrounding healthy skin.
- Field-directed treatment: Apply gel approximately 1 mm thick to the treatment field. Apply gel to the lesions and the skin in-between the lesions. Additionally, cover approximately 5 mm of the surrounding healthy area.
Do not exceed an application area of 60 cm 2 and do not use more than 6 grams of AMELUZ (3 tubes) at one time. The gel can be applied to healthy skin around the lesions. Avoid application near mucous membranes such as the eyes, nostrils, mouth, and ears (keep a distance of 1 cm from these areas). In case of accidental contact with mucous membranes, thoroughly rinse with water [ see Warnings and Precautions (5.7) ].
Allow the gel to dry for approximately 10 minutes before applying occlusive dressing.
 Figure 2: Drug application
Figure 2: Drug application
Step 3. Occlusion for 3 Hours
Cover the area where the gel has been applied with a light-blocking, occlusive dressing. Following 3 hours of occlusion, remove the dressing and wipe off any remaining gel.
 Figure 3: Occlusion
Figure 3: Occlusion
Step 4. Illumination with Red Light
For patient and medical personnel, wear suitable protective eyewear during illumination. Avoid staring directly into the light source [ see Warnings and Precautions (5.3) ].
Illuminate the treatment area with the BF-RhodoLED or RhodoLED XL lamp immediately after removing occlusion and any remaining gel. BF-RhodoLED and RhodoLED XL lamps are red light sources with a narrow spectrum around 635 nm that deliver a light dose of approximately 37 J/cm 2 . Calibration by the operator is not needed; the illumination time is calculated automatically. Physical measures such as cooling with an air stream or nebulized water may help reduce pain during illumination.
Either the BF-RhodoLED or RhodoLED XL lamp can be used:
- BF-RhodoLED has an effective treatment area of 6 x 16 cm when an area of 8 x 18 cm is illuminated. Position the lamp head 5-8 cm from the skin’s surface. Larger areas can be illuminated in several steps.
- RhodoLED XL has a curved configuration with an effective treatment area up to 23 x 29 cm. Position the lamp head of the RhodoLED XL 11-14 cm from the skin’s surface. This usually allows a full-face illumination with the use of 5 panels. The smallest recommended number of panels to be used are 3 adjacent panels (see chapter 8.4.6 of RhodoLED XL user manual).
Healthy untreated skin surrounding the AK lesions does not need protection during illumination.
 Figure 4A: Illumination with BF-RhodoLED
Figure 4A: Illumination with BF-RhodoLED
 Figure 4B: Illumination with RhodoLED XL
Figure 4B: Illumination with RhodoLED XL
If for any reason, the lesions cannot be illuminated within 3 hours after AMELUZ application, rinse off the gel with saline and water. For 2 days, protect the lesion sites and surrounding skin from sunlight or prolonged or intense light (e.g., tanning beds, sun lamps).
DOSAGE FORMS AND STRENGTHS
Topical gel: 10% aminolevulinic acid hydrochloride as a white-to-yellowish gel in 2-gram tubes.
USE IN SPECIFIC POPULATIONS
Pregnancy
Risk Summary
There are no available data on AMELUZ use in pregnant women to inform a drug associated risk. Animal reproduction studies were not conducted with aminolevulinic acid. Systemic absorption of aminolevulinic acid in humans is negligible following topical administration of AMELUZ under maximal clinical use conditions [see Clinical Pharmacology (12.3) ] . It is not expected that maternal use of AMELUZ will result in fetal exposure to the drug.
The background risk of major birth defects and miscarriage for the indicated population are unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Lactation
Risk Summary
No data are available regarding the presence of aminolevulinic acid in human milk, the effects of aminolevulinic acid on the breastfed infant or on milk production. However, breastfeeding is not expected to result in exposure of the child to the drug due to the negligible systemic absorption of aminolevulinic acid in humans following topical administration of AMELUZ under maximal clinical use conditions [see Clinical Pharmacology (12.3) ] . The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for AMELUZ and any potential adverse effects on the breastfeeding child from AMELUZ or from the underlying maternal condition.
Pediatric Use
Safety and effectiveness in pediatric patients below the age of 18 have not been established. AK is not a condition generally seen in the pediatric population.
Geriatric Use
Of the 384 subjects exposed to AMELUZ in randomized, multicenter clinical trials, 83% (318/384) of the subjects were 65 years old and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, but greater sensitivity of some older individuals cannot be ruled out.
CONTRAINDICATIONS
AMELUZ is contraindicated in patients with:
- Known hypersensitivity to porphyrins.
- Known hypersensitivity to any of the components of AMELUZ, which includes soybean phosphatidylcholine [see Warnings and Precautions (5.1) ] .
- Porphyria. AMELUZ use may cause uncontrolled phototoxic effects [see Warnings and Precautions (5.5) ] .
- Photodermatoses. PDT may worsen the phototoxic or photoallergic reactions [see Warnings and Precautions (5.5) ] .
WARNINGS AND PRECAUTIONS
- Hypersensitivity: Hypersensitivity reactions have been reported with the use of AMELUZ prior to photodynamic therapy (PDT). If allergic reaction occurs, wash off AMELUZ and institute appropriate therapy (5.1 ).
- Transient Amnestic Episodes: Transient amnestic episodes have been reported with use of AMELUZ in combination with PDT. Advise patients to contact their healthcare provider if amnesia or confusion occurs after treatment (5.2 ).
- Risk of BF-RhodoLED or RhodoLED XL Lamp Induced Eye Injury: Patients and healthcare providers must wear protective eyewear before operating BF-RhodoLED or RhodoLED XL lamp (5.3 ).
- Ophthalmic Adverse Reactions: Avoid direct contact of AMELUZ with the eyes (5.4 ).
- Increased Photosensitivity: Protect treated lesions from sunlight exposure for 48 hours post treatment (5.5 ).
- Risk of Bleeding in Patients with Coagulation Disorders: Take special care to avoid bleeding during lesion preparation in patients with inherited or acquired coagulation disorders (5.6 ).
- Mucous Membrane Irritation: Avoid direct contact of AMELUZ with the mucous membranes (5.7 ).
Hypersensitivity
Several cases of hypersensitivity were reported during postmarketing use of AMELUZ prior to PDT illumination [ see Adverse Reactions (6.2) ]. If allergic reactions occur, clean the area of skin where the product was applied and institute appropriate therapy. Inform patients and their caregivers that AMELUZ may cause hypersensitivity, potentially including severe courses (anaphylaxis).
Transient Amnestic Episodes
Transient amnestic episodes have been reported during postmarketing use of AMELUZ in combination with photodynamic therapy. Inform patients and their caregivers that AMELUZ in combination with photodynamic therapy may cause transient amnestic episodes. Advise them to contact the healthcare provider if the patient develops amnesia after treatment.
Risk of BF-RhodoLED or RhodoLED XL Lamp Induced Eye Injury
BF-RhodoLED or RhodoLED XL lamp may cause eye irritation, glare, or injury. Before operating the lamp, personnel must refer to the user manual for specific warnings, cautions, and instructions. Eye exposure to the BF-RhodoLED or RhodoLED XL light must be prevented. Protective eye equipment must be used by patient, healthcare providers and any person present during the illumination period. Avoid staring directly into the light source.
Ophthalmic Adverse Reactions
Eyelid edema and dry eyes have occurred after PDT with AMELUZ. PDT with AMELUZ can cause ophthalmic adverse reactions. Avoid direct contact of AMELUZ with the eyes. Rinse eyes with water in case of accidental contact.
Increased Photosensitivity
AMELUZ increases photosensitivity. Avoid sunlight, prolonged or intense light (e.g., tanning beds, sun lamps) on lesions and surrounding skin treated with AMELUZ for approximately 48 hours following treatment, whether exposed to illumination or not. Concomitant use of AMELUZ with other known photosensitizing agents may increase the risk of phototoxic reaction to PDT [see Drug Interactions (7) ] .
Risk of Bleeding in Patients with Coagulation Disorders
AMELUZ has not been tested on patients with inherited or acquired coagulation disorders.
Take special care to avoid bleeding during lesion preparation in such patients [see Dosage and Administration (2.3) ] . Any bleeding must be stopped before application of the gel.
Risk of Mucous Membrane Irritation
AMELUZ can cause mucous membrane irritation. AMELUZ is intended for topical use only. Avoid direct contact of AMELUZ to the mucous membranes. Rinse with water in case of accidental contact.
ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of the labeling:
- Hypersensitivity [ see Warnings and Precautions (5.1) ].
- Transient Amnestic Episodes [ see Warnings and Precautions (5.2) ] .
- Risk of BF-RhodoLED or RhodoLED XL Lamp Induced Eye Injury [see Warnings and Precautions (5.3) ] .
- Ophthalmic Adverse Reactions [see Warnings and Precautions (5.4) ].
- Increased Photosensitivity [see Warnings and Precautions (5.5) ] .
Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The clinical program for AMELUZ included three double-blind and placebo-controlled phase 3 trials (Trials 1, 2, and 3), enrolling a total of 299 subjects that were treated with narrow band light. Trial subjects were adults greater than or equal to 49 years of age, and the majority had Fitzpatrick skin type I, II, or III. No subjects had Fitzpatrick skin type V or VI. Approximately 86% of subjects were male, and all subjects were White.
For all three phase 3 trials, the enrolled subjects had mild to moderate AKs (Olsen grade 1 and 2) with 4 to 8 lesions on the face and scalp. Overall, 212 AMELUZ-treated subjects (n=32, n=55, and n=125) and 87 subjects receiving placebo (n=16, n=32, n=39) were illuminated with BF-RhodoLED or similar narrow spectrum lamps. For these trials, the maximal dose was one tube of AMELUZ (2 g) and the size of the application area was up to 20 cm 2 .
Local skin reactions at the application site were observed in about 99.5% of subjects treated with AMELUZ and narrow spectrum lamps. The most frequent adverse reactions during and after PDT were application site erythema, pain, burning, irritation, edema, pruritus, exfoliation, scab, induration, and vesicles.
Most adverse reactions occurred during illumination or shortly afterwards, were generally of mild or moderate intensity, and lasted for 1 to 4 days in most cases; in some cases, however, they persisted for 1 to 2 weeks or even longer. Severe pain/burning occurred in up to 30% of subjects. In one case, the adverse reactions required interruption or discontinuation of the illumination.
Table 1 presents the incidence of common (≥1%, <10%) and very common (≥10%) adverse reactions at the application site in randomized, multicenter trials which evaluated a maximal dose of one tube of AMELUZ (2 g) and an application area up to 20 cm 2 .
Adverse reaction | Vehicle n=87 | AMELUZ n=212 |
Adverse reactions at the application site | ||
Erythema Pain/Burning Irritation Edema Pruritus Exfoliation Scab Induration Vesicles Paresthesia Hyperalgesia Reaction Discomfort Erosion Discharge Bleeding Pustules | 34 (39%) 26 (30%) 17 (20%) 3 (3%) 14 (16%) 4 (5%) 2 (2%) 0 (0%) 1 (1%) 2 (2%) 0 (0%) 2 (2%) 0 (0%) 0 (0%) 0 (0%) 0 (0%) 0 (0%) | 195 (92%) 195 (92%) 153 (72%) 75 (35%) 72 (34%) 41 (19%) 41 (19%) 26 (12%) 25 (12%) 18 (9%) 13 (6%) 8 (4%) 7 (3%) 6 (3%) 4 (2%) 3 (1%) 3 (1%) |
Common (≥1%, <10%) adverse reactions not at the application site for AMELUZ maximal dose of one tube (2 g) and application area up to 20 cm 2 were headache, skin exfoliation, chills and eyelid edema.
Less common (≥0.1%, <1%) adverse reactions at the application site for AMELUZ maximal dose of one tube (2 g) and application area up to 20 cm 2 were hemorrhage and swelling. The adverse reactions not at the application site were blister, feeling hot, pruritus, pyrexia, scab, nervousness, pain, petechiae, rash pustular, skin erosion and ulcer.
In a clinical trial designed to investigate the sensitization potential of aminolevulinic acid with 216 healthy subjects, 13 subjects (6%) developed allergic contact dermatitis after continuous exposure for 21 days with doses of aminolevulinic acid that were higher than doses normally used in the treatment of AK.
In two open label clinical trials to evaluate safety and tolerability, 116 subjects with actinic keratosis (AK) on the face and scalp were treated with three tubes (6 g) of AMELUZ applied to a 60 cm² area. Most adverse reactions observed were consistent with those reported in trials using one tube (2 g) of AMELUZ on a 20 cm² area (see Table 1). Additional reactions which occurred in ≥1% of subjects when three tubes (6 g) of AMELUZ were applied to a 60 cm² area included dry eyes, photosensitivity, and application site discoloration, dryness, papules, and fissures.
The frequencies of certain adverse reactions at the application site in these trials—exfoliation, itching, scabbing, and erosion—were more than 10% higher with the larger dose (three tubes, 6 g) and treatment area (60 cm²) compared to the frequencies observed in the trials for the smaller dose (one tube, 2 g) and area (20 cm²). Severe application site pain was reported by 41% of the subjects treated with three tubes (6 g) on a 60 cm² area. Most cases were reported during PDT illumination. A total of 15% of subjects discontinued illumination due to adverse reactions.
Postmarketing Experience
The following adverse reactions have been reported during post-approval use of AMELUZ. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Skin and subcutaneous tissue disorders: allergic dermatitis, application site inflammation, application site discoloration.
Eye disorders: eye irritation, diplopia, ocular hyperemia, photophobia, and blurred vision.
General disorders and administration site conditions: fatigue.
Immune System disorders: hypersensitivity.
Nervous system disorders: dysaesthesia, transient amnestic episodes.
DRUG INTERACTIONS
There have been no formal studies of the interaction of AMELUZ with other drugs. It is possible that concomitant use of other known photosensitizing agents such as St. John’s wort, griseofulvin, thiazide diuretics, sulfonylureas, phenothiazines, sulphonamides, quinolones and tetracyclines may enhance the phototoxic reaction to PDT [see Warnings and Precautions (5.3) ] .
DESCRIPTION
AMELUZ (aminolevulinic acid hydrochloride) topical gel, 10% is a non-sterile white-to-yellowish gel. The gel formulation contains a nanoemulsion.
Aminolevulinic acid, a porphyrin precursor, is a white to off-white crystalline solid. It is readily soluble in water, methanol, and dimethylformamide. Its chemical name is 5-amino-4-oxo-pentanoic acid hydrochloride, molecular weight is 167.59 and molecular formula is C 5 H 9 NO 3 ·HCl. The structural formula of aminolevulinic acid hydrochloride is represented below:

Each gram of AMELUZ contains 100 mg of aminolevulinic acid hydrochloride (equivalent to 78 mg aminolevulinic acid) as the active ingredient and the following inactive ingredients: xanthan gum, soybean phosphatidylcholine, polysorbate 80, medium-chain triglycerides, isopropyl alcohol, dibasic sodium phosphate, monobasic sodium phosphate, sodium benzoate and purified water.
CLINICAL PHARMACOLOGY
Mechanism of Action
Photoactivation following topical application of AMELUZ occurs when aminolevulinic acid (prodrug) is metabolized to protoporphyrin IX (PpIX), a photoactive compound which accumulates in the skin. When exposed to red light of a suitable wavelength and energy, PpIX is activated resulting in an excited state of porphyrin molecules. In the presence of oxygen, reactive oxygen species are formed which causes damage to cellular components, and eventually destroys the cells. AMELUZ photodynamic therapy of AK lesions utilizes photoactivation of topically applied AMELUZ resulting from BF-RhodoLED or RhodoLED XL illumination, which provides a red light of narrow spectrum and a light dose of approximately 37 J/cm 2 .
Pharmacodynamics
The pharmacodynamics of AMELUZ in the treatment of actinic keratosis are unknown.
Pharmacokinetics
Aminolevulinic acid acts as a prodrug to the photoactive metabolite protoporphyrin IX (PpIX).
Pharmacokinetics (PK) of aminolevulinic acid and PpIX were evaluated in two trials:
In the first trial, a single dose of one entire tube of AMELUZ (2 grams) was applied under occlusion for 3 hours followed by PDT to a total area of 20 cm 2 in 12 adult subjects with mild to moderate AK with at least 10 AK lesions on the face or forehead. The mean ± SD baseline plasma aminolevulinic acid concentration was 20.16 ± 16.53 ng/mL. In most subjects, an up to 2.5-fold increase of aminolevulinic acid plasma concentrations was observed during the first 3 hours after AMELUZ application. The mean ± SD area under the concentration time curve (AUC 0-t ) and maximum concentration (C max ) for baseline corrected aminolevulinic acid (n=12) were 142.83 ± 75.50 ng·h/mL and 27.19 ± 20.02 ng/mL, respectively. The median T max (time at which C max occurred) was 3 hours.
The mean ± SD baseline plasma PpIX concentration was 3.27 ± 2.40 ng/mL (n=12). The majority (about 55%) of the PpIX concentrations were below the limit of quantification (LOQ = 1 ng/mL) and baseline corrected values were negative in all subjects except for one. The baseline corrected AUC 0-t and C max in the single subject was 0.07 ng·h/mL and 0.29 ng/mL, respectively.
In the second trial, a single dose of three entire tubes of AMELUZ (6 grams) was applied under occlusion for 3 hours followed by PDT to a total area of 60 cm 2 in 16 adult subjects with mild to severe AK with at least 12 AK lesions on the face and scalp. The mean ± SD baseline plasma aminolevulinic acid concentration was 13.53 ± 1.58 ng/mL. In most subjects, an up to 2.5-fold increase of aminolevulinic acid plasma concentrations was observed during the first 3 hours after AMELUZ application. The mean ± SD area under the concentration time curve (AUC 0-t ) and maximum concentration (C max ) for baseline corrected aminolevulinic acid (n=16) were 134.24 ± 87.97 ng·h/mL and 33.85 ± 21.85 ng/mL, respectively. The median T max (time at which C max occurred) was 3 hours. The mean ± SD baseline plasma PpIX concentration was 1.93 ± 0.42 ng/mL (n=15). The mean ± SD baseline corrected C max of PpIX was 0.67 ± 0.78 ng/mL (n=13) with a median T max of 4 hours. The baseline corrected PpIX concentrations in 14 of 15 subjects were < 1 ng/mL.
NONCLINICAL TOXICOLOGY
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies to evaluate the carcinogenic potential of AMELUZ or aminolevulinic acid have not been performed.
Aminolevulinic acid revealed no evidence of mutagenic or clastogenic potential based on the results of three in vitro genotoxicity tests (Ames assay, HPRT test in V79 cells, and Human lymphocyte chromosomal aberration assay) and one in vivo genotoxicity test (mouse micronucleus assay). These genotoxicity studies were conducted without exposure to light. There is a literature report that indicates that aminolevulinic acid may cause genotoxic effects in the presence and in the absence of activating light. These genotoxic effects are likely caused by the formation of reactive oxygen species.
Animal fertility studies have not been conducted with aminolevulinic acid because of the negligible systemic absorption of aminolevulinic acid in humans following topical administration of AMELUZ under maximal clinical use conditions.
CLINICAL STUDIES
The efficacy and safety of AMELUZ in combination with PDT using a narrow spectrum (red light lamp) source were evaluated in three randomized, multicenter trials (Trials 1, 2, and 3). Trials 2 and 3 were vehicle-controlled and double-blind. Trial 1 was double-blind with respect to vehicle and observer-blind regarding the active comparator arm. All clinical trials included a follow-up assessment after 6 and 12 months.
In these trials, 212 subjects with 4 to 8 mild to moderate AK lesions on the face/forehead and/or bald scalp were treated with up to one tube (2 grams) of AMELUZ on an application area up to 20 cm² and a narrow band spectrum lamp. Subjects ranged from 49 to 87 years of age (mean 71 years), and 92% had Fitzpatrick skin type I, II, or III. No subjects had Fitzpatrick skin type V or VI. Approximately 86% of subjects were male, and all subjects were White.
All sessions were comprised of lesion preparation to roughen the surface and remove crusts, application of AMELUZ with occlusion for 3 hours, and removal of the residual gel. Subsequently, the entire treatment area was illuminated with a narrow spectrum red light source, a lamp of either 630 nm or 633 nm and a light dose of approximately 37 J/cm 2 . In Trial 3, illumination was performed with BF-RhodoLED, a red light source with a narrow spectrum around 635 nm and a light dose of approximately 37 J/cm 2 .
In all trials, the lesions that were not completely cleared 12 weeks after the initial treatment were treated a second time with an identical regimen. In the trials, 42% (88/212) of subjects needed a second treatment.
The primary endpoint for all trials was complete clearance 12 weeks after the last PDT. The results of Trials 1, 2 and 3 are presented in Table 2.
AMELUZ | Vehicle | |
Trial 1 | 106/125 (85%) | 5/39 (13%) |
Trial 2 | 27/32 (84%) | 2/16 (13%) |
Trial 3 | 50/55 (91%) | 7/32 (22%) |
Subjects who achieved complete clearance at 12 weeks after the last PDT entered a 12-month follow-up period. In the three trials, subjects who received AMELUZ with the narrowband PDT and achieved complete clearance 12 weeks after the last PDT had recurrence rates of 14%, 11%, and 25%, respectively (at 6 months) and 40%, 22%, and 37%, respectively (at 12 months).
Recurrence was defined as the percentage of subjects with at least one recurrent lesion during the 6-month or 12-month follow-up period in subjects with completely cleared lesions 12 weeks after the last PDT.
HOW SUPPLIED/STORAGE AND HANDLING
AMELUZ (aminolevulinic acid hydrochloride) topical gel, 10% is a white-to-yellowish gel. The drug product is supplied in an aluminum tube with a white, high density polyethylene (HDPE) screw cap. Each tube contains 2 g of gel.
NDC 70621-101-01 2 g tube
NDC 70621-101-10 Cardbox containing one 2 g tube
NDC 70621-101-20 Cardbox containing ten 2 g tubes
Store AMELUZ in a refrigerator, 2°C – 8°C (36°F – 46°F). Excursions permitted to 15°C – 30°C (59°F – 86°F).
After opening, AMELUZ can be stored for up to 12 weeks in a refrigerator at 2°C – 8°C (36°F – 46°F) if the tube is tightly closed.
INSTRUCTION FOR USE

User Manual
Manufacturer: Biofrontera Pharma GmbH Distributor: Biofrontera Inc.
Foreword
Thank you for choosing the BF-RhodoLED ® LED lamp for your photodynamic therapy. BF-RhodoLED ® has been developed in accordance with applicable technical standards to provide high energy efficiency as well as constant light emission at the desired wavelength. Your lamp must be installed and maintained by a qualified Biofrontera technician and used only in accordance with the instructions in this manual. You may request the latest printed version covering this model from Biofrontera at any time. Our drug Ameluz ® and medical device BF-RhodoLED ® have been approved in combination for photodynamic therapy; the only approved use of our lamp is in combination with Ameluz ® gel. This user manual provides important BF-RhodoLED ® product details, cautions and warnings, and operating instructions. For Ameluz ® , please use its US prescribing information. The Ameluz ® prescribing information includes information on how to use the gel along with contraindications, warnings and precautions and dosage and administration.
Thorough reading and use of both this user manual and the Ameluz ® USPI is required prior to treatment. Our combination Ameluz ® /BF-RhodoLED ® for photodynamic therapy is only to be used by physicians or healthcare professionals.
For further questions about Ameluz ® , BF-RhodoLED ® or the combination therapy please contact your sales representative or Biofrontera ( Ameluz-US@biofrontera.com ). See contact information below or visit us at http://www.biofrontera.us.com/
Sales representative: Name: Tel.: E-mail: | Biofrontera Inc . 120 Presidential Way Suite 330 Woburn, MA 01801 USA Phone: (844) 426-3589 |
Warranty and Disclaimers Please see the terms and conditions of your contract for this information.
Manufacturer Biofrontera Pharma GmbH, Hemmelrather Weg 201, 51377 Leverkusen, Germany
"AMELUZ, RHODOLED and BF-RhodoLED are registered trademarks of Biofrontera Pharma GmbH. ©2024 Biofrontera Pharma GmbH. All rights reserved.”
1 Intended Use
BF-RhodoLED ® is a red light emitting LED lamp which is used exclusively in combination with Ameluz ® gel for lesion-directed and field-directed treatment of actinic keratoses (AKs) of mild-to-moderate severity on the face and scalp.
2 BF-RhodoLED ® - General Description
BF-RhodoLED ® is comprised of three main components, the lamp head, the easily adjustable scissor arm, and the mobile frame with castors for smooth transport. In addition, the lamp has a convenient touchscreen monitor, storage shelf, aids for positioning the lamp head and a variable speed patient fan that can be regulated throughout the treatment.
3 Warnings and Precautions
 | The BF-RhodoLED ® has been developed for use in photodynamic therapy in combination with Ameluz® gel (excitation wavelength approx. 635 nm). Any other use or combination of use is prohibited. |
 | During PDT side-effects such as application site erythema, pain, irritation, edema, pruritus, exfoliation, scab, induration and vesicles may occur. For further information please see Ameluz ® USPI chapter 6. |
 | The user manual must be read carefully prior to using the BF-RhodoLED ® . |
 | The BF-RhodoLED ® may only be used by healthcare professionals who have received adequate training in its use. |
 | To avoid eye irritation, glare, or injury, protective eye equipment must be used by patient, healthcare providers and any person present during the illumination period. |
 | Neither the BF-RhodoLED ® nor the power supply unit should be exposed to excessive mechanical stress or serviced by unauthorized personnel. Assembly and servicing may only be carried out by Biofrontera qualified professionals. |
 | Risk of trapping fingers! The mobility of the adjustable scissor arm may be obstructed by objects placed in or near slots ! Depending on positioning, the scissor arm slots below each connecting portion of the scissor arm and to the right of the support rail can cause fingers or objects to become trapped. When transporting or adjusting the lamp please ensure that fingers or other objects are NOT placed in or near the slots (see Figure 1 ). |
 | Beware of Tipping! If the castors are in locked position, the lamp will tip over if exposed to lateral force. Leaning against the device or using it for support is not permitted. Do not place the lamp on uneven or unstable surfaces. Do not place heavy objects on the storage shelf (maximum load up to 8 kg) or use for sitting, leaning or pushing, as this can lead to the device tipping over. |
 | In order to avoid the risk of electrical shock, the device may only be connected to a power supply with a grounded connection. |
 | Neither the BF-RhodoLED ® nor its touchscreen monitor may come into contact with water as this may lead to severe damage or electrical shock (exception: cleaning with a damp cloth). |
 | The plexiglass plate on the lamp head may NOT be touched during or directly after treatment as, in the event of faulty operation, the maximum temperature can reach approx. 73 °C (163.4 °F). |
 | Do not position the lamp so that it is difficult to unplug the power plug of the medical device. The lamp can only be disconnected from the supply grid by pulling the power plug. |
 | The BF-RhodoLED ® should be operated and housed indoors in areas with temperatures between 0°C and 40°C (32°F and 104°F), and relative humidity of 10 % to 90 %. |
 | Medical electronic devices require special precautionary measures with regard to the electro-magnetic compatibility (EMC); to avoid elect-magnetic disturbances, please do not place the device close to other electrical devices. |
 | There is a risk of tripping over the power cord or the lamp base. When transporting or when device is not in use, unplug and keep the power cord wound around the star knobs. |
 | Ensure that the touchscreen monitor is always returned to the fixture intended for this purpose, located on the storage shelf. Storing the touchscreen monitor by hanging it can lead to damage to the spiral cord and the plug fitting. |
 | The touchscreen monitor should not be exposed to strong external pressure or sharp objects. |
 | A person trained in operation of the BF-RhodoLED ® must always be present during treatment. |
 | The device must be protected against unauthorized use. |
| Both the maintenance and the assembly of the BF-RhodoLED ® and its components (including the opening of individual components) may only be carried out by authorized personnel. In the event of servicing or assembly being carried out by any other party, Biofrontera assumes no responsibility or liability regarding the safety or use of the device. |
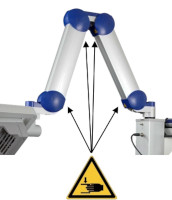
Figure 1 : The scissor arm in parked position. Arrows indicate potential points where fingers can get trapped. (The appearance of devices from batches 001 to 003 slightly differs. Appearance and location of the labels remain unaltered).
4 BF-RhodoLED ® Technical Description
4.1 Light Emitting Diodes (LEDs)
The light-field of the BF-RhodoLED ® consists of a total of 128 LEDs and lenses (arranged in a rectangle), which emit a uniform, bundled, visible red light with a typical peak wavelength of approximately 635 nm. The half-band width of the lamp is 20 nm.
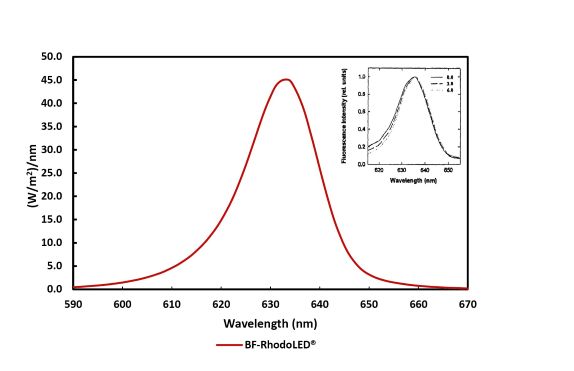
Figure 2 : Typical emission spectrum of the LEDs of the BF-RhodoLED ® . Insert: Fluorescence excitation spectrum of PPIX in cells 0, 3 and 6 mm below the tissue surface (Moan et al., 1996)
The BF-RhodoLED ® LEDs are calibrated so that the skin being treated receives a light dosage of approximately 37 J/cm 2 under the following conditions:
- Illumination time of 10 minutes
- Treatment distance of 5-8 cm (Optimum 6 cm)
The illumination area of the LED lamp is 8 x 18 cm. As the intensity decreases towards the edge of this area, the effective treatment area is reduced to 6 x 16 cm.
4.2 Lamp Components
The BF-RhodoLED ® LED lamp consists of the following components ( Figure 3 ):
- Lamp head with holding bracket
- Scissor arm
- Storage shelf with control unit (touchscreen monitor)
- Mobile frame and lamp base (include support rail, castors and power supply)
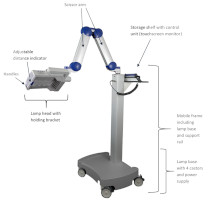
Figure 3 : BF-RhodoLED ® main components. (The appearance of devices from batches 001 to 003 slightly differs.)
The swivel range to the left and right of the vertical rail is +/- 24°. The lamp head can be adjusted horizontally and vertically and tilted sideways. The scissor arm with internal gas springs allows seamless adjustment of the lamp head to any position. 5 Eye Protection
In order to avoid eye irritation, glare or injury, protective eye equipment must be used by patient, healthcare providers and any person present during the illumination period. Do not stare into the light source! The operator and other persons present must wear protective glasses with a visible light transmission (VLT) of approximately 10%. The patient must wear eye protection such as disposable eye protection pads or eye caps with an optical density for visible light of 6 or higher. Both options are effective and comfortable for use during treatment. Note: Eyewear is not part of the medical device. Please carefully read any accompanying usage information before using any eye protection.
 | In order to avoid eye irritation, glare or injury, protective eye equipment must be used by patient, healthcare providers and any person present during the illumination period. |
6 BF-RhodoLED ® PDT Summarized Step-by-Step Instructions
These instructions should be used in conjunction with detailed operating instructions below (chapter 7). The section titles and numbers listed next to the instruction refer to the detailed instructions and/or descriptions that correspond with each step. Note: Every user of the BF-RhodoLED ® has to be trained in operating the device. The initial training is provided by Biofrontera during set-up of the BF-RhodoLED ® . Recurrent training is not mandatory but can be provided by Biofrontera employees. Before treating a patient with Ameluz, please ensure that BF-RhodoLED ® is in good working order.
| STEP 1) | Plug in lamp. |
| STEP 2) | Turn on the lamp and wait for home screen to appear (see chapter 7.3) |
| STEP 3) | Check the proper functioning of the BF-RhodoLED ® . |
| STEP 4) | Treat the affected skin area: Application, incubation and removal of the topical medication following the USPI of Ameluz ® . |
| STEP 5) | Position patient comfortably and place protective eyewear on patient (see chapter 5). |
| STEP 6) | Position the lamp and adjust the lamp head over the skin area to be treated (distance 5-8 cm) (see chapter 7.5.1). |
| STEP 7) | Put on protective eyewear and make sure anyone remaining in the treatment room also wears protective eyewear (see chapter 5). |
| STEP 8) | Start treatment by pressing "Treatment" on the home screen, press "Start" on the treatment screen, and press "Start" once again in the profile screen. The illumination period of 10 minutes begins (see chapter 7.5.3). Remind the patient to remain still throughout the illumination period. If sound tone during treatment is activated, explain the beep sequence to the patient. If the patient needs a break, press "Break" on the profile standard screen. To resume treatment, press "Start" on the profile standard screen (see chapter 7.5.3). To abort the illumination, press "Stop" on the profile standard screen. Adjust fan speed as needed by patient by pressing plus or minus 1% or 10% on the profile standard screen. Stay with the patient at all times during the illumination period. |
| STEP 9) | At the end of the illumination period, remove patient's protective eyewear and follow further instructions in the Ameluz ® USPI. |
7 Detailed Operating Instructions
Note: Every user of the BF-RhodoLED ® has to be trained in operating the device. The initial training is provided by Biofrontera during set-up of the BF-RhodoLED ® . Recurrent training is not mandatory but can be provided by Biofrontera employees. The device must be protected against unauthorized use.
 | In order to avoid eye irritation, glare or injury, protective eye equipment must be used by patient, healthcare providers and any person present during the illumination period. |
7.1 Transport of the lamp
The transportation of the lamp shall be done when the scissor arm is completely folded. During transportation one hand shall hold on to the support rail or storage shelf while the other hand holds the scissor arm ( Figure 4 ).
Figure 4 : Transport of the lamp. (The appearance of devices from batches 001 to 003 slightly differs.)
7.2 Movement area of the scissor arm and the lamp head
The scissor arm has a swivel range of +/- 24 degrees to the left and right. The swivel range is limited by a pin at the connection between scissor arm/ support rail and scissor arm/ lamp head ( Figure 5 ). The lamp head can be adjusted horizontally and vertically and tilted sideways, as it has a swivel range of +/- 90 degrees in all three dimensions.

Figure 5 : Limiting pins of the scissor arm and the lamp head. (The appearance of devices from batches 001 to 003 slightly differs. Appearance and location of the labels remain unaltered.)
7.3 Turning the Lamp On or Off
Note : Before turning on the lamp, the power cord must be plugged in. After connecting, the lamp can be turned on and used following the instructions below.
The on-off switch is located on the left side of the touchscreen monitor, close to its spiral cord. With the lamp plugged in, press the button to start the device.
Important: This push button can also be used to turn the lamp off. Switching off the lamp can also be done by pressing the switch off ![]() button on the touchscreen (see chapter 7.4.2 "Home Screen").
button on the touchscreen (see chapter 7.4.2 "Home Screen").

Figure 6 : Touchscreen monitor
Note : The blue LED of the push button indicates that the device is in stand-by mode.
7.4 Touchscreen Monitor and Menu
When using the touchscreen, please firmly press the center of the buttons shown on the screen to ensure it is read correctly by the software. Do not press too quickly, the input may not be read by the software. The touchscreen can be used while wearing commercially available examination gloves.
7.4.1 Start Screen
The Biofrontera corporate logo and software version are displayed during the LED lamp operating system start-up ( Figure 7 ). The operating system takes approximately 30 seconds to load.

Figure 7 : Start screen
7.4.2 Home Screen
The home screen appears after the operating system has loaded. From the home screen you can select "Treatment", "Settings" or "Service" ( Figure 8 ). In the "Treatment" menu only one treatment profile "profile standard" is available. The illumination time and light intensity of this program are fixed (see 7.5.3 for further information). The "Settings" menu allows adjustment of language, time, date, audio signals and power saving settings (see 7.6 for further information). The "Service" menu may only be accessed by authorized personnel. You can turn the LED lamp off by pressing the ![]() button.
button.
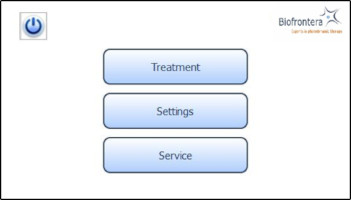
Figure 8 : Home screen
7.5 Conducting a Treatment
Prior to treating a patient with Ameluz please ensure that BF-RhodoLED ® is in good working order. Before starting a treatment, make sure the lamp is set-up in accordance with the operating instructions described in the following sections.
7.5.1 Distance Indicator
Position the lamp head over the skin area to be treated at a distance of 5-8 cm. Use the adjustable distance indicator and graduated scale bar to help measure and aid in achieving the correct distance ( Figure 9 ).
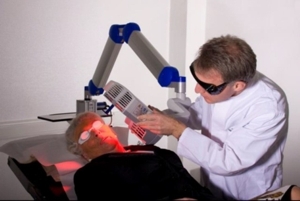

Figure 9 : Positioning of the lamp head to the treated skin area
7.5.2 Adjustment Light
To further aid positioning, use the adjustment light. On the touchscreen, press the following buttons in sequence (starting in the home screen): "Treatment" ( Figure 8 ), "Adjust" ( Figure 10 ) and "Start Adjustment" ( Figure 11 ). The adjustment light shows what the field of illumination will be during treatment but at a lower intensity (6x16 cm, approx. 2.4 x 6.3 inches). When finished, press "Stop adjustment" and then "Back" ( Figure 11 ) to return to the treatment menu ( Figure 10 ).
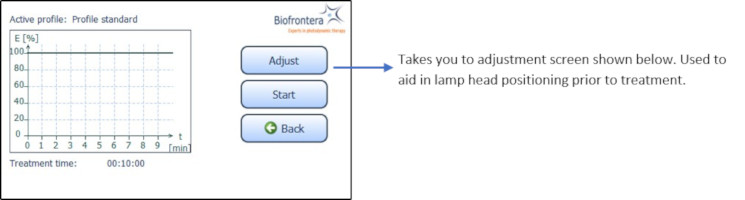
Figure 10 : Treatment menu

Figure 11 : Start and Stop adjustment
7.5.3 Treatment Menu
Press treatment to enter the treatment menu ( Figure 8 ). The standard illumination profile is depicted in a graph in the treatment menu. The x- and y-axis specify the treatment duration and light intensity respectively ( Figure 12 ).
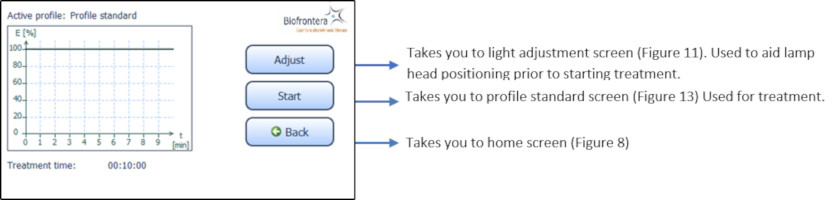
Figure 12 : Treatment menu
In the treatment menu ( Figure 12 ), press the "Start" button to access the profile standard screen.
7.5.4 Profile Standard Menu
In the profile screen menu, press "Start" to begin treatment ( Figure 13 ). You can interrupt the treatment at any time by pressing the "Break" button. Press the "Start" button to resume the treatment. Abort the current treatment by pressing the "Stop" button. If you press "Stop" prior to the end of the total treatment time, 10 minutes, the clock will reset and any interim treatment time will be not be saved.
After pressing "Stop" or upon completion of the full 10 minute treatment the software will automatically return you to the treatment menu.
The remaining treatment time is displayed to the right of the graph and the red vertical line within the graph indicates the time that has elapsed.
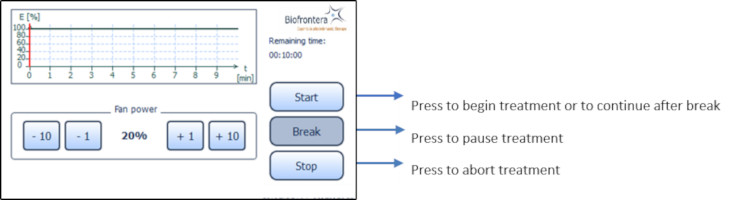
Figure 13 : Profile standard
7.5.5 Audio Signals during Treatment
During treatment the patient will hear audio signals, 1 beep indicating 25 %, 2 beeps indicating 50 % and 3 beeps indicating 75 % of the PDT is completed, and upon treatment completion the lamp will stop the illumination and 4 beeps will be heard (see also chapter 7.6.2).
7.5.6 Patient fan
You can control the speed of the patient fan both before and during treatment with the plus and minus buttons, in increments of 1 % or 10 %. The patient fan continues to run when the treatment is paused ("Break"). The fan can be stopped by pressing "Stop" but be aware the treatment will also be aborted and any elapsed treatment time will not be saved. 7.6 Settings Menu
The settings menu is invoked ( Figure 14 ) by pressing the "Settings" button in the home screen view (see Figure 12 ).
Figure 14 : Settings menu
You can configure the following parameters in this menu:
- Language
- Signals
- Energy service (Power management)
Switch back to the home screen menu via the "Back" button.
7.6.1 Language Settings
Select a language by tapping on the desired language. Use the arrow keys to scroll through the list. Confirm the selected language with the "OK" button ( Figure 15 ). Switch back to the settings menu without saving changes by pressing the "Back" button instead of "OK".
Figure 15 : Language setting 7.6.2 Sound Settings
Activate and deactivate the audio signals for typing, warnings and sounds during the treatment. To do this, select the desired fields. A check mark means the sound is on. Confirm with the "OK" button ( Figure 16 ) to save your settings or close the menu via the "Back" button to discard the setting. If "Turn off sound" is activated, all signal tones are switched off.
Important Note: Warning sounds will be emitted if an error occurs during operation of the lamp.
Figure 16 : Sound setting
7.6.3 Energy Service (Power Management)
Providing the standby function is not manually deactivated via the check box "Never turn off lamp automatically" located near the bottom of the screen, the lamp will turn off after 10 minutes of non-use ( Figure 17 ). You can change this time span in the menu "Energy service" via the plus and minus buttons. The settings are confirmed with the "OK" button and rejected with the "Back" button. In both cases you will return to the settings menu.
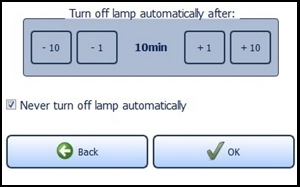
Figure 17 : Energy management
If the configured time expires, a request appears for 10 seconds asking if you wish to prevent shut down. The LED lamp turns off automatically afterwards.
7.7 Service Menu
Authorization is required to access the service menu. Only authorized personnel can access the service menu. 8 Error Messages
The BF-RhodoLED ® has an integrated monitoring function. In the event of a malfunction, an error message will appear in the message display. Possible error messages are set out in the table below.
| Error message | Possible causes | Measures |
| Lamp overheated! |
| Wait until the standard temperature has been reached. Check the ventilator and test the air inlets and outlets. Remove any visible foreign objects which may be present. In case of continuing impairment of cooling please contact your local sales representative or Biofrontera. |
| Lamp error! Please inform your supplier. |
| Further treatments may be carried out. However, please contact your local sales representative or Biofrontera immediately. |
| Communication error! |
| Confirm the communication error. Treatments can be continued in the treatment menu by pressing the "Start" button. Please contact your local sales representative or Biofrontera. |
| Lamp not calibrated! |
| The lamp has to be calibrated. Please contact your local sales representative or Biofrontera immediately. |
| Fehler-Nr.: 0084 Please inform your supplier. |
| The lamp head has to be repaired. Please contact your local sales representative or Biofrontera. |
| Firmware of light head is faulty or missing! Please inform your supplier. |
| An update of the firmware has to be performed. Please contact your local sales representative or Biofrontera. |
If your LED lamp no longer functions properly for some unknown reason, proceed as follows:
- Unplug the power supply for half a minute and then plug back into the outlet (This action prompts a software reboot).
- Turn the lamp back on using the touchscreen monitor and verify functionality.
Should the problem persist, please contact your sales representative or Biofrontera. The lamp may not be used until the problem has been rectified.
For all other malfunctions and difficulties with operation please contact your sales representative or Biofrontera. You will find the contact details on page 2.
9 Servicing
Servicing may only be performed by Biofrontera-authorized personnel. If servicing is done by third parties, BF-RhodoLED ® warranty is void and Biofrontera cannot be held responsible for potential malfunctions or damages of the device. It is recommended to have the lamp serviced after two years.
During service, the gas springs (gas pressure springs and gas traction springs) in the scissor arm as well as the general safety condition of the BF-RhodoLED® are checked.
Contact your sales representative or Biofrontera to schedule all service appointments.
10 Maintenance and Cleaning
The device may under no circumstances be cleaned with aggressive cleaning agents or solvents (e.g. acetone or highly concentrated ethanol), as these promote wear and tear of the surface (paint coating), safety signs and graduated scale bar as well as the logo. Please avoid allowing any penetration of liquids into the lamp, BF-RhodoLED ® is not waterproof and the electronics inside may be damaged.
Always unplug the lamp prior to cleaning and please observe the following cleaning practices:
| Daily: | Wipe the plexiglass screen located on the bottom of the lamp head with a damp soft cloth (e. g. a microfiber cloth). |
| Weekly: | Wipe the entire lamp. Do not use a very wet or dripping cleaning cloth. The unit is not waterproof, and damage could occur. Weekly cleaning can be carried out using a damp cloth. If a disinfection of the device is desired, a mild disinfecting agent can be used. Most commercially available products, which are suitable for plastics, should be compatible with BF-RhodoLED ® . A know exception is the agent "Incidin Plus TM " (active ingredient: Glucoprotamine) which can cause corrosion of the lamp head surfaces. Before using a disinfection agent, always refer to its manual to check for compatibility with plastic surfaces, especially with Plexiglas. Note: If you notice any deterioration of a surface of BF-RhodoLED after using a disinfectant, please stop further use and contact Biofrontera. |
| Please observe the following checks: | |
| Monthly: | Check lamp for obvious visible damage such as damaged cables, worn off labeling, cracks on the lamp housing, etc. Do not use a damaged device for treatment and please inform your supplier. |
11 Disposal Instructions
Follow all local and governmental disposal laws and regulations when disposing of the BF-RhodoLED ® .
12 Technical Data
12.1 Electrical connectors
Power supply unit:
- Primary wide-range input 100-240 VAC
- Secondary safety extra-low voltage output 48 VDC
- Secondary standby voltage output 5 VDC
Mother board for voltage management and control:
- Supply voltage input 48 VDC
- Standby voltage input 5 VDC plus Standby contact
- RS485 interface
- Output supply voltage HMI board 24 VDC
- 4 x ports LED board 8-pin (2 x LED circuits, temperature (3-pin))
- 4 x connector ventilator (2-pin)
LED board:
- 2 x ports mother board 8-pin (2 x LED circuits, temperature (3-pin))
Human Machine Interface (HMI) for operation:
- Supply voltage input 24 VDC
- RS485 interface
12.2 Specification
| Type | BF-RhodoLED ® Medical device class III; Device of protection class 1 |
| Certification | UL certified |
| Software Version No. | 1.6_US_CAN |
| Service life of the lamp | Approx. 4 years |
| Shelf life | 1.5 years |
| Number of LEDs | 128 |
| Typical peak wavelength | 635 nm ± 5 nm |
| Light dose (6 cm distance, 10 min) | Approx. 37 J/ cm 2 |
| Typical irradiance | 61 mW/cm 2 +/- 15 % |
| Max irradiance | 77 mW/cm 2 +/- 15 % (at 5 cm treatment distance) |
| Illuminated area | An area of 8x18 (approx. 3.1 x 7.1 inches) cm is illuminated. The effective treatment area is 6x16 cm (approx. 2.4 x 6.3 inches). |
| Max. deviation of the intensity over the treatment area | Approx. 15 % |
| Transport and storage conditions | Temperature: -30°C to +60°C (-22°F to +140°F) Atmospheric pressure: 500 hPa - 1060 hPa Relative humidity: 10% - 90%, non-condensing |
| Operating conditions | Temperature: 0°C to +40°C (32°F to +104°F) Atmospheric pressure: 700 hPa - 1060 hPa Operational altitude: ≤ 2000 m above NN Relative humidity: 10% - 90%, non-condensing This PDT lamp is suitable for continuous operation. |
| Operating voltage | 120-240 VAC, 50/60 Hz |
| Output voltage | 48 VDC |
| Power consumption | In calibrated normal operation: approx. 140 VA In standby mode: approx. 85 VA |
| Over-voltage category | II |
| Degree of contamination | 2 |
| Material group (CTI) | IIIb |
| Protection class | IP20 |
| Size of the lamp | 95 x 62 x 168 cm (37 x 24 x 66 inches) Devices of batch 001 to 003: 127 x 56 x 168 cm (50 x 22 x 66 inches) |
| Total lamp weight | Approx. 52 kg (115 lbs) Devices of batch 001 to 003: approx. 48 kg (106 lbs) |
For further information please directly contact Biofrontera. 13 Labeling and Symbols
13.1 Labeling on the Lamp
The product sticker is located on the reverse side of the lamp base (see Figure 18 ). It contains manufacturer and product specifications (classification, serial and order number and the power supply used) as well as IP (Ingress) protection. In addition, a unique device identifier label is placed next to the product sticker.

Figure 18 : Product and UDI label. (The appearance of devices from batches 001 to 003 slightly differs. Appearance and location of the labels remain unaltered.)
Labels with safety instructions, special disposal instructions and further instructions are represented in the following figures.
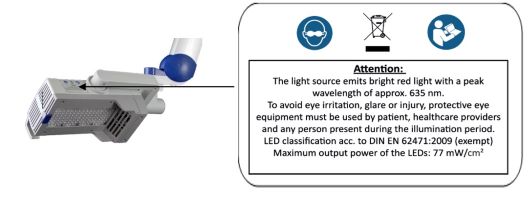
Figure 19 : Instructions and notes on the lamp head
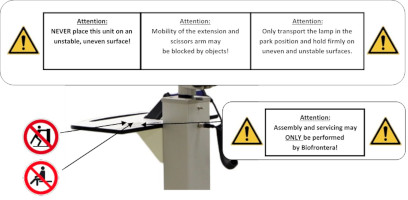
Figure 20 : Instructions on the storage shelf. (The appearance of devices from batches 001 to 003 slightly differs. Appearance and location of the labels remain unaltered.)
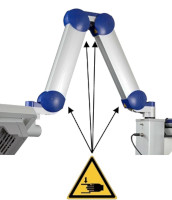
Figure 21 : Instructions on the scissors arm. (The appearance of devices from batches 001 to 003 slightly differs. Appearance and location of the labels remain unaltered.)
Instructions for existing ground connector connections (see Figure 22 ) are located:
- Next to the grounding screws, which are located within and next to the power supply cover box beneath the mobile castor, respectively
- On the power supply cover box (see Figure 23 )
Figure 22 : Instructions for ground connector connection

Figure 23 : BF-RhodoLED ® . (The appearance of devices from batches 001 to 003 slightly differs. Appearance and location of the labels remain unaltered.)
Instructions on the existing potential compensator conductor is located on the carrier bar beneath the potential compensator conductor (see Figure 24 ).

Figure 24 : Potential compensator conductor. (The appearance of devices from batches 001 to 003 slightly differs. Appearance and location of the labels remain unaltered.)
All depicted symbols are summarized in a table in section 13.2 "Explanation of symbols".
13.2 Description of Symbols
| Symbol | Description |
 | Eye protection is required. |
| IP20 | IP (Ingress Protection): Protection class of the housing against contact, foreign bodies and water 2 = against ingress of solid foreign bodies ≥ 12,5 mm Ø and larger 0 = Not protected against water |
 | Technical and electric devices must not be disposed of with household waste. Please pay attention to the applicable disposal instructions found in the user manual. |
 | Symbol for existing ground connector connections |
 | Follow the instructions for use! |
 | General warning signs:
|
 | Warning sign - risk of trapping fingers! |
 | Symbol for existing potential compensation conductor Function/Application: The purpose of a potential compensation conductor is to connect all conducting, metallic parts of a system to each other by the protective earthing in order to achieve the same electrical potential for the connected parts. Protection against contact voltage is thus guaranteed. Releasing the potential compensation conductor is forbidden. It should be firmly fitted to the support splint. Please observe the requirements for ME-systems set out in EN 60601-1 when using the potential compensation cable (chapter 16). |
 | Please do not stand on or push. There is a risk that the device may fall over through of the force applied. |
 | Please do not sit on the equipment. There is a risk that the device may fall over through the force applied. |
13.3 Labeling on the Packaging
- Attention: Protect from moisture and keep dry!
- Attention: Do not throw the package, damage possible!
- Attention: Please transport and store at -30°C/ -22°F to +60°C/ 140°F, a relative humidity of 10 % to 90 % and at an atmospheric pressure of 500 hPa (50 kPa) - 1060 hPa (106 kPa).
- This side up!
- Caution, one end of the package is heavy

- Contains a lithium metal battery (optionally, not required starting from batch 007)

14 Electromagnetic compatibility (EMC)
BF-RhodoLED ® meets the EMC requirements of the international standard EN60601-1-2:2015 Chapter 7 and 8. Other electrical devices can have an impact on the device. Using accessories that have not been approved can have a negative impact on the device and change the electromagnetic compatibility. BF-RhodoLED ® should not be used directly alongside or between other electrical devices.
| Guidelines and manufacturer's declaration - electromagnetic emissions |
BF-RhodoLED ® is intended for operation in the electromagnetic environment detailed below. The customer or operator of the device should ensure that it is operated in this environment. |
| Emission readings | Conformity | Electromagnetic environment - guidelines |
| HF emissions CISPR 11 | Group 1 | BF-RhodoLED ® only uses HF energy for its internal functionality. For this reason, its HF emission is very low and it is not likely that neighbouring electronic devices would be disturbed. |
HF emissions in accordance with CISPR 11 Harmonics in accordance with IEC 61000-3-2 Voltage fluctuations/flickers in accordance with IEC 61000 3-3 | Class B Class A In compliance | BF-RhodoLED ® is intended for operation in non-residential facilities and such like that are connected directly to a public low-voltage supply network that also supplies residential buildings. |
| Guidelines and manufacturer's declaration - electromagnetic immunity |
BF-RhodoLED ® is intended for operation in the electromagnetic environment detailed below. The customer or operator of the device should ensure that it is operated in this environment. |
| Immunity tests | IEC 60601 test levels | Compliance level | Electromagnetic environment - guidelines |
| Static discharge in accordance with IEC 61000-4-2 | ±8 kV contact discharge ±2 kV, ±4 kV, ±8 kV and ±15 kV air discharge | ±8 kV contact discharge ±2 kV, ±4 kV, ±8 kV and ±15 kV air discharge | Floors should be made of wood or concrete or finished with ceramic tiles. If the floor is finished with synthetic material, the relative air humidity must be at least 30 %. |
| Fast, transient electrical disturbances/bursts in accordance with IEC 61000-4-4 | ±2 kV for mains lines ±1 kV for input and output lines | ±2 kV for mains lines | The quality of the supply voltage should correspond to that of a typical commercial or hospital environment. |
| Surges in accordance with IEC 61000-4-5 | ±1 kV voltage outer conductor - outer conductor ±2 kV outer conductor - earth | ±1 kV voltage outer conductor - outer conductor ±2 kV outer conductor - earth | The quality of the supply voltage should correspond to that of a typical commercial or hospital environment. |
| Voltage drops, brief interruptions and fluctuations in the supply voltage in accordance with IEC 61000-4-11 | 0 % U T for 0.5 cycles at phase angles of 0°, 45°, 90°, 135°, 180°, 225°, 270° and 315° (applicable only to me equipment connected to single-phase a.c. mains) 0 % U T for 1 cycle 70 % U T for 25/30 cycles 0 % U T for 250/300 cycles | 0 % U T for 0.5 cycles at phase angles of 0°, 45°, 90°, 135°, 180°, 225°, 270° and 315° (applicable only to me equipment connected to single-phase a.c. mains) 0 % U T for 1 cycle 70 % U T for 25/30 cycles 0 % U T for 250/300 cycles | The quality of the supply voltage should correspond to that of a typical commercial or hospital environment. If the operator of the device needs functionality to be maintained despite interruptions in the energy supply, it is advisable to run the device on an uninterruptable power supply or a battery. |
| Power frequency (50/60 Hz) magnetic field in accordance with IEC 61000-4-8 | 3 A/m | 3 A/m | Mains frequency magnetic fields should correspond to the values typically found in the commercial and hospital environment. |
Comment: U T is the mains AC voltage prior to application of the test level |
| Guidelines and manufacturer's declaration - electromagnetic immunity |
| BF-RhodoLED ® is intended for operation in the electromagnetic environment detailed below. The customer or operator of the device should ensure that it is operated in this environment. |
| Immunity tests | IEC 60601 test levels | Compliance level | Electromagnetic environment - guidelines |
Conducted HF disturbances in accordance with IEC 61000-4-6 Radiated HF disturbances in accordance with IEC 61000-4-3 | 3 V 150 kHz to 80 MHz 6 V ISM bands 0.15 - 80 MHz 80 % AM with 1 kHz 3 V/m 80 MHz to 2.7 GHz 80 % AM with 1 kHz | 3 V 6 V 3 V/m | Portable and mobile communication devices should not be used in closer proximity of the unit or its cables than the recommended safety distance of 300 mm. Interference may occur in the vicinity of equipment marked with the following symbol.
|
| Comment 1: These guidelines may not be applicable in all cases. The propagation of electromagnetic values is influenced by absorptions and reflections of buildings, objects, and people. |
Recommended safety distances between portable and mobile HF telecommunication devices and the BF-RhodoLED ® |
The BF-RhodoLED ® is intended for use in the electromagnetic environment in which the HF disturbances are controlled. The customer or operator of the device can help to avoid electromagnetic interference by maintaining the minimum safe distance between portable and mobile high-frequency telecommunication devices (transmitters) and the device. Portable RF communications equipment (radios, including their accessories such as antenna cables and external antennas) should not be used at a distance less than 30 cm (or 12 inches) from the manufacturer's specified parts and leads of the BF-RhodoLED ® . Failure to do so may result in a reduction in the performance of the device. BF-RhodoLED ® should not be used directly alongside or between other electrical equipment. |
| Guidelines and manufacturer's declaration - proximity fields from RF wireless communication equipment |
BF-RhodoLED ® is intended for operation in the electromagnetic environment detailed below. The customer or operator of the device should ensure that it is operated in this environment. |
| Service | Frequency range/ discrete frequencies MHz | Compliance level [V/m] |
| TETRA 400 | 390 | 27 |
| GMRS 460, FRS 460 | 430 | 28 |
| LTE Band 13, 17 | 734 746 788 | 9 |
| GSM 800/900, TETRA 800, iDEN 820, CDMA, LTE Band 5 | 821 862 948 | 28 |
| GSM 1800, CDMA 1900, GSM 1900,DECT, LTE Band 1,3,4, 25 | 1730 1825 1970 | 28 |
| Bluetooth, WLAN, 802.11 b/g/n, RFID 2450, LTE Band 7 | 2452 | 28 |
| WLAN 802.11 a/n | 5240 5500 5700 | 9 |
Version 2.7
Mechanism of Action
Photoactivation following topical application of AMELUZ occurs when aminolevulinic acid (prodrug) is metabolized to protoporphyrin IX (PpIX), a photoactive compound which accumulates in the skin. When exposed to red light of a suitable wavelength and energy, PpIX is activated resulting in an excited state of porphyrin molecules. In the presence of oxygen, reactive oxygen species are formed which causes damage to cellular components, and eventually destroys the cells. AMELUZ photodynamic therapy of AK lesions utilizes photoactivation of topically applied AMELUZ resulting from BF-RhodoLED or RhodoLED XL illumination, which provides a red light of narrow spectrum and a light dose of approximately 37 J/cm 2 .
