Butrans prior authorization resources
Most recent state uniform prior authorization forms
Butrans patient education
Patient toolkit
Dosage & administration
DOSAGE AND ADMINISTRATION
- BUTRANS should be prescribed only by healthcare professionals who are knowledgeable about the use of extended release/long-acting opioids and how to mitigate the associated risks. (2.1 )
- BUTRANS doses of 7.5, 10, 15, and 20 mcg/hour are only for use in patients receiving, for one week or longer, daily opioid doses up to 80 mg/day of oral morphine or an equianalgesic dose of another opioid. (2.1 )
- Use the lowest effective dosage for the shortest duration of time consistent with individual patient treatment goals. Reserve titration to higher doses of BUTRANS for patients in whom lower doses are insufficiently effective and in whom the expected benefits of using a higher dose opioid clearly outweigh the substantial risks. (2.1 , 5 )
- Initiate the dosing regimen for each patient individually, taking into account the patients underlying cause and severity of pain, prior analgesic treatment and response, and risk factors for addiction, abuse, and misuse. (2.1 , 5.1 )
- Respiratory depression can occur at any time during opioid therapy, especially when initiating and following dosage increases with BUTRANS. Consider this risk when selecting an initial dose and when making dose adjustments. (2.1 , 5.1 )
- For patients who are not opioid tolerant, initiate treatment with a 5 mcg/hour patch. (2.1 )
- Instruct patients to wear BUTRANS for 7 days and to wait a minimum of 3 weeks before applying to the same site. (2.1 )
- Discuss opioid overdose reversal agents and options for acquiring them with the patient and/or caregiver, both when initiating and renewing treatment with BUTRANS, especially if the patient has additional risk factors for overdose, or close contacts at risk for exposure and overdose. (2.2 , 5.1 , 5.2 , 5.3 )
- Periodically reassess patients receiving BUTRANS to evaluate the continued need for opioid analgesics to maintain pain control, for the signs or symptoms of adverse reactions, and for the development of addiction, abuse, or misuse. (2.3 )
- Do not rapidly reduce or abruptly discontinue BUTRANS in a physically dependent patient because rapid reduction or abrupt discontinuation of opioid analgesics has resulted in serious withdrawal symptoms, uncontrolled pain, and suicide. (2.5 , 5.19 )
Important Dosage and Administration Information
- BUTRANS should be prescribed only by healthcare professionals who are knowledgeable about the use of extended-release/long-acting opioids and how to mitigate the associated risks.
- BUTRANS doses of 7.5, 10, 15, and 20 mcg/hour are only for use in patients who are receiving, for one week or longer, daily opioid doses up to 80 mg/day of oral morphine or an equianalgesic dose of another opioid.
- Use the lowest effective dosage for the shortest duration of time consistent with individual patient's treatment goals [see Warnings and Precautions (5) ] . Because the risk of overdose increases as opioid doses increase, reserve titration to higher doses of BUTRANS for patients in whom lower doses are insufficiently effective and in whom the expected benefits of using a higher dose opioid clearly outweigh the substantial risks.
- Initiate the dosing regimen for each patient individually, taking into account the patient's underlying cause and severity of pain, prior analgesic treatment and response, and risk factors for addiction, abuse, and misuse [see Warnings and Precautions (5.1) ] .
- Respiratory depression can occur at any time during opioid therapy, especially when initiating and following dosage increases with BUTRANS. Consider this risk when selecting an initial dose and when making dose adjustments [see Warnings and Precautions (5.2) ].
- BUTRANS is for transdermal use (on intact skin) only. Each BUTRANS patch is intended to be worn for 7 days.
- Instruct patients not to use BUTRANS if the pouch seal is broken or the patch is cut, damaged, or changed in any way and not to cut BUTRANS.
- Instruct patients to avoid exposing BUTRANS to external heat sources, hot water, or prolonged direct sunlight [see Warnings and Precautions (5.6) ] .
Patient Access to an Opioid Overdose Reversal Agent for the Emergency Treatment of Opioid Overdose
Inform patients and caregivers about opioid overdose reversal agents (e.g., naloxone, nalmefene). Discuss the importance of having access to an opioid overdose reversal agent, especially if the patient has risk factors for overdose (e.g., concomitant use of CNS depressants, a history of opioid use disorder, or prior opioid overdose) or if there are household members (including children) or other close contacts at risk for accidental ingestion or opioid overdose. The presence of risk factors for overdose should not prevent the management of pain in any patient [see Warnings and Precautions(5.1 , 5.2 , 5.3) ] .
Discuss the options for obtaining an opioid overdose reversal agent (e.g., prescription, over-the-counter, or as part of a community-based program) [see Warnings and Precautions (5.2) ] .
There are important differences among the opioid overdose reversal agents, such as route of administration, product strength, approved patient age range, and pharmacokinetics. Be familiar with these differences, as outlined in the approved labeling for those products, prior to recommending or prescribing such an agent.
Initial Dosage
It is safer to underestimate a patient's 24-hour oral buprenorphine dosage and provide rescue medication (e.g., immediate-release opioid) than to overestimate the 24-hour buprenorphine dosage and manage an adverse reaction due to an overdose. While useful tables of opioid equivalents are readily available, there is inter-patient variability in the potency of opioid drugs and opioid formulations. Frequently reevaluate patients for signs and symptoms of opioid withdrawal and for signs of oversedation/toxicity after converting patients to BUTRANS.
Use of BUTRANS in Patients who are not Opioid Tolerant
Unless otherwise listed below, initiate treatment with BUTRANS with a 5 mcg/hour patch.
Conversion from Other Opioid Analgesics to BUTRANS
When BUTRANS therapy is initiated, discontinue all other opioid analgesics other than those used on an as-needed basis for breakthrough pain when appropriate.
There is a potential for buprenorphine to precipitate withdrawal in patients who are already on opioids.
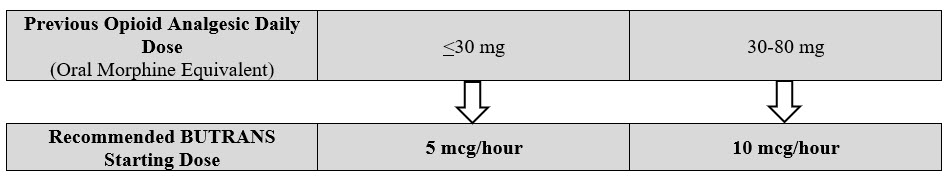
- Prior Total Daily Dose of Opioid Less than 30 mg of Oral Morphine Equivalents per Day :
Initiate treatment with BUTRANS 5 mcg/hour at the next dosing interval (see Table 1 below, middle column). - Prior Total Daily Dose of Opioid Between 30 mg to 80 mg of Oral Morphine Equivalents per Day:
Taper the patient's current around-the-clock opioids for up to 7 days to no more than 30 mg of morphine or equivalent per day before beginning treatment with BUTRANS. Then initiate treatment with BUTRANS 10 mcg/hour at the next dosing interval (see Table 1 below, right column). Patients may use short-acting analgesics as needed until analgesic efficacy with BUTRANS is attained. - Prior Total Daily Dose of Opioid Greater than 80 mg of Oral Morphine Equivalents per Day :
BUTRANS 20 mcg/hour may not provide adequate analgesia for patients requiring greater than 80 mg/day oral morphine equivalents. Consider the use of an alternate analgesic.
Table 1: Initial BUTRANS Dose
Conversion from Methadone to BUTRANS
Regular evaluation is of particular importance when converting from methadone to other opioid agonists. The ratio between methadone and other opioid agonists may vary widely as a function of previous dose exposure. Methadone has a long half-life and can accumulate in the plasma.
Titration and Maintenance of Therapy
Individually titrate BUTRANS to a dose that provides adequate analgesia and minimizes adverse reactions. Continually reevaluate patients receiving BUTRANS to assess the maintenance of pain control, signs and symptoms of opioid withdrawal and other adverse reactions, as well as reassessing for the development of addiction, abuse, or misuse [see Warnings and Precautions (5.1 , 5.19) ] . Frequent communication is important among the prescriber, other members of healthcare team, the patient, and the caregiver/family during periods of changing analgesic requirements, including initial titration. During use of opioid therapy for an extended period of time, periodically reassess the continued need for opioid analgesics.
The minimum BUTRANS titration interval is 72 hours, based on the pharmacokinetic profile and time to reach steady state levels [see Clinical Pharmacology (12.3) ] .
The maximum BUTRANS dose is 20 mcg/hour. Do not exceed a dose of one 20 mcg/hour BUTRANS system due to the risk of QTc interval prolongation. In a clinical trial, BUTRANS 40 mcg/hour (given as two BUTRANS 20 mcg/hour systems) resulted in prolongation of the QTc interval [see Warnings and Precautions (5.17) , Clinical Pharmacology (12.2) ].
Patients who experience breakthrough pain may require a dosage adjustment increase of BUTRANS or may need rescue medication with an appropriate dose of an immediate-release analgesic.
If the level of pain increases after dose stabilization, attempt to identify the source of increased pain before increasing the BUTRANS dose. If after increasing the dosage, unacceptable opioid- related adverse reactions are observed (including an increase in pain after dosage increase) consider reducing the dosage [see Warnings and Precautions (5) ]. Adjust the dosage to obtain an appropriate balance between the management of pain and opioid-related adverse reactions.
Because steady-state plasma concentrations are achieved within 72 hours, BUTRANS dosage may be adjusted every 3 days. Dose adjustments may be made in 5 mcg/hour, 7.5 mcg/hour, or 10 mcg/hour increments by using no more than two patches of the 5 mcg/hour, or 7.5 mcg/hour, or 10 mcg/hour system(s). The total dose from both patches should not exceed 20 mcg/hour. For the use of two patches, instruct patients to remove their current patch, and apply the two new patches at the same time, adjacent to one another at a different application site [see Dosage and Administration (2.7) ].
Safe Reduction or Discontinuation of BUTRANS
Do not rapidly reduce or abruptly discontinue BUTRANS in patients who may be physically dependent on opioids. Rapid reduction or abrupt discontinuation of opioid analgesics in patients who are physically dependent on opioids has resulted in serious withdrawal symptoms, uncontrolled pain, and suicide. Rapid reduction or abrupt discontinuation has also been associated with attempts to find other sources of opioid analgesics, which may be confused with drug-seeking for abuse. Patients may also attempt to treat their pain or withdrawal symptoms with illicit opioids, such as heroin, and other substances.
When a decision has been made to decrease the dose or discontinue therapy in an opioid-dependent patient taking BUTRANS, there are a variety of factors that should be considered, including the total daily dose of opioid (including BUTRANS) the patient has been taking, the duration of treatment, the type of pain being treated, and the physical and psychological attributes of the patient. It is important to ensure ongoing care of the patient and to agree on an appropriate tapering schedule and follow-up plan so that patient and provider goals and expectations are clear and realistic. When opioid analgesics are being discontinued due to a suspected substance use disorder, evaluate and treat the patient, or refer for evaluation and treatment of the substance use disorder. Treatment should include evidence-based approaches, such as medication assisted treatment of opioid use disorder. Complex patients with comorbid pain and substance use disorders may benefit from referral to a specialist.
There are no standard opioid tapering schedules that are suitable for all patients. Good clinical practice dictates a patient-specific plan to taper the dose of the opioid gradually. For patients on BUTRANS who are physically opioid-dependent, initiate the taper by a small enough increment (e.g., no greater than 10% to 25% of the total daily dose) to avoid withdrawal symptoms, and proceed with dose-lowering at an interval of every 2 to 4 weeks. Patients who have been taking opioids for briefer periods of time may tolerate a more rapid taper.
It may be necessary to provide the patient with lower dosage strengths to accomplish a successful taper. Reassess the patient frequently to manage pain and withdrawal symptoms, should they emerge. Common withdrawal symptoms include restlessness, lacrimation, rhinorrhea, yawning, perspiration, chills, myalgia, and mydriasis. Other signs and symptoms also may develop, including irritability, anxiety, backache, joint pain, weakness, abdominal cramps, insomnia, nausea, anorexia, vomiting, diarrhea, or increased blood pressure, respiratory rate, or heart rate. If withdrawal symptoms arise, it may be necessary to pause the taper for a period of time or raise the dose of the opioid analgesic to the previous dose, and then proceed with a slower taper. In addition, evaluate patients for any changes in mood, emergence of suicidal thoughts, or use of other substances.
When managing patients taking opioid analgesics, particularly those who have been treated for an extended period of time, and/or with high doses for chronic pain, ensure that a multimodal approach to pain management, including mental health support (if needed), is in place prior to initiating an opioid analgesic taper. A multimodal approach to pain management may optimize the treatment of chronic pain, as well as assist with the successful tapering of the opioid analgesic [see Warnings and Precautions (5.19) , Drug Abuse and Dependence (9.3) ] .
Patients with Hepatic Impairment
BUTRANS has not been evaluated in patients with severe hepatic impairment. As BUTRANS is only intended for 7-day application, consider use of an alternate analgesic that may permit more flexibility with the dosing in patients with severe hepatic impairment [see Warnings and Precautions (5.14) , Use in Specific Populations (8.6) , Clinical Pharmacology (12.3) ].
Administration of BUTRANS
- Instruct patients to apply immediately after removal from the individually sealed pouch. Instruct patients not to use BUTRANS if the pouch seal is broken or the patch is cut, damaged, or changed in any way. See the Instructions for Use for step-by-step instructions for applying BUTRANS.
- Apply BUTRANS to the upper outer arm, upper chest, upper back or the side of the chest. These 4 sites (each present on both sides of the body) provide 8 possible application sites. Rotate BUTRANS among the 8 described skin sites. After BUTRANS removal, wait a minimum of 21 days before reapplying to the same skin site [see Clinical Pharmacology (12.3) ].
- Apply BUTRANS to a hairless or nearly hairless skin site. If none are available, the hair at the site should be clipped, not shaven. Do not apply BUTRANS to irritated skin. If the application site must be cleaned, clean the site with water only. Do not use soaps, alcohol, oils, lotions, or abrasive devices. Allow the skin to dry before applying BUTRANS.
- Incidental exposure of the BUTRANS patch to water, such as while bathing or showering is acceptable based on experience during clinical studies.
- If problems with adhesion of BUTRANS occur, the edges may be taped with first aid tape. If problems with lack of adhesion continue, the patch may be covered with waterproof or semipermeable adhesive dressings suitable for 7 days of wear.
- If BUTRANS falls off during the 7-day dosing interval, dispose of the transdermal system properly and place a new BUTRANS patch on at a different skin site.
- When changing the system, instruct patients to remove BUTRANS and dispose of it properly [see Dosage and Administration (2.8) ] .
- If the buprenorphine-containing adhesive matrix accidentally contacts the skin, instruct patients or caregivers to wash the area with water and not to use soap, alcohol, or other solvents to remove the adhesive because they may enhance the absorption of the drug.
Disposal Instructions
Patients should refer to the Instructions for Use for proper disposal of BUTRANS. Dispose of used and unused patches by following the instructions on the Patch-Disposal Unit that is packaged with the BUTRANS patches.
Alternatively, patients can dispose of used patches by folding the adhesive side of the patch to itself, then flushing the patch down the toilet immediately upon removal. Unused patches should be removed from their pouches, the protective liners removed, the patches folded so that the adhesive side of the patch adheres to itself, and immediately flushed down the toilet.
Patients should dispose of any patches remaining from a prescription as soon as they are no longer needed.

By using PrescriberAI, you agree to the AI Terms of Use.
Butrans prescribing information
WARNING: SERIOUS AND LIFE-THREATENING RISKS FROM USE OF BUTRANS
Addiction, Abuse, and Misuse
Because the use of BUTRANS ® exposes patients and other users to the risks of opioid addiction, abuse, and misuse, which can lead to overdose and death, assess each patient's risk prior to prescribing and reassess all patients regularly for the development of these behaviors and conditions [see Warnings and Precautions (5.1) ].
Life-Threatening Respiratory Depression
Serious, life-threatening, or fatal respiratory depression may occur with use of BUTRANS, especially during initiation or following a dosage increase . To reduce the risk of respiratory depression, proper dosing and titration of BUTRANS are essential. Misuse or abuse of BUTRANS by chewing, swallowing, snorting or injecting buprenorphine extracted from the transdermal system will result in the uncontrolled delivery of buprenorphine and pose a significant risk of overdose and death [see Warnings and Precautions (5.2) ].
Accidental Exposure
Accidental exposure of even one dose of BUTRANS, especially in children, can result in a fatal overdose of buprenorphine [see Warnings and Precautions (5.2) ] .
Risks From Concomitant Use With Benzodiazepines Or Other CNS Depressants
Concomitant use of opioids with benzodiazepines or other central nervous system (CNS) depressants, including alcohol, may result in profound sedation, respiratory depression, coma, and death. Reserve concomitant prescribing of BUTRANS and benzodiazepines or other CNS depressants for use in patients for whom alternative treatment options are inadequate [see Warnings and Precautions (5.3) , Drug Interactions (7) ].
Neonatal Opioid Withdrawal Syndrome (NOWS)
Advise pregnant women using opioids for an extended period of time of the risk of Neonatal Opioid Withdrawal Syndrome, which may be life-threatening if not recognized and treated. Ensure that management by neonatology experts will be available at delivery [see Warnings and Precautions (5.4) ] .
Opioid Analgesic Risk Evaluation and Mitigation Strategy (REMS)
Healthcare providers are strongly encouraged to complete a REMS-compliant education program and to counsel patients and caregivers on serious risks, safe use, and the importance of reading the Medication Guide with each prescription [see Warnings and Precautions (5.5) ].
INDICATIONS AND USAGE
BUTRANS is indicated for the management of severe and persistent pain that requires an opioid analgesic and that cannot be adequately treated with alternative options, including immediate-release opioids.
Limitations of Use
- Because of the risks of addiction, abuse, misuse, overdose, and death, which can occur at any dosage or duration and persist over the course of therapy [see Warnings and Precautions (5.1) ] , reserve opioid analgesics, including BUTRANS, for use in patients for whom alternative treatment options are ineffective, not tolerated, or would be otherwise inadequate to provide sufficient management of pain.
- BUTRANS is not indicated as an as-needed (prn) analgesic
DOSAGE AND ADMINISTRATION
- BUTRANS should be prescribed only by healthcare professionals who are knowledgeable about the use of extended release/long-acting opioids and how to mitigate the associated risks. (2.1 )
- BUTRANS doses of 7.5, 10, 15, and 20 mcg/hour are only for use in patients receiving, for one week or longer, daily opioid doses up to 80 mg/day of oral morphine or an equianalgesic dose of another opioid. (2.1 )
- Use the lowest effective dosage for the shortest duration of time consistent with individual patient treatment goals. Reserve titration to higher doses of BUTRANS for patients in whom lower doses are insufficiently effective and in whom the expected benefits of using a higher dose opioid clearly outweigh the substantial risks. (2.1 , 5 )
- Initiate the dosing regimen for each patient individually, taking into account the patients underlying cause and severity of pain, prior analgesic treatment and response, and risk factors for addiction, abuse, and misuse. (2.1 , 5.1 )
- Respiratory depression can occur at any time during opioid therapy, especially when initiating and following dosage increases with BUTRANS. Consider this risk when selecting an initial dose and when making dose adjustments. (2.1 , 5.1 )
- For patients who are not opioid tolerant, initiate treatment with a 5 mcg/hour patch. (2.1 )
- Instruct patients to wear BUTRANS for 7 days and to wait a minimum of 3 weeks before applying to the same site. (2.1 )
- Discuss opioid overdose reversal agents and options for acquiring them with the patient and/or caregiver, both when initiating and renewing treatment with BUTRANS, especially if the patient has additional risk factors for overdose, or close contacts at risk for exposure and overdose. (2.2 , 5.1 , 5.2 , 5.3 )
- Periodically reassess patients receiving BUTRANS to evaluate the continued need for opioid analgesics to maintain pain control, for the signs or symptoms of adverse reactions, and for the development of addiction, abuse, or misuse. (2.3 )
- Do not rapidly reduce or abruptly discontinue BUTRANS in a physically dependent patient because rapid reduction or abrupt discontinuation of opioid analgesics has resulted in serious withdrawal symptoms, uncontrolled pain, and suicide. (2.5 , 5.19 )
Important Dosage and Administration Information
- BUTRANS should be prescribed only by healthcare professionals who are knowledgeable about the use of extended-release/long-acting opioids and how to mitigate the associated risks.
- BUTRANS doses of 7.5, 10, 15, and 20 mcg/hour are only for use in patients who are receiving, for one week or longer, daily opioid doses up to 80 mg/day of oral morphine or an equianalgesic dose of another opioid.
- Use the lowest effective dosage for the shortest duration of time consistent with individual patient's treatment goals [see Warnings and Precautions (5) ] . Because the risk of overdose increases as opioid doses increase, reserve titration to higher doses of BUTRANS for patients in whom lower doses are insufficiently effective and in whom the expected benefits of using a higher dose opioid clearly outweigh the substantial risks.
- Initiate the dosing regimen for each patient individually, taking into account the patient's underlying cause and severity of pain, prior analgesic treatment and response, and risk factors for addiction, abuse, and misuse [see Warnings and Precautions (5.1) ] .
- Respiratory depression can occur at any time during opioid therapy, especially when initiating and following dosage increases with BUTRANS. Consider this risk when selecting an initial dose and when making dose adjustments [see Warnings and Precautions (5.2) ].
- BUTRANS is for transdermal use (on intact skin) only. Each BUTRANS patch is intended to be worn for 7 days.
- Instruct patients not to use BUTRANS if the pouch seal is broken or the patch is cut, damaged, or changed in any way and not to cut BUTRANS.
- Instruct patients to avoid exposing BUTRANS to external heat sources, hot water, or prolonged direct sunlight [see Warnings and Precautions (5.6) ] .
Patient Access to an Opioid Overdose Reversal Agent for the Emergency Treatment of Opioid Overdose
Inform patients and caregivers about opioid overdose reversal agents (e.g., naloxone, nalmefene). Discuss the importance of having access to an opioid overdose reversal agent, especially if the patient has risk factors for overdose (e.g., concomitant use of CNS depressants, a history of opioid use disorder, or prior opioid overdose) or if there are household members (including children) or other close contacts at risk for accidental ingestion or opioid overdose. The presence of risk factors for overdose should not prevent the management of pain in any patient [see Warnings and Precautions(5.1 , 5.2 , 5.3) ] .
Discuss the options for obtaining an opioid overdose reversal agent (e.g., prescription, over-the-counter, or as part of a community-based program) [see Warnings and Precautions (5.2) ] .
There are important differences among the opioid overdose reversal agents, such as route of administration, product strength, approved patient age range, and pharmacokinetics. Be familiar with these differences, as outlined in the approved labeling for those products, prior to recommending or prescribing such an agent.
Initial Dosage
It is safer to underestimate a patient's 24-hour oral buprenorphine dosage and provide rescue medication (e.g., immediate-release opioid) than to overestimate the 24-hour buprenorphine dosage and manage an adverse reaction due to an overdose. While useful tables of opioid equivalents are readily available, there is inter-patient variability in the potency of opioid drugs and opioid formulations. Frequently reevaluate patients for signs and symptoms of opioid withdrawal and for signs of oversedation/toxicity after converting patients to BUTRANS.
Use of BUTRANS in Patients who are not Opioid Tolerant
Unless otherwise listed below, initiate treatment with BUTRANS with a 5 mcg/hour patch.
Conversion from Other Opioid Analgesics to BUTRANS
When BUTRANS therapy is initiated, discontinue all other opioid analgesics other than those used on an as-needed basis for breakthrough pain when appropriate.
There is a potential for buprenorphine to precipitate withdrawal in patients who are already on opioids.
- Prior Total Daily Dose of Opioid Less than 30 mg of Oral Morphine Equivalents per Day : Initiate treatment with BUTRANS 5 mcg/hour at the next dosing interval (see Table 1 below, middle column).
- Prior Total Daily Dose of Opioid Between 30 mg to 80 mg of Oral Morphine Equivalents per Day: Taper the patient's current around-the-clock opioids for up to 7 days to no more than 30 mg of morphine or equivalent per day before beginning treatment with BUTRANS. Then initiate treatment with BUTRANS 10 mcg/hour at the next dosing interval (see Table 1 below, right column). Patients may use short-acting analgesics as needed until analgesic efficacy with BUTRANS is attained.
- Prior Total Daily Dose of Opioid Greater than 80 mg of Oral Morphine Equivalents per Day : BUTRANS 20 mcg/hour may not provide adequate analgesia for patients requiring greater than 80 mg/day oral morphine equivalents. Consider the use of an alternate analgesic.
Table 1: Initial BUTRANS Dose

Conversion from Methadone to BUTRANS
Regular evaluation is of particular importance when converting from methadone to other opioid agonists. The ratio between methadone and other opioid agonists may vary widely as a function of previous dose exposure. Methadone has a long half-life and can accumulate in the plasma.
Titration and Maintenance of Therapy
Individually titrate BUTRANS to a dose that provides adequate analgesia and minimizes adverse reactions. Continually reevaluate patients receiving BUTRANS to assess the maintenance of pain control, signs and symptoms of opioid withdrawal and other adverse reactions, as well as reassessing for the development of addiction, abuse, or misuse [see Warnings and Precautions (5.1 , 5.19) ] . Frequent communication is important among the prescriber, other members of healthcare team, the patient, and the caregiver/family during periods of changing analgesic requirements, including initial titration. During use of opioid therapy for an extended period of time, periodically reassess the continued need for opioid analgesics.
The minimum BUTRANS titration interval is 72 hours, based on the pharmacokinetic profile and time to reach steady state levels [see Clinical Pharmacology (12.3) ] .
The maximum BUTRANS dose is 20 mcg/hour. Do not exceed a dose of one 20 mcg/hour BUTRANS system due to the risk of QTc interval prolongation. In a clinical trial, BUTRANS 40 mcg/hour (given as two BUTRANS 20 mcg/hour systems) resulted in prolongation of the QTc interval [see Warnings and Precautions (5.17) , Clinical Pharmacology (12.2) ].
Patients who experience breakthrough pain may require a dosage adjustment increase of BUTRANS or may need rescue medication with an appropriate dose of an immediate-release analgesic.
If the level of pain increases after dose stabilization, attempt to identify the source of increased pain before increasing the BUTRANS dose. If after increasing the dosage, unacceptable opioid- related adverse reactions are observed (including an increase in pain after dosage increase) consider reducing the dosage [see Warnings and Precautions (5) ]. Adjust the dosage to obtain an appropriate balance between the management of pain and opioid-related adverse reactions.
Because steady-state plasma concentrations are achieved within 72 hours, BUTRANS dosage may be adjusted every 3 days. Dose adjustments may be made in 5 mcg/hour, 7.5 mcg/hour, or 10 mcg/hour increments by using no more than two patches of the 5 mcg/hour, or 7.5 mcg/hour, or 10 mcg/hour system(s). The total dose from both patches should not exceed 20 mcg/hour. For the use of two patches, instruct patients to remove their current patch, and apply the two new patches at the same time, adjacent to one another at a different application site [see Dosage and Administration (2.7) ].
Safe Reduction or Discontinuation of BUTRANS
Do not rapidly reduce or abruptly discontinue BUTRANS in patients who may be physically dependent on opioids. Rapid reduction or abrupt discontinuation of opioid analgesics in patients who are physically dependent on opioids has resulted in serious withdrawal symptoms, uncontrolled pain, and suicide. Rapid reduction or abrupt discontinuation has also been associated with attempts to find other sources of opioid analgesics, which may be confused with drug-seeking for abuse. Patients may also attempt to treat their pain or withdrawal symptoms with illicit opioids, such as heroin, and other substances.
When a decision has been made to decrease the dose or discontinue therapy in an opioid-dependent patient taking BUTRANS, there are a variety of factors that should be considered, including the total daily dose of opioid (including BUTRANS) the patient has been taking, the duration of treatment, the type of pain being treated, and the physical and psychological attributes of the patient. It is important to ensure ongoing care of the patient and to agree on an appropriate tapering schedule and follow-up plan so that patient and provider goals and expectations are clear and realistic. When opioid analgesics are being discontinued due to a suspected substance use disorder, evaluate and treat the patient, or refer for evaluation and treatment of the substance use disorder. Treatment should include evidence-based approaches, such as medication assisted treatment of opioid use disorder. Complex patients with comorbid pain and substance use disorders may benefit from referral to a specialist.
There are no standard opioid tapering schedules that are suitable for all patients. Good clinical practice dictates a patient-specific plan to taper the dose of the opioid gradually. For patients on BUTRANS who are physically opioid-dependent, initiate the taper by a small enough increment (e.g., no greater than 10% to 25% of the total daily dose) to avoid withdrawal symptoms, and proceed with dose-lowering at an interval of every 2 to 4 weeks. Patients who have been taking opioids for briefer periods of time may tolerate a more rapid taper.
It may be necessary to provide the patient with lower dosage strengths to accomplish a successful taper. Reassess the patient frequently to manage pain and withdrawal symptoms, should they emerge. Common withdrawal symptoms include restlessness, lacrimation, rhinorrhea, yawning, perspiration, chills, myalgia, and mydriasis. Other signs and symptoms also may develop, including irritability, anxiety, backache, joint pain, weakness, abdominal cramps, insomnia, nausea, anorexia, vomiting, diarrhea, or increased blood pressure, respiratory rate, or heart rate. If withdrawal symptoms arise, it may be necessary to pause the taper for a period of time or raise the dose of the opioid analgesic to the previous dose, and then proceed with a slower taper. In addition, evaluate patients for any changes in mood, emergence of suicidal thoughts, or use of other substances.
When managing patients taking opioid analgesics, particularly those who have been treated for an extended period of time, and/or with high doses for chronic pain, ensure that a multimodal approach to pain management, including mental health support (if needed), is in place prior to initiating an opioid analgesic taper. A multimodal approach to pain management may optimize the treatment of chronic pain, as well as assist with the successful tapering of the opioid analgesic [see Warnings and Precautions (5.19) , Drug Abuse and Dependence (9.3) ] .
Patients with Hepatic Impairment
BUTRANS has not been evaluated in patients with severe hepatic impairment. As BUTRANS is only intended for 7-day application, consider use of an alternate analgesic that may permit more flexibility with the dosing in patients with severe hepatic impairment [see Warnings and Precautions (5.14) , Use in Specific Populations (8.6) , Clinical Pharmacology (12.3) ].
Administration of BUTRANS
- Instruct patients to apply immediately after removal from the individually sealed pouch. Instruct patients not to use BUTRANS if the pouch seal is broken or the patch is cut, damaged, or changed in any way. See the Instructions for Use for step-by-step instructions for applying BUTRANS.
- Apply BUTRANS to the upper outer arm, upper chest, upper back or the side of the chest. These 4 sites (each present on both sides of the body) provide 8 possible application sites. Rotate BUTRANS among the 8 described skin sites. After BUTRANS removal, wait a minimum of 21 days before reapplying to the same skin site [see Clinical Pharmacology (12.3) ].
- Apply BUTRANS to a hairless or nearly hairless skin site. If none are available, the hair at the site should be clipped, not shaven. Do not apply BUTRANS to irritated skin. If the application site must be cleaned, clean the site with water only. Do not use soaps, alcohol, oils, lotions, or abrasive devices. Allow the skin to dry before applying BUTRANS.
- Incidental exposure of the BUTRANS patch to water, such as while bathing or showering is acceptable based on experience during clinical studies.
- If problems with adhesion of BUTRANS occur, the edges may be taped with first aid tape. If problems with lack of adhesion continue, the patch may be covered with waterproof or semipermeable adhesive dressings suitable for 7 days of wear.
- If BUTRANS falls off during the 7-day dosing interval, dispose of the transdermal system properly and place a new BUTRANS patch on at a different skin site.
- When changing the system, instruct patients to remove BUTRANS and dispose of it properly [see Dosage and Administration (2.8) ] .
- If the buprenorphine-containing adhesive matrix accidentally contacts the skin, instruct patients or caregivers to wash the area with water and not to use soap, alcohol, or other solvents to remove the adhesive because they may enhance the absorption of the drug.
Disposal Instructions
Patients should refer to the Instructions for Use for proper disposal of BUTRANS. Dispose of used and unused patches by following the instructions on the Patch-Disposal Unit that is packaged with the BUTRANS patches.
Alternatively, patients can dispose of used patches by folding the adhesive side of the patch to itself, then flushing the patch down the toilet immediately upon removal. Unused patches should be removed from their pouches, the protective liners removed, the patches folded so that the adhesive side of the patch adheres to itself, and immediately flushed down the toilet.
Patients should dispose of any patches remaining from a prescription as soon as they are no longer needed.
DOSAGE FORMS AND STRENGTHS
BUTRANS is a rectangular or square, beige-colored system consisting of a protective liner and functional layers. BUTRANS is available in five strengths:
- BUTRANS 5 mcg/hour Transdermal System (dimensions: 45 mm by 45 mm)
- BUTRANS 7.5 mcg/hour Transdermal System (dimensions: 58 mm by 45 mm)
- BUTRANS 10 mcg/hour Transdermal System (dimensions: 45 mm by 68 mm)
- BUTRANS 15 mcg/hour Transdermal System (dimensions: 59 mm by 72 mm)
- BUTRANS 20 mcg/hour Transdermal System (dimensions: 72 mm by 72 mm)
USE IN SPECIFIC POPULATIONS
Pregnancy
Risk Summary
Use of opioid analgesics for an extended period of time during pregnancy may cause neonatal opioid withdrawal syndrome [see Warnings and Precautions (5.4) ]. Available data with BUTRANS in pregnant women are insufficient to inform a drug-associated risk for major birth defects and miscarriage.
In animal reproduction studies, buprenorphine caused an increase in the number of stillborn offspring, reduced litter size, and reduced offspring growth in rats at maternal exposure levels that were approximately 10 times that of human subjects who received one BUTRANS 20 mcg/hour, the maximum recommended human dose (MRHD) [see Data ]. Based on animal data, advise pregnant women of the potential risk to a fetus.
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Clinical Considerations
Fetal/neonatal adverse reactions
Use of opioid analgesics for an extended period of time during pregnancy for medical or nonmedical purposes can result in physical dependence in the neonate and neonatal opioid withdrawal syndrome shortly after birth. Neonatal opioid withdrawal syndrome presents as irritability, hyperactivity and abnormal sleep pattern, high pitched cry, tremor, vomiting, diarrhea, and failure to gain weight. The onset, duration, and severity of neonatal opioid withdrawal syndrome vary based on the specific opioid used, duration of use, timing and amount of last maternal use, and rate of elimination of the drug by the newborn. Observe newborns for symptoms of neonatal opioid withdrawal syndrome and manage accordingly [see Warnings and Precautions (5.4) ].
Labor and Delivery
Opioids cross the placenta and may produce respiratory depression and psychophysiologic effects in neonates. An opioid overdose reversal agent, such as naloxone or nalmefene, must be available for reversal of opioid-induced respiratory depression in the neonate. BUTRANS is not recommended for use in women immediately prior to labor, when shorter acting analgesics or other analgesic techniques are more appropriate. Opioid analgesics, including BUTRANS, can prolong labor through actions that temporarily reduce the strength, duration, and frequency of uterine contractions. However, this effect is not consistent and may be offset by an increased rate of cervical dilatation, which tends to shorten labor.
Data
Animal Data
Studies in rats and rabbits demonstrated no evidence of teratogenicity following BUTRANS or subcutaneous (SC) administration of buprenorphine during the period of organogenesis. Rats were administered up to one BUTRANS 20 mcg/hour every 3 days (Gestation Days 6, 9, 12, & 15) or received daily SC buprenorphine up to 5 mg/kg (Gestation Days 6 to 17). Rabbits were administered four BUTRANS 20 mcg/hour every 3 days (Gestation Days 6, 9, 12, 15, 18, and 19) or received daily SC buprenorphine up to 5 mg/kg (Gestation Days 6-19). No teratogenicity was observed at any dose. AUC values for buprenorphine with BUTRANS application and SC injection were approximately 110 and 140 times, respectively, that of human subjects who received the MRHD of one BUTRANS 20 mcg/hour.
In a pre- and post-natal study conducted in pregnant and lactating rats, administration of buprenorphine either as BUTRANS or SC buprenorphine was associated with toxicity to offspring. Buprenorphine was present in maternal milk. Pregnant rats were administered 1/4 of one BUTRANS 5 mcg/hour every 3 days or received daily SC buprenorphine at doses of 0.05, 0.5, or 5 mg/kg from Gestation Day 6 to Lactation Day 21 (weaning). Administration of BUTRANS or SC buprenorphine at 0.5 or 5 mg/kg caused maternal toxicity and an increase in the number of stillborns, reduced litter size, and reduced offspring growth at maternal exposure levels that were approximately 10 times that of human subjects who received the MRHD of one BUTRANS 20 mcg/hour. Maternal toxicity was also observed at the no observed adverse effect level (NOAEL) for offspring.
Lactation
Risk Summary
Because of the potential for serious adverse reactions, including excess sedation and respiratory depression in a breastfed infant, advise patients that breastfeeding is not recommended during treatment with BUTRANS.
Clinical Considerations
Monitor infants exposed to BUTRANS through breast milk for excess sedation and respiratory depression. Withdrawal symptoms can occur in breastfed infants when maternal administration of buprenorphine is stopped or when breast-feeding is stopped.
Females and Males of Reproductive Potential
Infertility
Use of opioids for an extended period of time may cause reduced fertility in females and males of reproductive potential. It is not known whether these effects on fertility are reversible [see Adverse Reactions (6.2) , Clinical Pharmacology (12.2) , Nonclinical Toxicology (13.1) ] .
Pediatric Use
The safety and efficacy of BUTRANS in patients under 18 years of age has not been established. BUTRANS has been evaluated in an open-label clinical trial in pediatric patients. However, definitive conclusions are not possible because of the small sample size.
Geriatric Use
Of the total number of subjects in the clinical trials (5,415), BUTRANS was administered to 1,377 patients aged 65 years and older. Of those, 457 patients were 75 years of age and older. In the clinical program, the incidences of selected BUTRANS-related AEs were higher in older subjects. The incidences of application site AEs were slightly higher among subjects <65 years of age than those ≥65 years of age for both BUTRANS and placebo treatment groups.
In a single-dose study of healthy elderly and healthy young subjects treated with BUTRANS 10 mcg/hour, the pharmacokinetics were similar. In a separate dose-escalation safety study, the pharmacokinetics in the healthy elderly and hypertensive elderly subjects taking thiazide diuretics were similar to those in the healthy young adults. In the elderly groups evaluated, adverse event rates were similar to or lower than rates in healthy young adult subjects, except for constipation and urinary retention, which were more common in the elderly. Although specific dose adjustments on the basis of advanced age are not required for pharmacokinetic reasons, use caution in the elderly population to ensure safe use [see Clinical Pharmacology (12.3) ] .
Respiratory depression is the chief risk for elderly patients treated with opioids and has occurred after large initial doses were administered to patients who were not opioid-tolerant or when opioids were co-administered with other agents that depress respiration. Titrate the dosage of BUTRANS slowly in geriatric patients and frequently reevaluate the patient for signs of central nervous system and respiratory depression [see Warnings and Precautions (5.10) ] .
Hepatic Impairment
In a study utilizing intravenous buprenorphine, peak plasma levels (C max ) and exposure (AUC) of buprenorphine in patients with mild and moderate hepatic impairment did not increase as compared to those observed in subjects with normal hepatic function. BUTRANS has not been evaluated in patients with severe hepatic impairment. As BUTRANS is intended for 7-day dosing, consider the use of alternate analgesic therapy in patients with severe hepatic impairment [see Dosage and Administration (2.6) and Clinical Pharmacology (12.3) ].
CONTRAINDICATIONS
BUTRANS is contraindicated in patients with:
- Significant respiratory depression [see Warnings and Precautions (5.2) ]
- Acute or severe bronchial asthma in an unmonitored setting or in the absence of resuscitative equipment [see Warnings and Precautions (5.10) ]
- Known or suspected gastrointestinal obstruction, including paralytic ileus [see Warnings and Precautions (5.15) ]
- Hypersensitivity (e.g., anaphylaxis) to buprenorphine [see Warnings and Precautions (5.18) , Adverse Reactions (6) ]
WARNINGS AND PRECAUTIONS
- Opioid-Induced Hyperalgesia and Allodynia : Opioid-Induced Hyperalgesia (OIH) occurs when an opioid analgesic paradoxically causes an increase in pain, or an increase in sensitivity to pain. If OIH is suspected, carefully consider appropriately decreasing the dose of the current opioid analgesic, or opioid rotation. (5.9 )
- Life Threatening Respiratory Depression in Patients with Chronic Pulmonary Disease or in Elderly, Cachectic, or Debilitated Patients : Regularly evaluate particularly during initiation and titration. (5.10 )
- Adrenal Insufficiency : If diagnosed, treat with physiologic replacement of corticosteroids, and wean patient off of the opioid. (5.11 )
- Severe Hypotension : Regularly evaluate during dose initiation and titration. Avoid use of BUTRANS in patients with circulatory shock (5.12 )
- Risks of Use in Patients with Increased Intracranial Pressure, Brain Tumors, Head Injury, or Impaired Consciousness : Monitor for sedation and respiratory depression. Avoid use of BUTRANS in patients with impaired consciousness or coma. (5.13 )
Addiction, Abuse, and Misuse
BUTRANS contains buprenorphine, a Schedule III controlled substance. As an opioid, BUTRANS exposes users to the risks of addiction, abuse, and misuse [see Drug Abuse and Dependence (9) ] .
Although the risk of addiction in any individual is unknown, it can occur in patients appropriately prescribed BUTRANS. Addiction can occur at recommended doses and if the drug is misused or abused. The risk of opioid-related overdose or overdose-related death is increased with higher opioid doses, and this risk persists over the course of therapy. In postmarketing studies, addiction, abuse, misuse, and fatal and non-fatal opioid overdose were observed in patients with long-term opioid use [see Adverse Reactions (6.2) ] .
Assess each patient's risk for opioid addiction, abuse, or misuse prior to prescribing BUTRANS, and reassess all patients receiving BUTRANS for the development of these behaviors and conditions. Risks are increased in patients with a personal or family history of substance abuse (including drug or alcohol abuse or addiction) or mental illness (e.g., major depression). The potential for these risks should not, however, prevent the proper management of pain in any given patient. Patients at increased risk may be prescribed opioids such as BUTRANS but use in such patients necessitates intensive counseling about the risks and proper use of BUTRANS, along with frequent reevaluation for signs of addiction, abuse, or misuse. Consider recommending or prescribing an opioid overdose reversal agent [see Dosage and Administration (2.2) , Warnings and Precautions (5.2) .
Abuse or misuse of BUTRANS by placing it in the mouth, chewing it, swallowing it, or using it in ways other than indicated may cause choking, overdose and death [see Overdosage (10) ] .
Opioids are sought for nonmedical use and are subject to diversion from legitimate prescribed use. Consider these risks when prescribing or dispensing BUTRANS. Strategies to reduce these risks include prescribing the drug in the smallest appropriate quantity and advising the patient on careful storage of the drug during the course of treatment and the proper disposal of unused drug. Contact local state professional licensing board or state-controlled substances authority for information on how to prevent and detect abuse or diversion of this product.
Life-Threatening Respiratory Depression
Serious, life-threatening, or fatal respiratory depression has been reported with the use of opioids, even when used as recommended. Respiratory depression, if not immediately recognized and treated, may lead to respiratory arrest and death. Management of respiratory depression may include close observation, supportive measures, and use of opioid overdose reversal agents, depending on the patient's clinical status [see Overdosage (10) ] . Carbon dioxide (CO 2 ) retention from opioid-induced respiratory depression can exacerbate the sedating effects of opioids.
While serious, life-threatening, or fatal respiratory depression can occur at any time during the use of BUTRANS, the risk is greatest during the initiation of therapy or following a dosage increase.
To reduce the risk of respiratory depression, proper dosing and titration of BUTRANS are essential [see Dosage and Administration (2) ] . Overestimating the BUTRANS dosage when converting patients from another opioid product can result in fatal overdose with the first dose.
Accidental exposure to BUTRANS, especially in children, can result in respiratory depression and death due to an overdose of buprenorphine.
Educate patients and caregivers on how to recognize respiratory depression and emphasize the importance of calling 911 or getting emergency medical help right away in the event of a known or suspected overdose .
Opioids can cause sleep-related breathing disorders including central sleep apnea (CSA) and sleep-related hypoxemia. Opioid use increases the risk of CSA in a dose-dependent fashion. In patients who present with CSA, consider decreasing the opioid dosage using best practices for opioid taper [see Dosage and Administration (2.5) ].
Patient Access to an Opioid Overdose Reversal Agent for the Emergency Treatment of Opioid Overdose
Inform patients and caregivers about opioid overdose reversal agents (e.g., naloxone, nalmefene). Discuss the importance of having access to an opioid overdose reversal agent, especially if the patient has risk factors for overdose (e.g., concomitant use of CNS depressants, a history of opioid use disorder, or prior opioid overdose) or if there are household members (including children) or other close contacts at risk for accidental ingestion or opioid overdose. The presence of risk factors for overdose should not prevent the management of pain in any patient [see Warnings and Precautions (5.1 , 5.3) ] .
Discuss the options for obtaining an opioid overdose reversal agent (e.g., prescription, over-the-counter, or as part of a community-based program).
There are important differences among the opioid overdose reversal agents, such as route of administration, product strength, approved patient age range, and pharmacokinetics. Be familiar with these differences, as outlined in the approved labeling for those products, prior to recommending or prescribing such an agent.
Educate patients and caregivers on how to recognize respiratory depression, and how to use an opioid overdose reversal agent for the emergency treatment of opioid overdose. Emphasize the importance of calling 911 or getting emergency medical help, even if an opioid overdose reversal agent is administered [see Dosage and Administration (2.2) , Warnings and Precautions (5.1 , 5.3) , Overdosage (10) ].
Risks from Concomitant Use with Benzodiazepines or Other CNS Depressants
Profound sedation, respiratory depression, coma, and death may result from the concomitant use of BUTRANS with benzodiazepines and/or other CNS depressants, including alcohol (e.g., non-benzodiazepine sedatives/hypnotics, anxiolytics, tranquilizers, muscle relaxants, general anesthetics, antipsychotics, gabapentinoids [gabapentin and pregabalin], and other opioids). Because of these risks, reserve concomitant prescribing of these drugs for use in patients for whom alternative treatment options are inadequate.
Observational studies have demonstrated that concomitant use of opioid analgesics and benzodiazepines increases the risk of drug-related mortality compared to use of opioid analgesics alone. Because of similar pharmacological properties, it is reasonable to expect similar risk with the concomitant use of other CNS depressant drugs with opioid analgesics [see Drug Interactions (7) ] .
If the decision is made to prescribe a benzodiazepine or other CNS depressant concomitantly with an opioid analgesic, prescribe the lowest effective dosages and minimum durations of concomitant use. In patients already receiving an opioid analgesic, prescribe a lower initial dose of the benzodiazepine or other CNS depressant than indicated in the absence of an opioid, and titrate based on clinical response. If an opioid analgesic is initiated in a patient already taking a benzodiazepine or other CNS depressant, prescribe a lower initial dose of the opioid analgesic, and titrate based on clinical response. Inform patients and caregivers of this potential interaction and educate them on the signs and symptoms of respiratory depression (including sedation).
If concomitant use is warranted, consider recommending or prescribing an opioid overdose reversal agent [see Dosage and Administration (2.2) , Warnings and Precautions (5.2) , Overdosage (10) ] .
Advise both patients and caregivers about the risks of respiratory depression and sedation when BUTRANS is used with benzodiazepines or other CNS depressants (including alcohol and illicit drugs). Advise patients not to drive or operate heavy machinery until the effects of concomitant use of the benzodiazepine or other CNS depressant have been determined. Screen patients for risk of substance use disorders, including opioid abuse and misuse, and warn them of the risk for overdose and death associated with the use of additional CNS depressants including alcohol and illicit drugs [see Drug Interactions (7) , Patient Counseling Information (17) ] .
Neonatal Opioid Withdrawal Syndrome
Use of BUTRANS for an extended period of time during pregnancy can result in withdrawal in the neonate. Neonatal opioid withdrawal syndrome, unlike opioid withdrawal syndrome in adults, may be life-threatening if not recognized and treated, and requires management according to protocols developed by neonatology experts. Observe newborns for signs of neonatal opioid withdrawal syndrome and manage accordingly. Advise pregnant women using opioids for an extended period of time of the risk of neonatal opioid withdrawal syndrome and ensure that appropriate treatment will be available [see Use in Specific Populations (8.1) ].
Opioid Analgesic Risk Evaluation and Mitigation Strategy (REMS)
To ensure that the benefits of opioid analgesics outweigh the risks of addiction, abuse, and misuse, the Food and Drug Administration (FDA) has required a Risk Evaluation and Mitigation Strategy (REMS) for these products. Under the requirements of the REMS, drug companies with approved opioid analgesic products must make REMS-compliant education programs available to healthcare providers. Healthcare providers are strongly encouraged to do all of the following:
- Complete a REMS-compliant education program offered by an accredited provider of continuing education (CE) or another education program that includes all the elements of the FDA Education Blueprint for Health Care Providers Involved in the Management or Support of Patients with Pain.
- Discuss the safe use, serious risks, and proper storage and disposal of opioid analgesics with patients and/or their caregivers every time these medicines are prescribed. The Patient Counseling Guide (PCG) can be obtained at this link: www.fda.gov/OpioidAnalgesicREMSPCG.
- Emphasize to patients and their caregivers the importance of reading the Medication Guide that they will receive from their pharmacist every time an opioid analgesic is dispensed to them.
- Consider using other tools to improve patient, household, and community safety, such as patient-prescriber agreements that reinforce patient-prescriber responsibilities.
To obtain further information on the opioid analgesic REMS and for a list of accredited REMS CME/CE, call 1-800-503-0784, or log on to www.opioidanalgesicrems.com. The FDA Blueprint can be found at www.fda.gov/OpioidAnalgesicREMSBlueprint.
Risks of Use with Application of External Heat
Advise patients and their caregivers to avoid exposing the BUTRANS application site and surrounding area to direct external heat sources, such as heating pads or electric blankets, heat or tanning lamps, saunas, hot tubs, and heated water beds while wearing the system because an increase in absorption of buprenorphine may occur [see Clinical Pharmacology (12.3) ]. Advise patients against exposure of the BUTRANS application site and surrounding area to hot water or prolonged exposure to direct sunlight. There is a potential for temperature-dependent increases in buprenorphine released from the system resulting in possible overdose and death.
Risk of Use in Patients with Fever
Regularly evaluate patients wearing BUTRANS systems who develop fever or increased core body temperature due to strenuous exertion for opioid side effects and adjust the BUTRANS dose if signs of respiratory or central nervous system depression occur.
Application Site Skin Reactions
In rare cases, severe application site skin reactions with signs of marked inflammation including "burn," "discharge," and "vesicles" have occurred. Time of onset varies, ranging from days to months following the initiation of BUTRANS treatment. Instruct patients to promptly report the development of severe application site reactions and discontinue therapy.
Opioid-Induced Hyperalgesia and Allodynia
Opioid-Induced Hyperalgesia (OIH) occurs when an opioid analgesic paradoxically causes an increase in pain, or an increase in sensitivity to pain. This condition differs from tolerance, which is the need for increasing doses of opioids to maintain a defined effect [see Drug Abuse and Dependence (9.3) ]. Symptoms of OIH include (but may not be limited to) increased levels of pain upon opioid dosage increase, decreased levels of pain upon opioid dosage decrease, or pain from ordinarily non-painful stimuli (allodynia). These symptoms may suggest OIH only if there is no evidence of underlying disease progression, opioid tolerance, opioid withdrawal, or addictive behavior.
Cases of OIH have been reported, both with short-term and longer-term use of opioid analgesics. Though the mechanism of OIH is not fully understood, multiple biochemical pathways have been implicated. Medical literature suggests a strong biologic plausibility between opioid analgesics and OIH and allodynia. If a patient is suspected to be experiencing OIH, carefully consider appropriately decreasing the dose of the current opioid analgesic or opioid rotation (safely switching the patient to a different opioid moiety) [see Dosage and Administration (2.5) , Warnings and Precautions (5.19) ].
Life-Threatening Respiratory Depression in Patients with Chronic Pulmonary Disease or in Elderly, Cachectic, or Debilitated Patients
The use of BUTRANS in patients with acute or severe bronchial asthma in an unmonitored setting or in the absence of resuscitative equipment is contraindicated.
Patients with Chronic Pulmonary Disease: BUTRANS-treated patients with significant chronic obstructive pulmonary disease or cor pulmonale, and those with a substantially decreased respiratory reserve, hypoxia, hypercapnia, or pre-existing respiratory depression are at increased risk of decreased respiratory drive including apnea, even at recommended dosages of BUTRANS [see Warnings and Precautions (5.2) ].
Elderly, Cachectic, or Debilitated Patients: Life-threatening respiratory depression is more likely to occur in elderly, cachectic, or debilitated patients because they may have altered pharmacokinetics or altered clearance compared to younger, healthier patients [see Warnings and Precautions (5.2) ].
Regularly evaluate patients particularly when initiating and titrating BUTRANS and when BUTRANS is given concomitantly with other drugs that depress respiration [see Warnings and Precautions (5.2 , 5.3) Drug Interactions (7) ] . Alternatively, consider the use of non-opioid analgesics in these patients.
Adrenal Insufficiency
Cases of adrenal insufficiency have been reported with opioid use, more often following greater than one month of use. Presentation of adrenal insufficiency may include non-specific symptoms and signs including nausea, vomiting, anorexia, fatigue, weakness, dizziness, and low blood pressure. If adrenal insufficiency is suspected, confirm the diagnosis with diagnostic testing as soon as possible. If adrenal insufficiency is diagnosed, treat with physiologic replacement doses of corticosteroids. Wean the patient off of the opioid to allow adrenal function to recover and continue corticosteroid treatment until adrenal function recovers. Other opioids may be tried as some cases reported use of a different opioid without recurrence of adrenal insufficiency. The information available does not identify any particular opioids as being more likely to be associated with adrenal insufficiency.
Severe Hypotension
BUTRANS may cause severe hypotension including orthostatic hypotension and syncope in ambulatory patients. There is an increased risk in patients whose ability to maintain blood pressure has already been compromised by a reduced blood volume or concurrent administration of certain CNS depressant drugs (e.g., phenothiazines or general anesthetics) [see Drug Interactions (7) ] . Regularly evaluate these patients for signs of hypotension after initiating or titrating the dosage of BUTRANS. In patients with circulatory shock, BUTRANS may cause vasodilation that can further reduce cardiac output and blood pressure. Avoid the use of BUTRANS in patients with circulatory shock.
Risks of Use in Patients with Increased Intracranial Pressure, Brain Tumors, Head Injury or Impaired Consciousness
In patients who may be susceptible to the intracranial effects of CO 2 retention (e.g., those with evidence of increased intracranial pressure or brain tumors), BUTRANS may reduce respiratory drive, and the resultant CO 2 retention can further increase intracranial pressure. Monitor such patients for signs of sedation and respiratory depression, particularly when initiating therapy with BUTRANS.
Opioids may also obscure the clinical course in a patient with a head injury. Avoid the use of BUTRANS in patients with impaired consciousness or coma.
Hepatotoxicity
Cases of cytolytic hepatitis and hepatitis with jaundice have been observed in individuals receiving sublingual buprenorphine for the treatment of opioid dependence, both in clinical trials and in post-marketing adverse event reports. The spectrum of abnormalities ranges from transient asymptomatic elevations in hepatic transaminases to case reports of hepatic failure, hepatic necrosis, hepatorenal syndrome, and hepatic encephalopathy. In many cases, the presence of pre-existing liver enzyme abnormalities, infection with hepatitis B or hepatitis C virus, concomitant usage of other potentially hepatotoxic drugs, and ongoing injection drug abuse may have played a causative or contributory role. For patients at increased risk of hepatotoxicity (e.g., patients with a history of excessive alcohol intake, intravenous drug abuse or liver disease), obtain baseline liver enzyme levels and monitor periodically and during treatment with BUTRANS.
Risks of Gastrointestinal Conditions
BUTRANS is contraindicated in patients with known or suspected gastrointestinal obstruction, including paralytic ileus.
The buprenorphine in BUTRANS may cause spasm of the sphincter of Oddi. Opioids may cause increases in the serum amylase. Regularly evaluate patients with biliary tract disease, including acute pancreatitis, for worsening symptoms.
Cases of opioid-induced esophageal dysfunction (OIED) have been reported in patients taking opioids. The risk of OIED may increase as the dose and/or duration of opioids increases. Regularly evaluate patients for signs and symptoms of OIED (e.g., dysphagia, regurgitation, non-cardiac chest pain) and, if necessary, adjust opioid therapy as clinically appropriate [see Clinical Pharmacology (12.2) ] .
Increased Risk of Seizures in Patients with Seizure Disorders
The buprenorphine in BUTRANS may increase the frequency of seizures in patients with seizure disorders, and may increase the risk of seizures in other clinical settings associated with seizures. Regularly evaluate patients with a history of seizure disorders for worsened seizure control during BUTRANS therapy.
QTc Prolongation
Thorough QT studies with buprenorphine products have demonstrated QT prolongation ≤15 msec. This QTc prolongation effect does not appear to be mediated by hERG channels. Based on these two findings, buprenorphine is unlikely to be pro-arrhythmic when used alone in patients without risk factors. The risk of combining buprenorphine with other QT-prolonging agents is not known.
Consider these observations in clinical decisions when prescribing BUTRANS to patients with risk factors such as hypokalemia, bradycardia, recent conversion from atrial fibrillation, congestive heart failure, digitalis therapy, baseline QT prolongation, subclinical long-QT syndrome, or severe hypomagnesemia.
Anaphylactic/Allergic Reactions
Cases of acute and chronic hypersensitivity to buprenorphine have been reported both in clinical trials and in the post-marketing experience. The most common signs and symptoms include rashes, hives, and pruritus. Cases of bronchospasm, angioneurotic edema, and anaphylactic shock have been reported. A history of hypersensitivity to buprenorphine is a contraindication to the use of BUTRANS.
Withdrawal
Do not rapidly reduce or abruptly discontinue buprenorphine in a patient physically dependent on opioids. When discontinuing BUTRANS in a physically dependent patient, gradually taper the dosage. Rapid tapering of buprenorphine in a patient physically dependent on opioids may lead to a withdrawal syndrome and return of pain [see Dosage and Administration (2.5) , Drug Abuse and Dependence (9.3) ] .
Additionally, the use of BUTRANS, a partial agonist opioid analgesic, in patients who are receiving a full opioid agonist analgesic may reduce the analgesic effect and/or precipitate withdrawal symptoms. Avoid concomitant use of BUTRANS with a full opioid agonist analgesic.
Risks of Driving and Operating Machinery
BUTRANS may impair the mental and physical abilities needed to perform potentially hazardous activities such as driving a car or operating machinery. Warn patients not to drive or operate dangerous machinery unless they are tolerant to the effects of BUTRANS and know how they will react to the medication.
Use in Addiction Treatment
BUTRANS has not been studied and is not approved for use in the management of addictive disorders.
ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in the labeling:
- Addiction, Abuse, and Misuse [see Warnings and Precautions (5.1) ]
- Life-Threatening Respiratory Depression [see Warnings and Precautions (5.2) ]
- Interactions with Benzodiazepines or Other CNS Depressants [see Warnings and Precautions (5.3) ]
- Neonatal Opioid Withdrawal Syndrome [see Warnings and Precautions (5.4) ]
- Application Site Skin Reactions [see Warnings and Precautions (5.8) ]
- Opioid induced Hyperalgesia and Allodynia [see Warnings and Precautions (5.9) ]
- Adrenal Insufficiency [see Warnings and Precautions (5.11) ]
- Severe Hypotension [see Warnings and Precautions (5.12) ]
- Hepatotoxicity [see Warnings and Precautions (5.14) ]
- Gastrointestinal Effects [see Warnings and Precautions (5.15) ]
- Seizures [see Warnings and Precautions (5.16) ]
- QTc Prolongation [see Warnings and Precautions (5.17) ]
- Anaphylactic/Allergic Reactions [see Warnings and Precautions (5.18) ]
Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
A total of 5,415 patients were treated with BUTRANS in controlled and open-label chronic pain clinical trials. Nine hundred twenty-four subjects were treated for approximately six months and 183 subjects were treated for approximately one year. The clinical trial population consisted of patients with persistent moderate to severe pain.
The most common serious adverse drug reactions (all <0.1%) occurring during clinical trials with BUTRANS were: chest pain, abdominal pain, vomiting, dehydration, and hypertension/blood pressure increased.
The most common adverse events (≥2%) leading to discontinuation were: nausea, dizziness, vomiting, headache, and somnolence.
The most common adverse reactions (≥5%) reported by patients in clinical trials comparing BUTRANS 10 or 20 mcg/hour to placebo are shown in Table 2, and comparing BUTRANS 20 mcg/hour to BUTRANS 5 mcg/hour are shown in Table 3 below:
| Open-Label Titration Period | Double-Blind Treatment Period | ||
|---|---|---|---|
| BUTRANS | BUTRANS | Placebo | |
| MedDRA Preferred Term | (N = 1024) | (N = 256) | (N = 283) |
| Nausea | 23% | 13% | 10% |
| Dizziness | 10% | 4% | 1% |
| Headache | 9% | 5% | 5% |
| Application site pruritus | 8% | 4% | 7% |
| Somnolence | 8% | 2% | 2% |
| Vomiting | 7% | 4% | 1% |
| Constipation | 6% | 4% | 1% |
| Open-Label Titration Period | Double-Blind Treatment Period | ||
|---|---|---|---|
| BUTRANS | BUTRANS 20 | BUTRANS 5 | |
| MedDRA Preferred Term | (N = 1160) | (N = 219) | (N = 221) |
| Nausea | 14% | 11% | 6% |
| Application site pruritus | 9% | 13% | 5% |
| Headache | 9% | 8% | 3% |
| Somnolence | 6% | 4% | 2% |
| Dizziness | 5% | 4% | 2% |
| Constipation | 4% | 6% | 3% |
| Application site erythema | 3% | 10% | 5% |
| Application site rash | 3% | 8% | 6% |
| Application site irritation | 2% | 6% | 2% |
The following table lists adverse reactions that were reported in at least 2.0% of patients in four placebo/active-controlled titration-to-effect trials.
| MedDRA Preferred Term | BUTRANS (N = 392) | Placebo (N = 261) |
|---|---|---|
| Nausea | 21% | 6% |
| Application site pruritus | 15% | 12% |
| Dizziness | 15% | 7% |
| Headache | 14% | 9% |
| Somnolence | 13% | 4% |
| Constipation | 13% | 5% |
| Vomiting | 9% | 1% |
| Application site erythema | 7% | 2% |
| Application site rash | 6% | 6% |
| Dry mouth | 6% | 2% |
| Fatigue | 5% | 1% |
| Hyperhidrosis | 4% | 1% |
| Peripheral edema | 3% | 1% |
| Pruritus | 3% | 0% |
| Stomach discomfort | 2% | 0% |
The adverse reactions seen in controlled and open-label studies are presented below in the following manner: most common (≥5%), common (≥1% to <5%), and less common (<1%).
The most common adverse reactions (≥5%) reported by patients treated with BUTRANS in the clinical trials were nausea, headache, application site pruritus, dizziness, constipation, somnolence, vomiting, application site erythema, dry mouth, and application site rash.
The common (≥1% to <5%) adverse reactions reported by patients treated with BUTRANS in the clinical trials organized by MedDRA (Medical Dictionary for Regulatory Activities) System Organ Class were:
Gastrointestinal disorders : diarrhea, dyspepsia, and upper abdominal pain
General disorders and administration site conditions : fatigue, peripheral edema, application site irritation, pain, pyrexia, chest pain, and asthenia
Infections and infestations : urinary tract infection, upper respiratory tract infection, nasopharyngitis, influenza, sinusitis, and bronchitis
Injury, poisoning and procedural complications : fall
Metabolism and nutrition disorders : anorexia
Musculoskeletal and connective tissue disorders : back pain, arthralgia, pain in extremity, muscle spasms, musculoskeletal pain, joint swelling, neck pain, and myalgia
Nervous system disorders : hypoesthesia, tremor, migraine, and paresthesia
Psychiatric disorders : insomnia, anxiety, and depression
Respiratory, thoracic and mediastinal disorders : dyspnea, pharyngolaryngeal pain, and cough
Skin and subcutaneous tissue disorders : pruritus, hyperhidrosis, rash, and generalized pruritus
Vascular disorders : hypertension
Other less common adverse reactions, including those known to occur with opioid treatment, that were seen in <1% of the patients in the BUTRANS trials include the following in alphabetical order:
Abdominal distention, abdominal pain, accidental injury, affect lability, agitation, alanine aminotransferase increased, angina pectoris, angioedema, apathy, application site dermatitis, asthma aggravated, bradycardia, chills, confusional state, contact dermatitis, coordination abnormal, dehydration, depersonalization, depressed level of consciousness, depressed mood, disorientation, disturbance in attention, diverticulitis, drug hypersensitivity, drug withdrawal syndrome, dry eye, dry skin, dysarthria, dysgeusia, dysphagia, euphoric mood, face edema, flatulence, flushing, gait disturbance, hallucination, hiccups, hot flush, hyperventilation, hypotension, hypoventilation, ileus, insomnia, libido decreased, loss of consciousness, malaise, memory impairment, mental impairment, mental status changes, miosis, muscle weakness, nervousness, nightmare, orthostatic hypotension, palpitations, psychotic disorder, respiration abnormal, respiratory depression, respiratory distress, respiratory failure, restlessness, rhinitis, sedation, sexual dysfunction, syncope, tachycardia, tinnitus, urinary hesitation, urinary incontinence, urinary retention, urticaria, vasodilatation, vertigo, vision blurred, visual disturbance, weight decreased, and wheezing.
Postmarketing Experience
The following adverse reactions have been identified during post approval use of buprenorphine. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Serotonin syndrome : Cases of serotonin syndrome, a potentially life-threatening condition, have been reported during concomitant use of opioids with serotonergic drugs.
Adrenal insufficiency : Cases of adrenal insufficiency have been reported with opioid use, more often following greater than one month of use.
Anaphylaxis : Anaphylaxis has been reported with ingredients contained in BUTRANS.
Androgen deficiency : Cases of androgen deficiency have occurred with use of opioids for an extended period of time. [see Clinical Pharmacology (12.2) ] .
Hyperalgesia and Allodynia : Cases of hyperalgesia and allodynia have been reported with opioid therapy of any duration [see Warnings and Precautions (5.9) ].
Hypoglycemia : Cases of hypoglycemia have been reported in patients taking opioids. Most reports were in patients with at least one predisposing risk factor (e.g., diabetes).
Opioid-induced esophageal dysfunction (OIED ): Cases of OIED have been reported in patients taking opioids and may occur more frequently in patients taking higher doses of opioids, and/or in patients taking opioids longer term [see Warnings and Precautions (5.15) ].
Adverse Reactions from Observational Studies
A prospective, observational cohort study estimated the risks of addiction, abuse, and misuse in patients initiating long-term use of Schedule II opioid analgesics between 2017 and 2021. Study participants included in one or more analyses had been enrolled in selected insurance plans or health systems for at least one year, were free of at least one outcome at baseline, completed a minimum number of follow-up assessments, and either: 1) filled multiple extended-release/long-acting opioid analgesic prescriptions during a 90-day period (n=978); or 2) filled any Schedule II opioid analgesic prescriptions covering at least 70 of 90 days (n=1,244). Those included also had no dispensing of the qualifying opioids in the previous 6 months.
Over 12 months:
- approximately 1% to 6% of participants across the two cohorts newly met criteria for addiction, as assessed with two validated interview-based measures of moderate-to-severe opioid use disorder based on Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) criteria, and
- approximately 9% and 22% of participants across the two cohorts newly met criteria for prescription opioid abuse and misuse [defined in Drug Abuse and Dependence (9.2)], respectively, as measured with a validated self-reported instrument.
A retrospective, observational cohort study estimated the risk of opioid- involved overdose or opioid overdose-related death in patients with new long-term use of Schedule II opioid analgesics from 2006 through 2016 (n=220,249). Included patients had been enrolled in either one of two commercial insurance programs, one managed care program, or one Medicaid program for at least 9 months. New long-term use was defined as having Schedule II opioid analgesic prescriptions covering at least 70 days' supply over the 3 months prior to study entry and none during the preceding 6 months. Patients were excluded if they had an opioid-involved overdose in the 9 months prior to study entry. Overdose was measured using a validated medical code-based algorithm with linkage to the National Death Index database. The 5-year cumulative incidence estimates for opioid-involved overdose or opioid overdose-related death ranged from approximately 1.5% to 4% across study sites, counting only the first event during follow-up. Approximately 17% of first opioid overdoses observed over the entire study period (5-11 years, depending on the study site) were fatal. Higher baseline opioid dose was the strongest and most consistent predictor of opioid-involved overdose or opioid overdose-related death. Study exclusion criteria may have selected patients at lower risk of overdose, and substantial loss to follow-up (approximately 80%) also may have biased estimates.
The risk estimates from the studies described above may not be generalizable to all patients receiving opioid analgesics, such as those with exposures shorter or longer than the duration evaluated in the studies.
DRUG INTERACTIONS
Table 5 Includes clinically significant drug interactions with BUTRANS.
| Benzodiazepines | |
|---|---|
| Clinical Impact: | There have been a number of reports regarding coma and death associated with the misuse and abuse of the combination of buprenorphine and benzodiazepines. In many, but not all of these cases, buprenorphine was misused by self-injection of crushed buprenorphine tablets. Preclinical studies have shown that the combination of benzodiazepines and buprenorphine altered the usual ceiling effect on buprenorphine-induced respiratory depression, making the respiratory effects of buprenorphine appear similar to those of full opioid agonists. |
| Intervention: | Regularly evaluate patients with concurrent use of BUTRANS and benzodiazepines. Warn patients that it is extremely dangerous to self-administer benzodiazepines while taking BUTRANS, and warn patients to use benzodiazepines concurrently with BUTRANS only as directed by their physician. |
| Benzodiazepines and Other Central Nervous System (CNS) Depressants | |
| Clinical Impact: | Due to additive pharmacologic effects, the concomitant use of benzodiazepines or other CNS depressants, including alcohol, can increase the risk of hypotension, respiratory depression, profound sedation, coma, and death [see Warnings and Precautions (5.3) ] . |
| Intervention: | Reserve concomitant prescribing of these drugs for use in patients for whom alternative treatment options are inadequate. Limit dosages and durations to the minimum required. Inform patients and caregivers of this potential interaction and educate them on the signs and symptoms of respiratory depression (including sedation). If concomitant use is warranted, consider recommending or prescribing an opioid overdose reversal agent [see Dosage and Administration (2.2) , Warnings and Precautions (5.1 , 5.2 , 5.3) ] . |
| Examples: | Benzodiazepines and other sedatives/hypnotics, anxiolytics, tranquilizers, muscle relaxants, general anesthetics, antipsychotics, gabapentinoids (gabapentin or pregabalin), other opioids, alcohol. |
| Inhibitors of CYP3A4 | |
| Clinical Impact: | The concomitant use of buprenorphine and CYP3A4 inhibitors can increase the plasma concentration of buprenorphine, resulting in increased or prolonged opioid effects, particularly when an inhibitor is added after a stable dose of BUTRANS is achieved. After stopping a CYP3A4 inhibitor, as the effects of the inhibitor decline, the buprenorphine plasma concentration will decrease [see Clinical Pharmacology (12.3) ] , potentially resulting in decreased opioid efficacy or a withdrawal syndrome in patients who had developed physical dependence to buprenorphine. |
| Intervention: | If concomitant use is necessary, consider dosage reduction of BUTRANS until stable drug effects are achieved. Evaluate patients at frequent intervals for respiratory depression and sedation. If a CYP3A4 inhibitor is discontinued, consider increasing the BUTRANS dosage until stable drug effects are achieved. Assess for signs of opioid withdrawal. |
| Examples: | Macrolide antibiotics (e.g., erythromycin), azole-antifungal agents (e.g., ketoconazole), protease inhibitors (e.g., ritonavir) |
| CYP3A4 Inducers | |
| Clinical Impact: | The concomitant use of buprenorphine and CYP3A4 inducers can decrease the plasma concentration of buprenorphine [see Clinical Pharmacology (12.3) ] , potentially resulting in decreased efficacy or onset of a withdrawal syndrome in patients who have developed physical dependence to buprenorphine. After stopping a CYP3A4 inducer, as the effects of the inducer decline, the buprenorphine plasma concentration will increase [see Clinical Pharmacology (12.3) ] , which could increase or prolong both therapeutic effects and adverse reactions and may cause serious respiratory depression. |
| Intervention: | If concomitant use is necessary, consider increasing the BUTRANS dosage until stable drug effects are achieved. Assess for signs of opioid withdrawal. If a CYP3A4 inducer is discontinued, consider BUTRANS dosage reduction and evaluate patients at frequent intervals for signs of respiratory depression and sedation. |
| Examples: | Rifampin, carbamazepine, phenytoin |
| Serotonergic Drugs | |
| Clinical Impact: | The concomitant use of opioids with other drugs that affect the serotonergic neurotransmitter system has resulted in serotonin syndrome. |
| Intervention: | If concomitant use is warranted, frequently evaluate the patient, particularly during treatment initiation and dose adjustment. Discontinue BUTRANS if serotonin syndrome is suspected. |
| Examples: | Selective serotonin reuptake inhibitors (SSRIs), serotonin and norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), triptans, 5-HT3 receptor antagonists, drugs that affect the serotonin neurotransmitter system (e.g., mirtazapine, trazodone, tramadol), certain muscle relaxants (i.e., cyclobenzaprine, metaxalone), monoamine oxidase inhibitors (those intended to treat psychiatric disorders and also others, such as linezolid and intravenous methylene blue). |
| Monoamine Oxidase Inhibitors (MAOIs) | |
| Clinical Impact: | MAOI interactions with opioids may manifest as serotonin syndrome or opioid toxicity (e.g., respiratory depression, coma) [see Warnings and Precautions (5.2) ] |
| Intervention: | The use of BUTRANS is not recommended for patients taking MAOIs or within 14 days of stopping such treatment. |
| Examples: | phenelzine, tranylcypromine, linezolid |
| Mixed Agonist/Antagonist Opioid Analgesics | |
| Clinical Impact: | May reduce the analgesic effect of BUTRANS and/or precipitate withdrawal symptoms. |
| Intervention: | Avoid concomitant use. |
| Examples: | butorphanol, nalbuphine, pentazocine |
| Muscle Relaxants | |
| Clinical Impact: | Buprenorphine may enhance the neuromuscular blocking action of skeletal muscle relaxants and produce an increased degree of respiratory depression. |
| Intervention: | Because respiratory depression may be greater than otherwise expected, decrease the dosage of BUTRANS and/or the muscle relaxant as necessary. Due to the risk of respiratory depression with concomitant use of skeletal muscle relaxants and opioids, consider recommending or prescribing an opioid overdose reversal agent [see Dosage and Administration (2.2) , Warnings and Precautions (5.2 , 5.3) ]. |
| Examples: | Cyclobenzaprine, metaxalone |
| Diuretics | |
| Clinical Impact: | Opioids can reduce the efficacy of diuretics by inducing the release of antidiuretic hormone. |
| Intervention: | Evaluate patients for signs of diminished diuresis and/or effects on blood pressure and increase the dosage of the diuretic as needed. |
| Anticholinergic Drugs | |
| Clinical Impact: | The concomitant use of opioid analgesics, including buprenorphine, and anticholinergic drugs may increase the risk of urinary retention and/or severe constipation, which may lead to paralytic ileus. |
| Intervention: | Evaluate patients for signs of urinary retention or reduced gastric motility when BUTRANS is used concomitantly with anticholinergic drugs. |
DESCRIPTION
BUTRANS is a transdermal system providing systemic delivery of buprenorphine, a mu opioid partial agonist analgesic, continuously for 7 days. The chemical name of buprenorphine is 6,14-ethenomorphinan-7-methanol, 17-(cyclopropylmethyl)- α-(1,1-dimethylethyl)-4, 5-epoxy-18, 19-dihydro-3-hydroxy-6-methoxy-α-methyl-, [5α, 7α, (S)]. The structural formula is:
The molecular weight of buprenorphine is 467.6; the empirical formula is C 29 H 41 NO 4 . Buprenorphine occurs as a white or almost white powder and is very slightly soluble in water, freely soluble in acetone, soluble in methanol and ether, and slightly soluble in cyclohexane. The pKa is 8.5 and the melting point is about 217°C.
System Components and Structure
Five different strengths of BUTRANS are available: 5, 7.5, 10, 15, and 20 mcg/hour (Table 6). The proportion of buprenorphine mixed in the adhesive matrix is the same in each of the five strengths. The amount of buprenorphine released from each system per hour is proportional to the active surface area of the system. The skin is the limiting barrier to diffusion from the system into the bloodstream.
| Buprenorphine Delivery Rate (mcg/hour) | Active Surface Area (cm 2 ) | Total Buprenorphine Content (mg) |
|---|---|---|
| BUTRANS 5 | 6.25 | 5 |
| BUTRANS 7.5 | 9.375 | 7.5 |
| BUTRANS 10 | 12.5 | 10 |
| BUTRANS 15 | 18.75 | 15 |
| BUTRANS 20 | 25 | 20 |
BUTRANS is a rectangular or square, beige-colored system consisting of a protective liner and functional layers. Proceeding from the outer surface toward the surface adhering to the skin, the layers are (1) a beige-colored web backing layer; (2) an adhesive rim without buprenorphine; (3) a separating layer over the buprenorphine-containing adhesive matrix; (4) the buprenorphine-containing adhesive matrix; and (5) a peel-off release liner. Before use, the release liner covering the adhesive layer is removed and discarded.
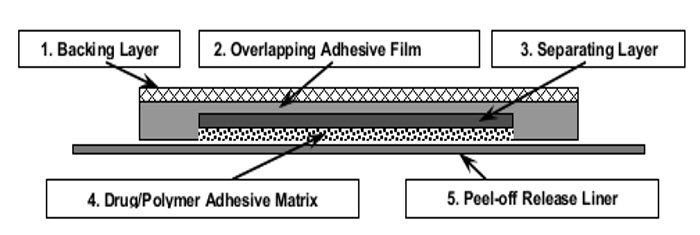
Figure 1: Cross-Section Diagram of BUTRANS (not to scale).
The active ingredient in BUTRANS is buprenorphine. The inactive ingredients in each system are: levulinic acid, oleyl oleate, povidone, and polyacrylate cross-linked with aluminum.
CLINICAL PHARMACOLOGY
Mechanism of Action
Buprenorphine is a partial agonist at the mu-opioid receptor and an antagonist at the kappa-opioid receptors, an agonist at delta-opioid receptors, and a partial agonist at ORL-1 (nociceptin) receptors. The contributions of these actions to its analgesic profile are unclear.
Pharmacodynamics
Effects on the Central Nervous System
Buprenorphine produces respiratory depression by direct action on brainstem respiratory centers. The respiratory depression involves a reduction in the responsiveness of the brainstem respiratory centers to both increases in carbon dioxide tension and electrical stimulation.
Buprenorphine causes miosis, even in total darkness. Pinpoint pupils are a sign of opioid overdose but are not pathognomonic (e.g., pontine lesions of hemorrhagic or ischemic origins may produce similar findings). Marked mydriasis rather than miosis may be seen with worsening hypoxia in overdose situations.
Effects on the Gastrointestinal Tract and Other Smooth Muscle
Buprenorphine causes a reduction in motility associated with an increase in smooth muscle tone in the antrum of the stomach and duodenum. Digestion of food in the small intestine is delayed and propulsive contractions are decreased. Propulsive peristaltic waves in the colon are decreased, while tone is increased to the point of spasm, resulting in constipation. Other opioid-induced effects may include a reduction in biliary and pancreatic secretions, spasm of sphincter of Oddi, and transient elevations in serum amylase, and opioid-induced esophageal dysfunction (OIED).
Effects on the Cardiovascular System
Buprenorphine produces peripheral vasodilation, which may result in orthostatic hypotension or syncope. Manifestations of histamine release and/or peripheral vasodilation may include pruritus, flushing, red eyes, sweating, and/or orthostatic hypotension.
Effects on Cardiac Electrophysiology
The effect of BUTRANS 10 mcg/hour and 2 x BUTRANS 20 mcg/hour on QTc interval was evaluated in a double-blind (BUTRANS vs. placebo), randomized, placebo and active-controlled (moxifloxacin 400 mg, open label), parallel-group, dose-escalating, single-dose study in 132 healthy male and female subjects aged 18 to 55 years. The dose escalation sequence for BUTRANS during the titration period was: BUTRANS 5 mcg/hour for 3 days, then BUTRANS 10 mcg/hour for 3 days, then BUTRANS 20 mcg/hour for 3 days, then 2 x BUTRANS 20 mcg/hour for 4 days. The QTc evaluation was performed during the third day of BUTRANS 10 mcg/hour and the fourth day of 2 x BUTRANS 20 mcg/hour when the plasma levels of buprenorphine were at steady state for the corresponding doses [see Warnings and Precautions (5.17) ] .
There was no clinically meaningful effect on mean QTc with a BUTRANS dose of 10 mcg/hour. A BUTRANS dose of 40 mcg/hour (given as two 20 mcg/hour BUTRANS Transdermal Systems) prolonged mean QTc by a maximum of 9.2 (90% CI: 5.2-13.3) msec across the 13 assessment time points.
Effects on the Endocrine System
Opioids inhibit the secretion of adrenocorticotropic hormone (ACTH), cortisol, and luteinizing hormone (LH) in humans [see Adverse Reactions (6.2) ] . They also stimulate prolactin, growth hormone (GH) secretion, and pancreatic secretion of insulin and glucagon.
Use of opioids for an extended period of time may influence the hypothalamic-pituitary-gonadal axis, leading to androgen deficiency that may manifest as low libido, impotence, erectile dysfunction, amenorrhea, or infertility. The causal role of opioids in the clinical syndrome of hypogonadism is unknown because the various medical, physical, lifestyle, and psychological stressors that may influence gonadal hormone levels have not been adequately controlled for in studies conducted to date [see Adverse Reactions (6.2) ] .
Effects on the Immune System
Opioids have been shown to have a variety of effects on components of the immune system in in vitro and animal models. The clinical significance of these findings is unknown. Overall, the effects of opioids appear to be modestly immunosuppressive.
Concentration–Efficacy Relationships
The minimum effective analgesic concentration will vary widely among patients, especially among patients who have been previously treated with opioid agonists. The minimum effective analgesic concentration of buprenorphine for any individual patient may increase over time due to an increase in pain, the development of a new pain syndrome, and/or the development of analgesic tolerance [see Dosage and Administration (2.1 , 2.4) ] .
Concentration–Adverse Reaction Relationships
There is a relationship between increasing buprenorphine plasma concentration and increasing frequency of dose-related opioid adverse reactions such as nausea, vomiting, CNS effects, and respiratory depression. In opioid-tolerant patients, the situation may be altered by the development of tolerance to opioid-related adverse reactions [see Dosage and Administration (2.1 , 2.3 , 2.4) ] .
Pharmacokinetics
Absorption
Each BUTRANS system provides delivery of buprenorphine for 7 days. Steady state was achieved during the first application by Day 3 (see Figure 2 ).
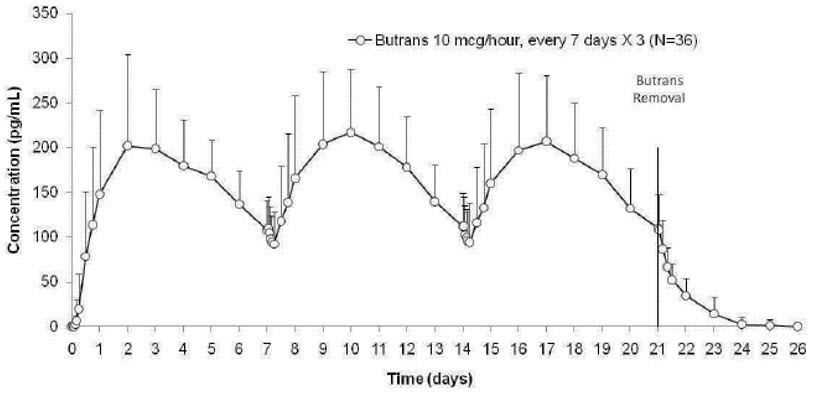
Figure 2 Mean (SD) Buprenorphine Plasma Concentrations Following Three Consecutive Applications of BUTRANS 10 mcg/hour (N = 36 Healthy Subjects)
BUTRANS 5, 10, and 20 mcg/hour provide dose-proportional total buprenorphine exposures (AUC) following 7-day applications. BUTRANS single 7-day application and steady-state pharmacokinetic parameters are summarized in Table 7. Plasma buprenorphine concentrations after titration showed no further change over the 60-day period studied.
| Single 7-day Application | AUC inf (pg.h/mL) | C max (pg/mL) |
|---|---|---|
| BUTRANS 5 mcg/hour | 12087 (37) | 176 (67) |
| BUTRANS 10 mcg/hour | 27035 (29) | 191 (34) |
| BUTRANS 20 mcg/hour | 54294 (36) | 471 (49) |
| Multiple 7-day Applications | AUC tau,ss (pg.h/mL) | C max,ss (pg/mL) |
| BUTRANS 10 mcg/hour, steady-state | 27543 (33) | 224 (35) |
Transdermal delivery studies showed that intact human skin is permeable to buprenorphine. In clinical pharmacology studies, the median time for BUTRANS 10 mcg/hour to deliver quantifiable buprenorphine concentrations (≥ 25 pg/mL) was approximately 17 hours.
The absolute bioavailability of BUTRANS relative to IV administration, following a 7-day application, is approximately 15% for all doses (BUTRANS 5, 10, and 20 mcg/hour).
Effects of Application Site
A study in healthy subjects demonstrated that the pharmacokinetic profile of buprenorphine delivered by BUTRANS 10 mcg/hour is similar when applied to the upper outer arm, upper chest, upper back, or the side of the chest [see Dosage and Administration (2.7) ] .
The reapplication of BUTRANS 10 mcg/hour after various rest periods to the same application site in healthy subjects showed that the minimum rest period needed to avoid variability in drug absorption is 3 weeks (21 days) [see Dosage and Administration (2.7) ] .
Effects of Heat
In a study of healthy subjects, application of a heating pad directly on the BUTRANS 10 mcg/hour system caused a 26% - 55% increase in blood concentrations of buprenorphine. Concentrations returned to normal within 5 hours after the heat was removed. For this reason, instruct patients not to apply heating pads directly to the BUTRANS system during system wear [see Warnings and Precautions (5.6) ].
Fever may increase the permeability of the skin, leading to increased buprenorphine concentrations during BUTRANS treatment. As a result, febrile patients are at increased risk for the possibility of BUTRANS-related reactions during treatment with BUTRANS. Monitor patients with febrile illness for adverse effects and consider dose adjustment [see Warnings and Precautions (5.7) ] . In a crossover study of healthy subjects receiving endotoxin or placebo challenge during BUTRANS 10 mcg/hour wear, the AUC and C max were similar despite a physiologic response of mild fever to endotoxin.
Distribution
Buprenorphine is approximately 96% bound to plasma proteins, mainly to alpha- and beta-globulin.
Studies of IV buprenorphine have shown a large volume of distribution (approximately 430 L), implying extensive distribution of buprenorphine.
CSF buprenorphine concentrations appear to be approximately 15-25% of concurrent plasma concentrations.
Elimination
Metabolism
Buprenorphine metabolism in the skin following BUTRANS application is negligible.
Buprenorphine primarily undergoes N -dealkylation by CYP3A4 to norbuprenorphine and glucuronidation by UGT-isoenzymes (mainly UGT1A1 and 2B7) to buprenorphine 3β- O -glucuronide. Norbuprenorphine, the major metabolite, is also glucuronidated (mainly UGT1A3) prior to excretion.
Norbuprenorphine is the only known active metabolite of buprenorphine. It has been shown to be a respiratory depressant in rats, but only at concentrations at least 50-fold greater than those observed following application to humans of BUTRANS 20 mcg/hour.
Excretion
Following IV administration, buprenorphine and its metabolites are secreted into bile and excreted in urine.
Following intramuscular administration of 2 mcg/kg dose of buprenorphine, approximately 70% of the dose was excreted in feces within 7 days. Approximately 27% was excreted in urine.
Following transdermal application, buprenorphine is eliminated via hepatic metabolism, with subsequent biliary excretion and renal excretion of soluble metabolites. After removal of BUTRANS, mean buprenorphine concentrations decrease approximately 50% within 10-24 hours, followed by decline with an apparent terminal half-life of approximately 26 hours. Since metabolism and excretion of buprenorphine occur mainly via hepatic elimination, reductions in hepatic blood flow induced by some general anesthetics (e.g., halothane) and other drugs may result in a decreased rate of hepatic elimination of the drug, leading to increased plasma concentrations. The total clearance of buprenorphine is approximately 55 L/hour in postoperative patients.
Drug Interaction Studies
Effect of CYP3A4 Inhibitors
In a drug-drug interaction study, BUTRANS 10 mcg/hour (single dose x 7 days) was co-administered with 200 mg ketoconazole, a strong CYP3A4 inhibitor or ketoconazole placebo twice daily for 11 days and the pharmacokinetics of buprenorphine and its metabolites were evaluated. Plasma buprenorphine concentrations did not accumulate during co-medication with ketoconazole 200 mg twice daily. Based on the results from this study, metabolism during therapy with BUTRANS is not expected to be affected by co-administration of CYP3A4 inhibitors [see Drug Interactions (7) ].
Antiretroviral agents have been evaluated for CYP3A4 mediated interactions with sublingual buprenorphine. Nucleoside reverse transcriptase inhibitors (NRTIs) and non-nucleoside reverse transcriptase inhibitors (NNRTIs) do not appear to have clinically significant interactions with buprenorphine. However, certain protease inhibitors (PIs) with CYP3A4 inhibitory activity such as atazanavir and atazanavir/ritonavir resulted in elevated levels of buprenorphine and norbuprenorphine when buprenorphine and naloxone were administered sublingually. C max and AUC for buprenorphine increased by up to 1.6 and 1.9 fold, and C max and AUC for norbuprenorphine increased by up to 1.6 and 2.0 fold respectively, when sublingual buprenorphine was administered with these PIs. Patients in this study reported increased sedation, and symptoms of opiate excess have been found in post-marketing reports of patients receiving buprenorphine and atazanavir with and without ritonavir concomitantly. It should be noted that atazanavir is both a CYP3A4 and UGT1A1 inhibitor. As such, the drug-drug interaction potential for buprenorphine with CYP3A4 inhibitors is likely to be dependent on the route of administration as well as the specificity of enzyme inhibition [see Drug Interactions (7) ].
Effect of CYP3A4 Inducers
The interaction between buprenorphine and CYP3A4 inducers has not been studied.
Specific Populations
Age: Geriatric Patients
Following a single application of BUTRANS 10 mcg/hour to 12 healthy young adults (mean age 32 years) and 12 healthy elderly subjects (mean age 72 years), the pharmacokinetic profile of BUTRANS was similar in healthy elderly and healthy young adult subjects, though the elderly subjects showed a trend toward higher plasma concentrations immediately after BUTRANS removal. Both groups eliminated buprenorphine at similar rates after system removal [see Use in Specific Populations (8.5) ] .
In a study of healthy young subjects, healthy elderly subjects, and elderly subjects treated with thiazide diuretics, BUTRANS at a fixed dose-escalation schedule (BUTRANS 5 mcg/hour for 3 days, followed by BUTRANS 10 mcg/hour for 3 days and BUTRANS 20 mcg/hour for 7 days) produced similar mean plasma concentration vs. time profiles for each of the three subject groups. There were no significant differences between groups in buprenorphine C max or AUC [see Use in Specific Populations (8.5) ].
Sex
In a pooled data analysis utilizing data from several studies that administered BUTRANS 10 mcg/hour to healthy subjects, no differences in buprenorphine C max and AUC or body-weight normalized C max and AUC were observed between males and females treated with BUTRANS.
Hepatic Impairment
The pharmacokinetics of buprenorphine following an IV infusion of 0.3 mg of buprenorphine were compared in 8 patients with mild impairment (Child-Pugh A), 4 patients with moderate impairment (Child-Pugh B) and 12 subjects with normal hepatic function. Buprenorphine and norbuprenorphine exposure did not increase in the mild and moderate hepatic impairment patients.
BUTRANS has not been evaluated in patients with severe (Child-Pugh C) hepatic impairment. [see Warnings and Precautions (5.14) , Use in Specific Populations (8.6) ] .
Renal Impairment
No studies in patients with renal impairment have been performed with BUTRANS.
In an independent study, the effect of impaired renal function on buprenorphine pharmacokinetics after IV bolus and after continuous IV infusion administrations was evaluated. It was found that plasma buprenorphine concentrations were similar in patients with normal renal function and in patients with impaired renal function or renal failure. In a separate investigation of the effect of intermittent hemodialysis on buprenorphine plasma concentrations in chronic pain patients with end-stage renal disease who were treated with a transdermal buprenorphine product (marketed outside the US) up to 70 mcg/hour, no significant differences in buprenorphine plasma concentrations before or after hemodialysis were observed.
No notable relationship was observed between estimated creatinine clearance rates and steady-state buprenorphine concentrations among patients during BUTRANS therapy.
NONCLINICAL TOXICOLOGY
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Buprenorphine administered daily by skin painting to Sprague Dawley rats for 100 weeks at dosages (20, 60, or 200 mg/kg) produced systemic exposures (based on AUC) that ranged from approximately 130 to 350 times that of human subjects administered the maximum recommended human dose (MRHD) of BUTRANS 20 mcg/hour. An increased incidence of benign testicular interstitial cell tumors, considered buprenorphine treatment-related, was observed in male rats compared with concurrent controls. The tumor incidence was also above the highest incidence in the historical control database of the testing facility. These tumors were noted at 60 mg/kg/day and higher at approximately 220 times the proposed MRHD based on AUC. The no observed effect level (NOEL) was 20 mg/kg/day (approximately 140 times the proposed MRHD based on AUC). The mechanism leading to the tumor findings and the relevance to humans is unknown.
Buprenorphine was administered by skin painting to hemizygous Tg.AC mice over a 6-month study period. At the dosages administered daily (18.75, 37.5, 150, or 600 mg/kg/day), buprenorphine was not carcinogenic or tumorigenic at systemic exposure to buprenorphine, based on AUC, of up to approximately 1000 times that of human subjects administered BUTRANS 20 mcg/hour, the MRHD.
Mutagenesis
Buprenorphine was not genotoxic in three in vitro genetic toxicology studies (bacterial mutagenicity test, mouse lymphoma assay, chromosomal aberration assay in human peripheral blood lymphocytes), and in one in vivo mouse micronucleus test.
Impairment of Fertility
BUTRANS (1/4 of a BUTRANS 5 mcg/hour, one BUTRANS 5 mcg/hour, or one BUTRANS 20 mcg/hour every 3 days in males for 4 weeks prior to mating for a total of 10 weeks and in females for 2 weeks prior to mating through Gestation Day 7) had no effect on fertility or general reproductive performance of rats at AUC-based exposure levels as high as approximately 65 times (females) and 100 times (males) that for human subjects who received BUTRANS 20 mcg/hour, the MRHD.
CLINICAL STUDIES
The efficacy of BUTRANS has been evaluated in four 12-week double-blind, controlled clinical trials in patients who were not opioid tolerant and opioid-experienced patients with moderate to severe chronic low back pain or osteoarthritis using pain scores as the primary efficacy variable. Two of these studies, described below, demonstrated efficacy in patients with low back pain. One study in low back pain and one study in osteoarthritis did not show a statistically significant pain reduction for either BUTRANS or the respective active comparators.
12-Week Study in Patients who were not Opioid Tolerant with Chronic Low Back Pain
A total of 1,024 patients with chronic low back pain who were suboptimally responsive to their non-opioid therapy entered an open-label, dose-titration period for up to four weeks. Patients initiated therapy with three days of treatment with BUTRANS 5 mcg/hour. After three days, if adverse events were tolerated, the dose was increased to BUTRANS 10 mcg/hour. If adverse effects were tolerated but adequate analgesia was not reached, the dose was increased to BUTRANS 20 mcg/hour for an additional 10-12 days. Patients who achieved adequate analgesia and tolerable adverse effects on BUTRANS 10 or 20 mcg/hour were then randomized to remain on their titrated dose of BUTRANS or matching placebo. Fifty-three percent of the patients who entered the open-label titration period were able to titrate to a tolerable and effective dose and were randomized into a 12-week, double-blind treatment period. Twenty-three percent of patients discontinued due to an adverse event from the open-label titration period and 14% discontinued due to lack of a therapeutic effect. The remaining 10% of patients were dropped due to various administrative reasons.
During the first seven days of double-blind treatment patients were allowed up to two tablets per day of immediate-release oxycodone 5 mg as supplemental analgesia to minimize opioid withdrawal symptoms in patients randomized to placebo. Thereafter, the supplemental analgesia was limited to either acetaminophen 500 mg or ibuprofen 200 mg at a maximum of four tablets per day. Sixty-six percent of the patients treated with BUTRANS completed the 12-week treatment compared to 70% of the patients treated with placebo. Of the 256 patients randomized to BUTRANS, 9% discontinued due to lack of efficacy and 16% due to adverse events. Of the 283 patients randomized to placebo, 13% discontinued due to lack of efficacy and 7% due to adverse events.
Of the patients who were randomized, the mean pain (SE) NRS scores were 7.2 (0.08) and 7.2 (0.07) at screening and 2.6 (0.08) and 2.6 (0.07) at pre-randomization (beginning of double-blind phase) for the BUTRANS and placebo groups, respectively.
The score for average pain over the last 24 hours at the end of the study (Week 12/Early Termination) was statistically significantly lower for patients treated with BUTRANS compared with patients treated with placebo. The proportion of patients with various degrees of improvement, from screening to study endpoint, is shown in Figure 3 below.
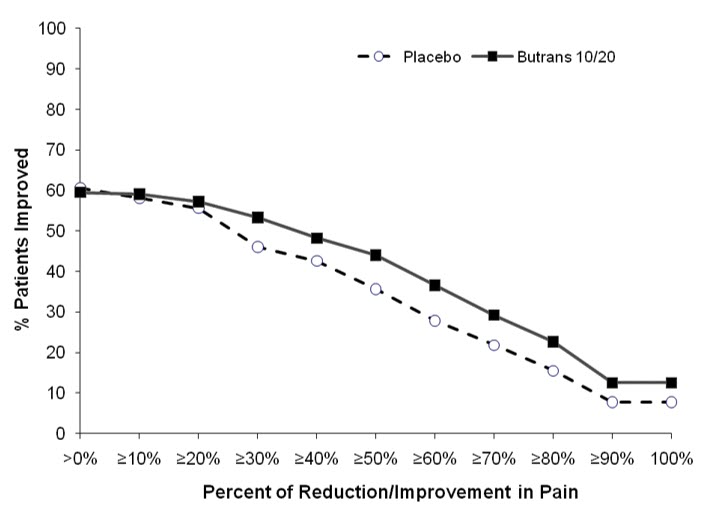
Figure 3: Percent Reduction in Pain Intensity
12-Week Study in Opioid-Experienced Patients with Chronic Low Back Pain
One thousand one hundred and sixty (1,160) patients on chronic opioid therapy (total daily dose 30-80 mg morphine equivalent) entered an open-label, dose-titration period with BUTRANS for up to 3 weeks, following taper of prior opioids. Patients initiated therapy with BUTRANS 10 mcg/hour for three days. After three days, if the patient tolerated the adverse effects, the dose was increased to BUTRANS 20 mcg/hour for up to 18 days. Patients with adequate analgesia and tolerable adverse effects on BUTRANS 20 mcg/hour were randomized to remain on BUTRANS 20 mcg/hour or were switched to a low-dose control (BUTRANS 5 mcg/hour) or an active control. Fifty-seven percent of the patients who entered the open-label titration period were able to titrate to and tolerate the adverse effects of BUTRANS 20 mcg/hour and were randomized into a 12-week double-blind treatment phase. Twelve percent of patients discontinued due to an adverse event and 21% discontinued due to lack of a therapeutic effect during the open-label titration period.
During the double-blind period, patients were permitted to take ibuprofen (200 mg tablets) or acetaminophen (500 mg tablets) every 4 hours as needed for supplemental analgesia (up to 3200 mg of ibuprofen and 4 grams of acetaminophen daily). Sixty-seven percent of patients treated with BUTRANS 20 mcg/hour and 58% of patients treated with BUTRANS 5 mcg/hour completed the 12-week treatment. Of the 219 patients randomized to BUTRANS 20 mcg/hour, 11% discontinued due to lack of efficacy and 13% due to adverse events. Of the 221 patients randomized to BUTRANS 5 mcg/hour, 24% discontinued due to lack of efficacy and 6% due to adverse events.
Of the patients who were able to be randomized in the double-blind period, the mean pain (SE) NRS scores were 6.4 (0.08) and 6.5 (0.08) at screening and were 2.8 (0.08) and 2.9 (0.08) at pre-randomization (beginning of Double-Blind Period) for the BUTRANS 5 mcg/hour and BUTRANS 20 mcg/hour, respectively.
The score for average pain over the last 24 hours at Week 12 was statistically significantly lower for subjects treated with BUTRANS 20 mcg/hour compared to subjects treated with BUTRANS 5 mcg/hour. A higher proportion of BUTRANS 20 mcg/hour patients (49%) had at least a 30% reduction in pain score from screening to study endpoint when compared to BUTRANS 5 mcg/hour patients (33%). The proportion of patients with various degrees of improvement from screening to study endpoint is shown in Figure 4 below.
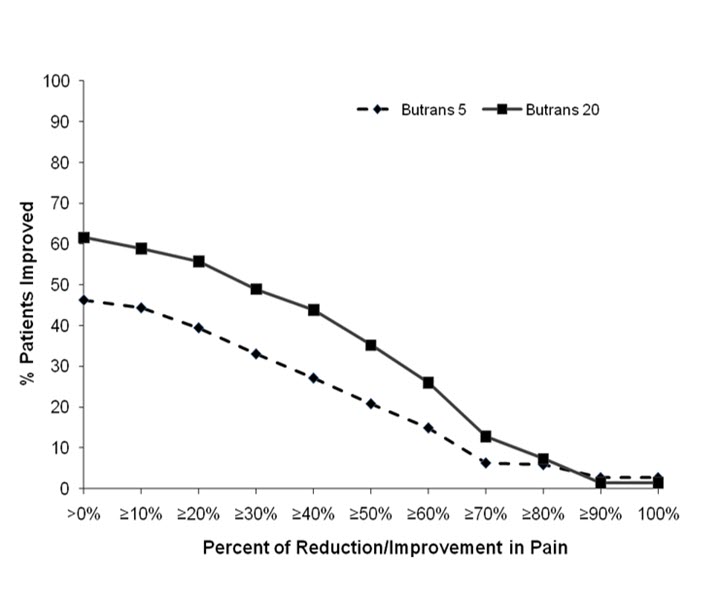
Figure 4: Percent Reduction in Pain Intensity
HOW SUPPLIED/STORAGE AND HANDLING
BUTRANS Transdermal System is supplied in cartons containing 4 individually packaged systems and a pouch containing 4 Patch-Disposal Units.
BUTRANS (buprenorphine) 5 mcg/hour Transdermal Systems are square, beige-colored adhesive patches measuring 45 mm by 45 mm. Each system is printed in blue with the BUTRANS logo and 5 mcg/hr and are supplied in a 4-count carton ( NDC 59011-750-04 ).
BUTRANS (buprenorphine) 7.5 mcg/hour Transdermal Systems are rectangular, beige-colored adhesive patches measuring 58 mm by 45 mm. Each system is printed in blue with the BUTRANS logo and 7.5 mcg/hr and are supplied in a 4-count carton ( NDC 59011-757-04 ).
BUTRANS (buprenorphine) 10 mcg/hour Transdermal Systems are rectangular, beige-colored adhesive patches measuring 68 mm by 45 mm. Each system is printed in blue with the BUTRANS logo and 10 mcg/hr and are supplied in a 4-count carton ( NDC 59011-751-04 ).
BUTRANS (buprenorphine) 15 mcg/hour Transdermal Systems are rectangular, beige-colored adhesive patches measuring 72 mm by 59 mm. Each system is printed in blue with the BUTRANS logo and 15 mcg/hr and are supplied in a 4-count carton ( NDC 59011-758-04 ).
BUTRANS (buprenorphine) 20 mcg/hour Transdermal Systems are square, beige-colored adhesive patches measuring 72 mm by 72 mm. Each system is printed in blue with the BUTRANS logo and 20mcg/hr and are supplied in a 4-count carton ( NDC 59011-752-04 ).
Store BUTRANS securely and dispose of properly [see Patient Counseling Information (17) ] .
Store at 25°C (77°F); excursions permitted between 15°C - 30°C (59°F - 86°F).
Instructions for Use BUTRANS ® (BYOO-trans) CIII (buprenorphine) Transdermal System
Be sure that you read, understand, and follow these Instructions for Use before you use BUTRANS. Talk to your healthcare provider or pharmacist if you have any questions.
Before Applying BUTRANS:
- Do not use soap, alcohol, lotions, oils, or other products to remove any leftover adhesive from a patch because this may cause more BUTRANS to pass through the skin.
- Each patch is sealed in its own protective pouch. Do not remove a patch from the pouch until you are ready to use it.
- Do not use a patch if the seal on the protective pouch is broken or if the patch is cut, damaged or changed in any way.
- BUTRANS patches are available in different strengths and patch sizes. Make sure you have the right strength patch that has been prescribed for you.
Where to apply BUTRANS:
- BUTRANS should be applied to the upper outer arm, upper chest, upper back, or the side of the chest ( See Figure A ). These 4 sites (located on both sides of the body) provide 8 possible BUTRANS application sites.
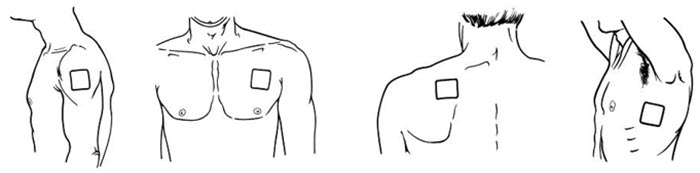 |
| Figure A |
- Do not apply more than 1 patch at the same time unless your doctor tells you to. However, if your healthcare provider tells you to do so, you may use 2 patches as prescribed, applied at the same site ( See Figure A for application sites) right next to each other ( See Figure B for an example of patch position when applying 2 patches). Always apply and remove the two patches together at the same time.
 |
| Figure B |
- You should change the skin site where you apply BUTRANS each week, making sure that at least 3 weeks (21 days) pass before you re-use the same skin site.
- Apply BUTRANS to a hairless or nearly hairless skin site . If needed, you can clip the hair at the skin site ( See Figure C ). Do not shave the area. The skin site should not be irritated. Use only water to clean the application site. You should not use soaps, alcohol, oils, lotions, or abrasive devices. Allow the skin to dry before you apply the patch.
 |
| Figure C |
- The skin site should be free of cuts and irritation (rashes, swelling, redness, or other skin problems).
When to apply a new patch:
- When you apply a new patch, write down the date and time that the patch is applied. Use this to remember when the patch should be removed.
- Change the patch at the same time of day, one week (exactly 7 days) after you apply it.
- After removing and disposing of the patch, write down the time it was removed and how it was disposed.
How to apply BUTRANS:
- If you are wearing a patch, remember to remove it before applying a new one.
- Each patch is sealed in its own protective pouch.
- If you are using two patches, remember to apply them at the same site right next to each other. Always apply and remove the two patches together at the same time.
- Use scissors to cut open the pouch along the dotted line ( See Figure D ) and remove the patch. Do not remove the patch from the pouch until you are ready to use it. Do not use patches that have been cut or damaged in any way.
 |
| Figure D |
- Hold the patch with the protective liner facing you.
- Gently bend the patch ( See Figures E and F ) along the faint line and slowly peel the larger portion of the liner, which covers the sticky surface of the patch.
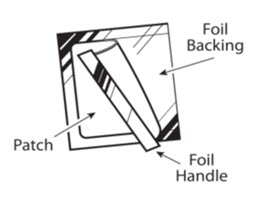 |
| Figure E |
 |
| Figure F |
- Do not touch the sticky side of the patch with your fingers.
- Using the smaller portion of the protective liner as a handle ( See Figure G ), apply the sticky side of the patch to one of the 8 body locations described above ( See " Where to apply BUTRANS " ).
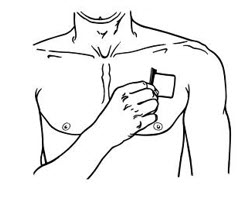 |
| Figure G |
- While still holding the sticky side down, gently fold back the smaller portion of the patch. Grasp an edge of the remaining protective liner and slowly peel it off ( See Figure H ).
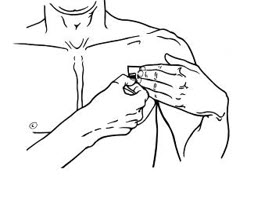 |
| Figure H |
- Press the entire patch firmly into place with the palm ( See Figure I ) of your hand over the patch, for about 15 seconds. Do not rub the patch.
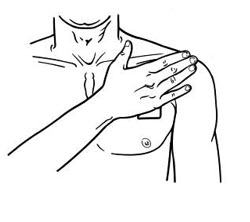 |
| Figure I |
- Make sure that the patch firmly sticks to the skin.
- Go over the edges with your fingers to assure good contact around the patch.
- If you are using two patches, follow the steps in this section to apply them right next to each other.
- Always wash your hands after applying or handling a patch.
- After the patch is applied, write down the date and time that the patch is applied. Use this to remember when the patch should be removed.
If the patch falls off right away after applying, throw it away and put a new one on at a different skin site ( See " Disposing of BUTRANS Patch ") .
If a patch falls off, do not touch the sticky side of the patch with your fingers. A new patch should be applied to a different site. Patches that fall off should not be re-applied . They must be thrown away correctly.
Short-term exposure of the BUTRANS patch to water, such as when bathing or showering, is permitted.
If the edges of the BUTRANS patch start to loosen:
- Apply first aid tape only to the edges of the patch.
- If problems with the patch not sticking continue, cover the patch with special see-through adhesive dressings (for example Bioclusive or Tegaderm).
- Remove the backing from the transparent adhesive dressing and place it carefully and completely over the BUTRANS patch, smoothing it over the patch and your skin.
- Never cover a BUTRANS patch with any other bandage or tape. It should only be covered with a special see-through adhesive dressing. Talk to your healthcare provider or pharmacist about the kinds of dressing that should be used.
If your patch falls off later, but before 1 week (7 days) of use, throw it away properly ( See " Disposing of a BUTRANS Patch ") and apply a new patch at a different skin site. Be sure to let your healthcare provider know that this has happened. Do not replace the new patch until 1 week (7 days) after you put it on (or as directed by your healthcare provider).
Disposing of BUTRANS Patch:
BUTRANS patches should be disposed of by using the Patch-Disposal Unit. Alternatively, the patches can be flushed down the toilet if a drug take-back option is not readily available.
To dispose of BUTRANS patches in household trash using the Patch-Disposal Unit: Remove your patch and follow the directions printed on the Patch-Disposal Unit ( See Figure J ) or see complete instructions below . Use one Patch-Disposal Unit for each patch.
 |
| Figure J |
- Peel back the disposal unit liner to show the sticky surface ( See Figure K ).
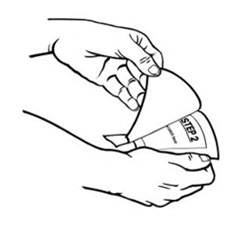 |
| Figure K |
- Place the sticky side of the used or unused patch to the indicated area on the disposal unit ( See Figure L ).
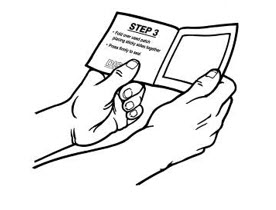 |
| Figure L |
- Close the disposal unit by folding the sticky sides together ( See Figure M ). Press firmly and smoothly over the entire disposal unit so that the patch is sealed within.
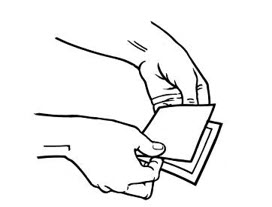 |
| Figure M |
- The closed disposal unit, with the patch sealed inside may be thrown away in the trash ( See Figure N ).
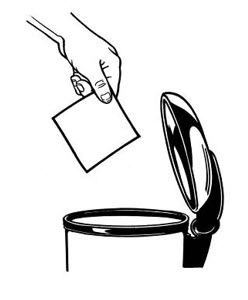 |
| Figure N |
Do not put expired, unwanted or unused patches in household trash without first sealing them in the Patch-Disposal Unit.
Always remove the leftover patches from their protective pouch and remove the protective liner. The pouch and liner can be disposed of separately in the trash and should not be sealed in the Patch-Disposal Unit.
To flush your BUTRANS patches down the toilet: Remove your BUTRANS patch, fold the sticky sides of a used patch together and flush it down the toilet right away ( See Figure O ).
| Figure O |
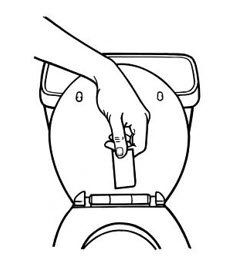 |
When disposing of unused BUTRANS patches you no longer need, remove the leftover patches from their protective pouch and remove the protective liner. Fold the patches in half with the sticky sides together, and flush the patches down the toilet.
Do not flush the pouch or the protective liner down the toilet. These items can be thrown away in the trash.
If you prefer not to flush the used patch down the toilet , and if there is not a drug take-back option readily available, you must use the Patch-Disposal Unit provided to you to discard the patch.
Never put used BUTRANS patches in the trash without first sealing them in the Patch-Disposal Unit.
This "Instructions for Use" has been approved by the U.S. Food and Drug Administration.
Distributed by: Purdue Pharma L.P., Stamford, CT 06901-3431
Revised: October 2019 ©2019, Purdue Pharma L.P.
Bioclusive is a trademark of Systagenix Wound Management (US), Inc. Tegaderm is a trademark of 3M.
Mechanism of Action
Buprenorphine is a partial agonist at the mu-opioid receptor and an antagonist at the kappa-opioid receptors, an agonist at delta-opioid receptors, and a partial agonist at ORL-1 (nociceptin) receptors. The contributions of these actions to its analgesic profile are unclear.