Caplyta prior authorization resources
Most recent state uniform prior authorization forms
Brand Resources
Caplyta patient education
Patient toolkit
Patient Support Program
Dosage & administration
DOSAGE AND ADMINISTRATION
Recommended Dosage
The recommended CAPLYTA dosage is 42 mg administered orally once daily with or without food. Dose titration is not needed.
Dosage Recommendations for Concomitant Use with Moderate or Strong CYP3A4 Inhibitors
The recommended CAPLYTA dosage in patients who receive [see Drug Interactions (7.1 ) ] :
- Strong CYP3A4 inhibitors is 10.5 mg once daily.
- Moderate CYP3A4 inhibitors is 21 mg once daily.
Dosage Recommendations for Patients with Hepatic Impairment
For patients with moderate hepatic impairment (HI) (Child-Pugh class B) or severe HI (Child-Pugh class C), the recommended CAPLYTA dosage is 21 mg once daily [see Use in Specific Populations (8.6) ] . The recommended CAPLYTA dosage in patients with mild HI is the same as those with normal hepatic function.

By using PrescriberAI, you agree to the AI Terms of Use.
Caplyta prescribing information
WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS; and SUICIDAL THOUGHTS AND BEHAVIORS
Increased Mortality in Elderly Patients with Dementia-Related Psychosis
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. CAPLYTA is not approved for the treatment of patients with dementia-related psychosis [see Warnings and Precautions (5.1) ].
Suicidal Thoughts and Behaviors
Antidepressants increased the risk of suicidal thoughts and behaviors in pediatric and young adults in short-term studies. Closely monitor all antidepressant-treated patients for clinical worsening, and for emergence of suicidal thoughts and behaviors [see Warnings and Precautions (5.2) ]. The safety and effectiveness of CAPLYTA have not been established in pediatric patients [see Use in Specific Populations (8.4) ].
- Indications and Usage, adjunctive treatment of major depressive disorder (1 ) 11/2025
INDICATIONS AND USAGE
CAPLYTA is indicated for:
- Treatment of schizophrenia in adults [see Clinical Studies (14.1) ] .
- Treatment of depressive episodes associated with bipolar I or II disorder (bipolar depression) in adults, as monotherapy and as adjunctive therapy with lithium or valproate [see Clinical Studies (14.2) ] .
- Adjunctive therapy with antidepressants for the treatment of major depressive disorder (MDD) in adults [see Clinical Studies (14.3 ) ].
DOSAGE AND ADMINISTRATION
Recommended Dosage
The recommended CAPLYTA dosage is 42 mg administered orally once daily with or without food. Dose titration is not needed.
Dosage Recommendations for Concomitant Use with Moderate or Strong CYP3A4 Inhibitors
The recommended CAPLYTA dosage in patients who receive [see Drug Interactions (7.1 ) ] :
- Strong CYP3A4 inhibitors is 10.5 mg once daily.
- Moderate CYP3A4 inhibitors is 21 mg once daily.
Dosage Recommendations for Patients with Hepatic Impairment
For patients with moderate hepatic impairment (HI) (Child-Pugh class B) or severe HI (Child-Pugh class C), the recommended CAPLYTA dosage is 21 mg once daily [see Use in Specific Populations (8.6) ] . The recommended CAPLYTA dosage in patients with mild HI is the same as those with normal hepatic function.
DOSAGE FORMS AND STRENGTHS
CAPLYTA capsules are available in three strengths:
- 42 mg: Blue cap and opaque white body imprinted with “ITI-007 42 mg”
- 21 mg: Opaque white cap and body imprinted with “ITI-007 21 mg”
- 10.5 mg: Opaque light pink cap and body imprinted with “ITI-007 10.5 mg”
USE IN SPECIFIC POPULATIONS
Pregnancy: May cause extrapyramidal and/or withdrawal symptoms in neonates with third trimester exposure. (8.1 )
Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to atypical antipsychotics, including CAPLYTA, during pregnancy. Healthcare providers are encouraged to advise patients to register by calling the National Pregnancy Registry for Atypical Antipsychotics at 1-866-961-2388 or visiting online at https://womensmentalhealth.org/research/pregnancyregistry/.
Risk Summary
Neonates exposed to antipsychotic drugs during the third trimester are at risk for extrapyramidal and/or withdrawal symptoms following delivery (see Clinical Considerations). Available data from case reports on CAPLYTA use in pregnant women are insufficient to establish any drug associated risks for birth defects, miscarriage, or adverse maternal or fetal outcomes. There are risks to the mother associated with untreated schizophrenia and with exposure to antipsychotics, including CAPLYTA, during pregnancy (see Clinical Considerations). In animal reproduction studies, no malformations were observed with oral administration of lumateperone to pregnant rats and rabbits during organogenesis at doses up to 2.4 and 9.7 times, respectively, the maximum recommended human dose (MRHD) of 42 mg/day on a mg/m 2 basis. When pregnant rats were administered lumateperone during the period of organogenesis through lactation, the number of perinatal deaths of pups was increased at 4.9 times the MRHD, with no adverse effects on pups at 2.4 times the MRHD (see Data) .
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Clinical Considerations
Disease Associated Maternal and/or Embryo/fetal Risk: There is risk to the mother from untreated schizophrenia, including increased risk of relapse, hospitalization, and suicide. Schizophrenia is associated with increased adverse perinatal outcomes, including preterm birth. It is not known if this is a direct result of the illness or other comorbid factors.
Fetal/neonatal Adverse Reactions: Extrapyramidal and/or withdrawal symptoms, including agitation, hypertonia, hypotonia, tremor, somnolence, respiratory distress, and feeding disorder have been reported in neonates who were exposed to antipsychotic drugs during the third trimester of pregnancy. These symptoms have varied in severity. Monitor neonates for extrapyramidal and/or withdrawal symptoms and manage symptoms appropriately. Some neonates recovered within hours or days without specific treatment; others required prolonged hospitalization.
Data
Animal Data
Pregnant rats were treated with oral doses of 3.5, 10.5, 21, and 63 mg/kg/day lumateperone (0.8, 2.4, 4.9, and 14.6 times the MRHD on a mg/m 2 basis) during the period of organogenesis. No malformations were observed with lumateperone at doses up to 2.4 times the MRHD. Findings of decreased body weight were observed in fetuses at 4.9 and 14.6 times the MRHD. Findings of incomplete ossification and increased incidences of visceral and skeletal variations were recorded in fetuses at 14.6 times the MRHD, a dose that induced maternal toxicity.
Pregnant rabbits were treated with oral doses of 2.1, 7, and 21 mg/kg/day lumateperone (1.0, 3.2, and 9.7 times the MRHD on a mg/m 2 basis) during the period of organogenesis. Lumateperone did not cause adverse developmental effects at doses up to 9.7 times the MRHD.
In a study in which pregnant rats were administered oral doses of 3.5, 10.5, and 21 mg/kg/day lumateperone (0.8, 2.4, and 4.9 times the MRHD on a mg/m 2 basis) during the period of organogenesis and through lactation, the number of live-born pups was decreased at 2.4 and 4.9 times the MRHD, and early postnatal deaths increased at a dose 4.9 times the MRHD. Impaired nursing and decreased body weight gain in pups were observed at 4.9 times, but not at 2.4 times, the MRHD.
Pregnant rats were treated with a human metabolite of lumateperone (reduced ketone metabolite) at oral doses of 15, 60, and 100 mg/kg/day (1.2, 19, and 27 times the exposure to this metabolite at the MRHD of lumateperone based on AUC plasma exposure) during the period of organogenesis. This metabolite did not cause adverse developmental effects at a dose 1.2 times the exposure at the MRHD of lumateperone; however, it caused an increase in visceral malformations (cleft palate) at 27 times and skeletal malformations at 19 times the exposure at the MRHD of lumateperone, a dose that induced maternal toxicity.
Lactation
Risk Summary
Lumateperone and its metabolites are present in human breast milk in low amounts. In a clinical lactation study, lumateperone was detected in human milk at an estimated daily infant dose 0.0004 mg/kg, with a relative infant dose (RID) of 0.06% the maternal weight-adjusted dosage. Several major circulating metabolites were similarly detected in breast milk in low amounts; however, aniline metabolites were not present in milk or maternal plasma at quantifiable levels (see Data) . There are no data on the effects of lumateperone on the breastfed infant or the effects on milk production.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for CAPLYTA and any potential adverse effects on the breastfed child from CAPLYTA or from the underlying maternal condition.
Data
A lactation study in 17 lactating women evaluated the concentrations of lumateperone and its metabolites in plasma and mature breast milk following a single dose of 42 mg CAPLYTA. The estimated daily infant dose of lumateperone in human milk was 0.0004 mg/kg/day (with assumed average milk consumption of 200 ml/kg/day). The mean relative infant dose (RID) (with assumed mean milk consumption of 200 mL/kg/day and average maternal weight of 71 kg) was 0.06% of the maternal weight-adjusted dosage. Several major circulating metabolites were also present in breast milk at estimated daily infant dose of 0.0004 mg/kg/day. Aniline metabolites were not present in milk or maternal plasma at quantifiable levels.
Females and Males of Reproductive Potential
Infertility
Based on findings from animal studies, lumateperone may impair male and female fertility [see Nonclinical Toxicology (13.1) ].
Pediatric Use
Safety and effectiveness of CAPLYTA have not been established in pediatric patients.
Antidepressants increased the risk of suicidal thoughts and behaviors in pediatric patients [see Boxed Warning , Warnings and Precautions (5.2) ].
Geriatric Use
Controlled clinical studies of CAPLYTA for the treatment of schizophrenia and as adjunctive therapy with antidepressants for the treatment of MDD did not include any patients aged 65 or older to determine whether or not they respond differently from younger adult patients.
Among the CAPLYTA-treated patients in clinical studies for the treatment of depressive episodes associated with bipolar depression, 20 (6%) were 65 to 74 years of age, and none were 75 years of age or older [see Clinical Studies (14.2) ]. These clinical studies did not include sufficient numbers of patients aged 65 years of age or older to determine whether or not they respond differently from younger adult patients.
Antipsychotic drugs increase the risk of death in elderly patients with dementia-related psychosis. CAPLYTA is not approved for the treatment of patients with dementia-related psychosis [see Boxed Warning , Warnings and Precautions (5.1 ) and (5.3 ) ] .
Elderly patients with dementia-related psychosis treated with antipsychotics have an increased risk of cerebrovascular adverse reactions (e.g., stroke, transient ischemic attack) including fatalities, compared to those treated with placebo [see Warnings and Precautions (5.1) ].
Antipsychotic drugs increase the risk of tardive dyskinesia and this risk appears to be highest among the elderly, particularly elderly women [see Warnings and Precautions (5.5) ].
The use of serotonin reuptake inhibitors (SRIs) has been associated with clinically significant hyponatremia in geriatric patients, who may be at greater risk for this adverse reaction. The concomitant use of CAPLYTA with an SRI may increase this risk [ see Drug Interactions (7.1) ].
Hepatic Impairment
Patients with moderate hepatic impairment (HI) (Child-Pugh class B) and severe HI (Child-Pugh class C) generally had higher exposure to lumateperone than patients with normal hepatic function; therefore, the recommended CAPLYTA dosage in patients with moderate or severe HI is lower than those with normal hepatic function [see Dosage and Administration (2.3 ) and Clinical Pharmacology (12.3 ) ] .
The recommended dosage in patients with mild HI (Child-Pugh class A) is the same as those with normal hepatic function.
CONTRAINDICATIONS
CAPLYTA is contraindicated in patients with history of hypersensitivity reaction to lumateperone or any components of CAPLYTA. Reactions have included pruritus, rash (e.g. allergic dermatitis, papular rash, and generalized rash), and urticaria.
WARNINGS AND PRECAUTIONS
- Cerebrovascular Adverse Reactions in Elderly Patients with Dementia-Related Psychosis: Increased incidence of cerebrovascular adverse reactions (e.g., stroke and transient ischemic attack). (5.3 )
- Neuroleptic Malignant Syndrome: If NMS is suspected, immediately discontinue CAPLYTA and provide intensive symptomatic treatment and monitoring. (5.4 )
- Tardive Dyskinesia: If signs and symptoms of TD occur consider discontinuing CAPLYTA treatment. (5.5 )
- Metabolic Changes: Monitor for hyperglycemia/diabetes mellitus, dyslipidemia, and weight gain. (5.6 )
- Leukopenia, Neutropenia, and Agranulocytosis : In patients with pre-existing low white blood cell count (WBC) or history of leukopenia or neutropenia, perform complete blood counts (CBC). Consider discontinuing CAPLYTA if clinically significant decline in WBC occurs in absence of other causative factors. Discontinue CAPLYTA in patients with clinically significant neutropenia or ANC < 1000/mm 3 and monitor closely until the neutropenia resolve. (5.7 )
- Orthostatic Hypotension and Syncope: Monitor heart rate and blood pressure in patients who are vulnerable to hypotension. (5.8 )
- Seizures: Use cautiously in patients with a history of seizures or with other conditions that lower seizure threshold. (5.10 )
- Potential for Cognitive and Motor Impairment: Use caution when operating machinery and driving a motor vehicle until patients are reasonably certain that therapy with CAPLYTA does not affect them adversely. (5.11 )
Increased Mortality in Elderly Patients with Dementia-Related Psychosis
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. In an analysis of 17 placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, the risk of death in antipsychotic drug-treated patients was 1.6 to 1.7 times the risk of death in placebo-treated patients. Over the course of a typical 10-week controlled trial, the incidence of death in antipsychotic-treated patients was about 4.5%, compared to an incidence of about 2.6% in placebo-treated patients. Although the causes of death varied, most of the deaths appeared to be either cardiovascular (e.g., heart failure, sudden death) or infectious (e.g., pneumonia).
CAPLYTA is not approved for the treatment of patients with dementia-related psychosis [see Indications and Usage (1) ] .
Suicidal Thoughts and Behaviors in Pediatric and Young Adult Patients
In pooled analyses of placebo-controlled trials of antidepressant drugs (SSRIs and other antidepressant classes) that included approximately 77,000 adult patients and 4,500 pediatric patients, the incidence of suicidal thoughts and behaviors in antidepressant-treated pediatric and young adult patients was greater than in placebo-treated patients. There were differences in absolute risk of suicidal thoughts and behaviors across the different uses, with the highest incidence in patients with MDD. The drug-placebo differences in the number of cases of suicidal thoughts and behaviors per 1,000 patients treated are provided in Table 1.
| Age Range | Drug-Placebo Difference in Number of Patients of Suicidal Thoughts or Behaviors per 1000 Patients Treated |
| Increases Compared to Placebo | |
| <18 years old | 14 additional patients |
| 18-24 years old | 5 additional patients |
| Decreases Compared to Placebo | |
| 25-64 years old | 1 fewer patient |
| > 65 years old | 6 fewer patients |
• CAPLYTA is not approved for use in pediatric patients.
It is unknown whether the risk of suicidal thoughts and behaviors in pediatric and young adult patients extends to longer-term use, i.e., beyond four months. However, there is substantial evidence from placebo-controlled maintenance trials in adults with MDD that antidepressants delay the recurrence of depression and that depression itself is a risk factor for suicidal thoughts and behaviors.
Monitor all antidepressant-treated patients, especially during the initial few months of anti-depressant drug therapy, and at times of dosage changes. Counsel family members or caregivers of patients to monitor for changes in behavior and to alert the health care provider. Consider changing the therapeutic regimen, including possibly discontinuing CAPLYTA, in patients whose depression is persistently worse, or who experience suicidal thoughts or behaviors.
Cerebrovascular Adverse Reactions, Including Stroke, in Elderly Patients with Dementia-Related Psychosis
In placebo-controlled trials, elderly patients with dementia-related psychosis treated with antipsychotics had a higher incidence of stroke and transient ischemic attack, including fatal stroke compared to those treated with placebo.
CAPLYTA is not approved for the treatment of patients with dementia-related psychosis [see Indications and Usage (1 ) ] .
Neuroleptic Malignant Syndrome
Neuroleptic Malignant Syndrome (NMS), a potentially fatal symptom complex, has been reported in association with administration of antipsychotic drugs. Clinical manifestations of NMS include hyperpyrexia, muscle rigidity, delirium, and autonomic instability. Additional signs may include elevated creatine phosphokinase, myoglobinuria (rhabdomyolysis), and acute renal failure.
If NMS is suspected, immediately discontinue CAPLYTA and provide intensive symptomatic treatment and monitoring.
Tardive Dyskinesia
Tardive dyskinesia (TD) may develop in patients treated with antipsychotic drugs, including CAPLYTA. TD can develop after a relatively brief treatment period at low dosages and may also occur after discontinuation of treatment. If antipsychotic treatment is discontinued, TD may partially or completely remit. Antipsychotic treatment, however, may suppress or partially suppress the signs and symptoms of TD, and may mask the underlying process. The effect that symptomatic suppression has upon the long-term course of TD is unknown.
The TD risk appears to be highest among the elderly, especially elderly women, but it is not possible to predict which patients are likely to develop TD. The TD risk and the likelihood that TD will become irreversible increase with the duration of antipsychotic drug treatment and the cumulative dosage.
Periodically reassess the need for continued treatment. If signs and symptoms of TD appear in CAPLYTA-treated patients, consider drug discontinuation. However, some patients may require CAPLYTA treatment despite the presence of TD.
Metabolic Changes
Antipsychotic drugs have caused metabolic changes, including hyperglycemia, diabetes mellitus, dyslipidemia, and weight gain.
Hyperglycemia and Diabetes Mellitus
Hyperglycemia, in some cases extreme and associated with ketoacidosis, hyperosmolar coma or death, has been reported in patients treated with antipsychotics. There have been reports of hyperglycemia in patients treated with CAPLYTA. Assess fasting plasma glucose before or soon after initiation of CAPLYTA and monitor periodically during long-term treatment.
- In pooled data from short-term (4- to 6-week), placebo-controlled trials of adult patients with schizophrenia, mean changes from baseline and the proportion of patients with shifts from normal to greater than normal levels of fasting glucose were similar in CAPLYTA-treated and placebo-treated patients. In an uncontrolled open-label trial of CAPLYTA for up to 1 year in adult patients with stable schizophrenia, the percentages of patients with shifts in fasting glucose and insulin values from normal to high were 8% and 12%, respectively. In this trial, 5% of CAPLYTA-treated patients with normal hemoglobin A1c (<6.5%) at baseline developed elevated levels (≥6.5%) post-baseline.
- In data from short-term (6-week), placebo-controlled monotherapy and adjunctive therapy bipolar depression trials, mean changes from baseline and the proportion of patients with shifts from normal to greater than normal levels of fasting glucose and insulin were similar in CAPLYTA-treated and placebo-treated patients.
- In pooled data from short-term (6-week), placebo-controlled adjunctive therapy MDD trials, mean changes from baseline and the proportion of patients with shifts from normal to greater than normal levels of fasting glucose were similar in CAPLYTA-treated and placebo-treated patients.
Dyslipidemia
Antipsychotics have caused adverse alterations in lipids. Before or soon after initiation of antipsychotic medications, obtain a fasting lipid profile at baseline and monitor periodically during treatment.
- In pooled data from short-term (4- to 6-week), placebo-controlled trials of adult patients with schizophrenia, mean changes from baseline and the proportion of patients with shifts to higher levels of fasting total cholesterol and triglycerides were similar in CAPLYTA-treated and placebo-treated patient. In an uncontrolled open-label trial of CAPLYTA for up to 1 year in patients with stable schizophrenia, the percentages of patients with a shift from normal to high were 8%, 5%, and 4% for total cholesterol, triglycerides, and LDL cholesterol, respectively.
- In data from short-term (6-week), placebo-controlled monotherapy and adjunctive therapy bipolar depression trials, mean changes from baseline and the proportion of patients with shifts to higher levels of fasting total cholesterol and triglycerides were similar in CAPLYTA-treated and placebo-treated patients. In an uncontrolled open-label trial of CAPLYTA for up to 6 months in patients with bipolar depression, the proportion of patients with a shift from normal to high were 10%, 5%, and 2% for total cholesterol, triglycerides, and LDL cholesterol, respectively.
- In pooled data from short-term (6-week), placebo-controlled adjunctive therapy MDD trials, mean changes from baseline and the proportion of patients with shifts to higher levels of fasting total cholesterol and triglycerides were similar in CAPLYTA-treated and placebo-treated patients.
Weight Gain
Weight gain has been observed with use of antipsychotics. Monitor weight at baseline and frequently thereafter.
- In pooled data from placebo-controlled trials of adult patients with schizophrenia, mean changes from baseline and the proportion of patients with an increase in weight ≥7% from baseline to end of study was similar in CAPLYTA-treated and placebo-treated patients. In an uncontrolled open-label trial of CAPLYTA for up to one year in patients with stable schizophrenia, the mean change in body weight was approximately -2 kg (SD 5.6) at Day 175 and approximately - 3.2 kg (SD 7.4) at Day 350.
- In data from short-term (6-week), placebo-controlled monotherapy and adjunctive therapy bipolar depression trials, mean changes from baseline and the proportion of patients with an increase in weight ≥7% from baseline to end of study were similar in CAPLYTA-treated and placebo-treated patients. In an uncontrolled open-label trial of CAPLYTA for up to 6 months in patients with bipolar depression, the mean change in body weight was -0.01 kg (SD 3.1) at Day 175.
- In pooled data from short-term (6-week), placebo-controlled adjunctive therapy MDD trials, mean changes from baseline and the proportion of patients with an increase in weight ≥7% from baseline to end of study were similar in CAPLYTA-treated and placebo-treated patients.
- In a long-term open-label adjunctive therapy MDD trial of CAPLYTA for up to 6 months, the mean change in body weight was -0.16 kg (SD 3.7) at Week 26.
Leukopenia, Neutropenia, and Agranulocytosis
Leukopenia and neutropenia have been reported during treatment with antipsychotic drugs, including CAPLYTA. Agranulocytosis (including fatal cases) has been reported with other antipsychotic drugs.
Possible risk factors for antipsychotic drug-leukopenia and neutropenia include pre-existing low white blood cell count (WBC) or absolute neutrophil count (ANC) and history of drug-induced leukopenia or neutropenia.
In patients with a pre-existing low WBC or ANC or a history of drug-induced leukopenia or neutropenia, perform complete blood count (CBC) monitoring during the first few months of CAPLYTA therapy. Consider discontinuing CAPLYTA in patients who have a clinically significant decline in WBC in the absence of other causative factors. Discontinue CAPLYTA in patients with clinically significant neutropenia or ANC < 1000/mm 3 and monitor closely until the neutropenia resolves.
Orthostatic Hypotension and Syncope
Atypical antipsychotics cause orthostatic hypotension and syncope. Generally, the risk is greatest during initial dose administration.
Orthostatic vital signs should be monitored in patients who are vulnerable to hypotension (e.g., elderly patients, patients with dehydration, hypovolemia, and concomitant treatment with antihypertensive drugs), patients with known cardiovascular disease (history of myocardial infarction, ischemic heart disease, heart failure, or conduction abnormalities), and patients with cerebrovascular disease. CAPLYTA has not been evaluated in patients with a recent history of myocardial infarction or unstable cardiovascular disease. Such patients were excluded from pre-marketing clinical trials.
- In pooled data from short-term (4- to 6-week), placebo-controlled schizophrenia trials, the frequencies of orthostatic hypotension in CAPLYTA-treated and placebo-treated patients were 0.7% and 0%, respectively. The incidence of syncope for CAPLYTA-treated and placebo-treated patients were 0.2% and 0.2%.
- In data from short-term (6-week), placebo-controlled monotherapy and adjunctive therapy bipolar depression trials, the frequencies of orthostatic hypotension for CAPLYTA-treated and placebo-treated patients were both 0%. The incidence of syncope for CAPLYTA and placebo were 0.3% and 0.5%, respectively in the monotherapy trials, and there were no reports of syncope in the adjunctive therapy trial.
- In pooled data from short-term (6-week), placebo-controlled adjunctive therapy MDD trials, the frequencies of orthostatic hypotension for CAPLYTA-treated and placebo-treated patients were 6.6% and 6.2%, respectively. The incidence of syncope for CAPLYTA-treated and placebo-treated patients were 0.2% and 0%, respectively.
Falls
Antipsychotics, including CAPLYTA, may cause somnolence, postural hypotension, and motor and sensory instability, which may lead to falls and, consequently, fractures and other injuries.
If patients have a condition (or take concomitant drugs) that could exacerbate these effects, complete fall risk assessments when initiating CAPLYTA treatment and periodically during long-term treatment.
Seizures
Like other antipsychotic drugs, CAPLYTA may cause seizures. The risk of antipsychotic drug-associated seizures is greatest in patients with a history of seizures or with conditions that lower the seizure threshold. Conditions that lower the seizure threshold may be more prevalent in older patients.
Use CAPLYTA cautiously in patients with a history of seizures or with other conditions that lower seizure threshold.
Potential for Cognitive and Motor Impairment
CAPLYTA, like other antipsychotics, may cause somnolence and has the potential to impair judgment, thinking, and motor skills. Patients should be cautioned about operating hazardous machinery and motor vehicles until they are reasonably certain that therapy with CAPLYTA does not affect them adversely.
- In short-term (i.e., 4- to 6-week), placebo-controlled clinical trials of patients with schizophrenia, somnolence and sedation were reported in 24% of CAPLYTA-treated patients, compared to 10% of placebo-treated patients.
- In short term (6-week), placebo-controlled monotherapy and adjunctive therapy bipolar depression clinical trials, somnolence and sedation were reported in 13% of CAPLYTA-treated patients, compared to 3% of placebo-treated patients.
- In short term (6-week), placebo-controlled adjunctive therapy MDD trials, somnolence and sedation were reported in 12% of CAPLYTA-treated patients, compared to 2% of placebo-treated patients.
Body Temperature Dysregulation
Atypical antipsychotics may disrupt the body’s ability to reduce core body temperature. Strenuous exercise, exposure to extreme heat, dehydration, and anticholinergic drugs may contribute to an elevation in core body temperature. Use CAPLYTA with caution in patients who may experience these conditions.
Dysphagia
Esophageal dysmotility and aspiration have been associated with antipsychotic drug use. Antipsychotic drugs, including CAPLYTA, should be used cautiously in patients at risk for aspiration.
ADVERSE REACTIONS
The following adverse reactions are discussed in detail in other sections of the labeling:
- Increased Mortality in Elderly Patients with Dementia-Related Psychosis [see Boxed Warning , Warnings and Precautions (5.1) ]
- Suicidal Thoughts and Behaviors [see Boxed Warning , Warnings and Precautions (5.2) ]
- Cerebrovascular Adverse Reactions, Including Stroke, in Elderly Patients with Dementia-related Psychosis [see Warnings and Precautions (5.3) ]
- Neuroleptic Malignant Syndrome [see Warnings and Precautions (5.4) ]
- Tardive Dyskinesia [see Warnings and Precautions (5.5) ]
- Metabolic Changes [see Warnings and Precautions (5.6) ]
- Leukopenia, Neutropenia, and Agranulocytosis [see Warnings and Precautions (5.7) ]
- Orthostatic Hypotension and Syncope [see Warnings and Precautions (5.8) ]
- Falls [see Warnings and Precautions (5.9) ]
- Seizures [see Warnings and Precautions (5.10) ]
- Potential for Cognitive and Motor Impairment [see Warnings and Precautions (5.11) ]
- Body Temperature Dysregulation [see Warnings and Precautions (5.12) ]
- Dysphagia [see Warnings and Precautions (5.13) ]
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of CAPLYTA has been evaluated in placebo-controlled clinical trials that included 3575 adult patients with schizophrenia, bipolar depression, or major depressive disorder, exposed to one or more CAPLYTA doses. A total of 852 CAPLYTA-treated patients had at least 6 months of treatment and 108 had at least 1 year of treatment with the 42-mg once daily dosage.
Adverse Reactions in Patients with Schizophrenia
The following adverse reactions are based on the pooled short-term (4- to 6-week), placebo-controlled studies in adult patients with schizophrenia in which CAPLYTA was administered at a dosage of 42 mg once daily (N=406) [see Clinical Studies (14.1) ].
There was no single adverse reaction that led to discontinuation that occurred at a rate of >2% in CAPLYTA-treated patients. The most common adverse reactions (incidence of at least 5% of CAPLYTA-treated patients and greater than twice the rate of placebo-treated patients) were somnolence/sedation and dry mouth. Adverse reactions (incidence of at least 2% in CAPLYTA-treated patients and greater than in placebo-treated patients) are shown in Table 2.
CAPLYTA 42 mg (N=406) | Placebo (N=412) | |
| Somnolence/Sedation | 24% | 10% |
| Nausea | 9% | 5% |
| Dry Mouth | 6% | 2% |
| Dizziness 1 | 5% | 3% |
| Creatine Phosphokinase Increased | 4% | 1% |
| Fatigue | 3% | 1% |
| Vomiting | 3% | 2% |
| Hepatic Transaminases Increased 2 | 2% | 1% |
| Decreased Appetite | 2% | 1% |
1 Dizziness, dizziness postural 2 ALT, AST, “hepatic enzymes” increased, or liver function test abnormal
Adverse Reactions in Patients with Bipolar Depression (CAPLYTA Monotherapy)
The following adverse reactions are based on the pooled short-term (6-week), placebo-controlled monotherapy bipolar depression studies in adult patients treated with CAPLYTA 42 mg once daily (N=372) [see Clinical Studies (14.2) ] .
There was no single adverse reaction leading to discontinuation that occurred at a rate of >2% in CAPLYTA-treated patients.
The most common adverse reactions (incidence of at least 5% of CAPLYTA-treated patients and greater than twice the rate in placebo-treated patients) were somnolence/sedation, dizziness, nausea, and dry mouth.
Adverse reactions associated with CAPLYTA (incidence of at least 2% in CAPLYTA-treated patients and greater than placebo-treated patients) are shown in Table 3.
| CAPLYTA 42 mg (N=372) | Placebo (N=374) | |
| Headache | 14% | 8% |
| Somnolence/Sedation | 13% | 3% |
| Dizziness 1 | 8% | 4% |
| Nausea | 8% | 3% |
| Dry mouth | 5% | 1% |
| Diarrhea | 4% | 2% |
| Vomiting | 4% | 0% |
| Abdominal pain 2 | 2% | 1% |
| Upper respiratory tract infection | 2% | 1% |
1 Dizziness, dizziness postural 2 Abdominal discomfort, abdominal pain, abdominal pain upper and lower
Adverse Reactions in Patients with Bipolar Depression (Concomitant Treatment with CAPLYTA and Lithium or Valproate)
The adverse reactions below are based on a 6-week, placebo-controlled adjunctive therapy bipolar depression study in adult patients treated with CAPLYTA 42 mg once daily + lithium or valproate OR placebo + lithium or valproate (N=177) [see Clinical Studies (14.2) ].
There was no single adverse reaction leading to discontinuation that occurred at a rate of >2% in patients in the CAPLYTA + lithium or valproate group.
The most common adverse reactions (incidence of at least 5% in patients treated with CAPLYTA + lithium or valproate and greater than twice the rate of patients treated with placebo + lithium or valproate) were somnolence/sedation, dizziness, nausea, and dry mouth.
Adverse reactions associated with CAPLYTA + lithium or valproate (incidence of at least 2% in patients treated with CAPLYTA + lithium or valproate and greater than with patients treated with placebo + lithium or valproate) are shown in Table 4.
CAPLYTA 42 mg + lithium or valproate (N=177) | Placebo + lithium or valproate (N=175) | |
| Somnolence/Sedation | 13% | 3% |
| Dizziness 1 | 11% | 2% |
| Nausea | 9% | 4% |
| Dry mouth | 5% | 1% |
| Vomiting | 4% | 0% |
| Diarrhea | 3% | 2% |
| Upper respiratory tract infection | 3% | 1% |
| Blurred vision | 3% | 1% |
| Increased blood prolactin | 2% | 0% |
1 Dizziness, dizziness postural
Adverse Reactions in Studies for Adjunctive Treatment of Major Depressive Disorder
The following adverse reactions are based on pooled short-term (6-week), placebo-controlled adjunctive therapy MDD studies in adult patients treated with CAPLYTA 42 mg orally once daily and background antidepressant therapy (ADT) or placebo and background ADT (N=483) [see Clinical Studies (14.3) ].
There was no single adverse reaction leading to discontinuation that occurred at an incidence of >2% in patients treated with CAPLYTA and background ADT.
The most common adverse reactions (incidence of at least 5% of patients treated with CAPLYTA and background ADT and greater than twice the incidence in patients treated with placebo and background ADT) were dizziness, dry mouth, somnolence/sedation, nausea, fatigue, and diarrhea.
Adverse reactions in the adjunctive therapy MDD studies (incidence of at least 2% of patients treated with CAPLYTA and background ADT and greater than in patients treated with placebo and background ADT) are shown in Table 5.
| CAPLYTA 42 mg + ADT (N=483) | Placebo + ADT (N=481) | |
| Headache 1 | 19% | 13% |
| Dizziness 2 | 17% | 5% |
| Dry Mouth | 13% | 3% |
| Somnolence/Sedation | 12% | 2% |
| Nausea | 9% | 4% |
| Fatigue | 8% | 2% |
| Diarrhea | 5% | 1% |
| Tremor | 4% | <1% |
| Vomiting | 3% | 2% |
| Vertigo | 3% | <1% |
| Insomnia | 3% | 2% |
1 Headache, migraine, tension headache 2 Dizziness, postural dizziness
Additional Adverse Reactions
Dystonia: Symptoms of dystonia, prolonged abnormal contractions of muscle groups, may occur in susceptible individuals during the first few days of treatment. Dystonic symptoms include: spasm of the neck muscles, sometimes progressing to tightness of the throat, swallowing difficulty, difficulty breathing, and/or protrusion of the tongue. Although these symptoms can occur at low doses, they occur more frequently and with greater severity with high potency and higher doses of first-generation antipsychotic drugs. An elevated risk of acute dystonia is observed in males and younger age groups.
Extrapyramidal Symptoms: In the short-term, placebo-controlled schizophrenia, bipolar depression, and adjunctive MDD studies, extrapyramidal (EPS) symptom data was objectively collected on the Simpson-Angus Scale (SAS) for EPS (total score ranges from 0 to 40), the Barnes Akathisia Rating Scale (BARS) for akathisia (total score ranges from 0 to 14), and the Abnormal Involuntary Movement Scale (AIMS) for dyskinesia (total score ranges from 0 to 28).
- In the 4- to 6-week, placebo-controlled schizophrenia trials, the frequency of EPS-related reported events including akathisia, extrapyramidal disorder, muscle spasms, restlessness, musculoskeletal stiffness, dyskinesia, dystonia, muscle twitching, tardive dyskinesia, tremor, drooling, and involuntary muscle contractions was 6.7% for the CAPLYTA-treated patients and 6.3% for placebo-treated patients. In these trials, the mean changes from baseline for CAPLYTA-treated patients and placebo-treated patients were 0.1 and 0 for the SAS, -0.1 and 0 for the BARS, and 0.1 and 0 for the AIMS, respectively.
- In the 6-week, monotherapy bipolar depression trials, the frequency of reported EPS-related reactions including muscle spasms, dyskinesia, extrapyramidal disorder, movement disorder, tremor, restlessness, and akathisia was 1.3% for CAPLYTA-treated patients and 1.1% for placebo-treated patients. In these trials, the mean changes from baseline for CAPLYTA-treated patients and placebo-treated patients were 0 and 0 for the SAS, -0.1 and -0.1 for the BARS, and 0 and 0 for the AIMS, respectively.
- In a 6-week, adjunctive therapy bipolar depression trial, the frequency of reported EPS-related reactions, including tremor, muscle spasms, akathisia, extrapyramidal disorder, gait disturbance, and restlessness was 4% for CAPLYTA-treated patients and 2.3% for placebo-treated patients. In the 6-week, monotherapy bipolar depression trials, the mean changes from baseline for CAPLYTA-treated patients and placebo-treated patients were 0 and 0 for the SAS, -0.1 and -0.1 for the BARS, and 0 and 0 for the AIMS, respectively. In this trial, the mean changes from baseline for CAPLYTA-treated patients and placebo-treated patients were 0 and 0 for the SAS, 0 and -0.1 for the BARS, and 0 and 0 for the AIMS, respectively.
- In the 6-week, adjunctive MDD trials, the frequency of reported EPS-related adverse reactions (tremor, bradykinesia, muscle spasms, gait disturbance, tongue spasm, muscle tightness and dyskinesia), excluding akathisia and restlessness, was 5% for CAPLYTA-treated patients and 0.8% for placebo-treated patients. The combined incidence of akathisia and restlessness was 1% for CAPLYTA-treated patients and 0.8% for placebo-treated patients. In these trials, the mean changes from baseline for CAPLYTA-treated patients and placebo-treated patients were 0 and 0 for the SAS, 0 and -0.1 for the BARS, and 0 and 0 for the AIMS, respectively.
Postmarketing Experience
The following adverse reaction has been identified during post-approval use of CAPLYTA. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to estimate their frequency or establish a causal relationship to drug exposure.
Central and Peripheral Nervous System Disorders : burning sensation, including skin burning sensation
DRUG INTERACTIONS
Drugs Having Clinically Important Interactions with CAPLYTA
Clinically important drug interactions with CAPLYTA are presented in Table 6.
| CYP3A4 Inducers• | |
| Prevention or Management | Avoid concomitant use of CAPLYTA with CYP3A4 inducers . |
| Clinical Impact | Concomitant use of CAPLYTA with CYP3A4 inducers decreases the exposure of lumateperone [see Clinical Pharmacology (12.3 ) ]. |
| Moderate or Strong CYP3A4 Inhibitors• | |
| Prevention or Management | Reduce the CAPLYTA dosage when used concomitantly with moderate or strong CYP3A4 inhibitors [see Dosage and Administration (2.2 ) ]. |
| Clinical Impact | Concomitant use of CAPLYTA with moderate or strong CYP3A4 inhibitors increases lumateperone exposure [see Clinical Pharmacology (12.3 ) ] , which may increase the risk of adverse reactions. |
| S erotonin Reuptake Inhibitors | |
| Prevention or Management | Increased monitoring for SRI- associated adverse reactions is recommended. |
| Clinical Impact | Although no clinically significant drug interactions with adjunctive SSRI/SNRIs in MDD were observed in CAPLYTA clinical trials, CAPLYTA’s moderate serotonin transporter (SERT) activity may increase the risk of SRI-associated adverse reactions (e.g., serotonin syndrome, hyponatremia). |
• See www.fda.gov/CYPandTransporterInteractingDrugs for examples of CYP3A4 Inducers and Moderate or Strong CYP3A4 Inhibitors
DESCRIPTION
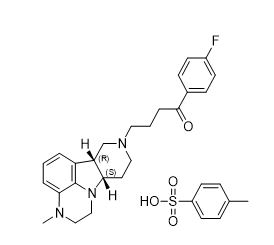
Lumateperone is an atypical antipsychotic present as lumateperone tosylate salt with the chemical name 4-((6b R ,10a S )-3-methyl-2,3,6b,9,10,10 a -hexahydro-1 H ,7 H -pyrido[3',4':4,5]pyrrolo[1,2,3- de ]quinoxalin-8-yl)-1-(4-fluoro-phenyl)-butan-1-one 4-methylbenzenesulfonate. Its molecular formula is C 31 H 36 FN 3 O 4 S, and its molecular weight is 565.71 g/mol with the following structure:
CAPLYTA (lumateperone) capsules are for oral administration. Each capsule contains:
- 42 mg of lumateperone (equivalent to 60 mg of lumateperone tosylate), or
- 21 mg of lumateperone (equivalent to 30 mg of lumateperone tosylate), or
- 10.5 mg of lumateperone (equivalent to 15 mg of lumateperone tosylate).
The capsules include the following inactive ingredients: croscarmellose sodium, gelatin, magnesium stearate, mannitol, and talc. Colorants include FD&C blue #1 and red #3 (42 mg), FDA/E172 black iron oxide, FDA/E172 red iron oxide and FD&C red #3 (10.5 mg), and titanium dioxide (42 mg, 21 mg and 10.5 mg).
CLINICAL PHARMACOLOGY
Mechanism of Action
The mechanism of action of lumateperone for the treatment of schizophrenia in adults, for the treatment of depressive episodes associated with bipolar depression (as monotherapy or as adjunctive therapy with lithium or valproate), and as adjunctive therapy with antidepressants for the treatment of MDD is unknown. However, the mechanism of action of lumateperone for these uses could be mediated through a combination of antagonist activity at central serotonin 5-HT 2A receptors, and partial agonist activity at central dopamine D 2 receptors.
Pharmacodynamics
Lumateperone has high binding affinity for serotonin 5-HT 2A receptors (K i = 0.54 nM) and moderate binding affinity for dopamine D 2 (K i = 32 nM) receptors. Lumateperone has moderate binding affinity for dopamine D 1 (K i = 41 nM) and D 4 and adrenergic alpha 1A and alpha 1B receptors (K i ≤ 100 nM), serotonin transporters (K i = 33 nM), and inhibits uptake of serotonin in cells expressing the human SERT (IC 50 = 150 nM). Lumateperone has low binding affinity (less than 50% inhibition at 100 nM) for muscarinic and histaminergic receptors.
In healthy human volunteers the mean cortical 5-HT 2A receptor occupancy and the mean striatal D 2 receptor occupancy were greater than 80% and approximately 12%, respectively, after a single 7 mg dose of CAPLYTA. D 2 receptor occupancy increased with a dose from 7 mg to 28 mg. In patients with schizophrenia treated with CAPLYTA 42 mg for 2 weeks, mean dorsal striatal D 2 receptor occupancy was 39%.
Cardiac Electrophysiology
QTcF interval was evaluated in a randomized, placebo- and active- (moxifloxacin 400 mg) controlled, four-arm crossover study utilizing concentration-QTc effect modeling in 33 patients with schizophrenia. The placebo-corrected change from baseline QTcF (90% two-sided upper confidence interval) values of 4.9 (8.9) and 15.8 (19.8) ms for a CAPLYTA 42 mg once daily oral dosage for five days and a CAPLYTA 126 mg once daily oral dosage (three times the recommended daily dosage) for five days, respectively.
Pharmacokinetics
Following once daily oral administration of CAPLYTA, lumateperone steady state is reached in about 5 days. Lumateperone steady-state exposure is approximately dose-proportional in the range of 3.5 mg to 56 mg (0.08 to 1.3 times the approved recommended daily dosage). A large inter-subject variability in lumateperone PK parameters was observed, with coefficients of variation for C max (peak plasma concentration) and AUC (area under the concentration vs time curve) ranging from 68% to 97% at steady state.
Absorption
After CAPLYTA dosing, the absolute bioavailability of lumateperone is about 4.4%. C max of lumateperone is reached approximately 1-2 hours after CAPLYTA dosing.
Effect of Food: Ingestion of a high-fat meal with CAPLYTA lowered lumateperone mean C max by 33% and increased mean AUC by 9%. Median T max was delayed about 1 hour (from 1 hour at fasted state to 2 hours in the presence of food).
Distribution
Protein binding of lumateperone is 97.4% at 5 µM (about 70-fold higher than therapeutic concentrations) in human plasma. The volume of distribution of lumateperone following intravenous administration is about 4.1 L/kg.
Elimination
The clearance of lumateperone is approximately 27.9 L/hour and the terminal half-life is about 18 hours after intravenous administration.
Metabolism: Lumateperone is extensively metabolized with more than twenty metabolites identified in vivo . After a single 14 C-labeled oral dose, lumateperone and glucuronidated metabolites represent about 2.8% and 51% of the total plasma radioactivity, respectively. In vitro studies show that multiple enzymes, including but not limited to uridine 5'-diphospho-glucuronosyltransferases (UDP-glucuronosyltransferase, UGT) 1A1, 1A4, and 2B15, aldoketoreductase (AKR)1C1, 1B10, and 1C4, and cytochrome P450 (CYP) 3A4, 2C8, and 1A2, are involved in the metabolism of lumateperone.
Excretion: In a human mass‑balance study, 58% and 29% of the radioactive dose was recovered in the urine and feces, respectively. Less than 1% of the dose was excreted as unchanged lumateperone in the urine.
Specific Populations
No clinically significant differences in the pharmacokinetics of lumateperone were observed based on age, sex, or race.
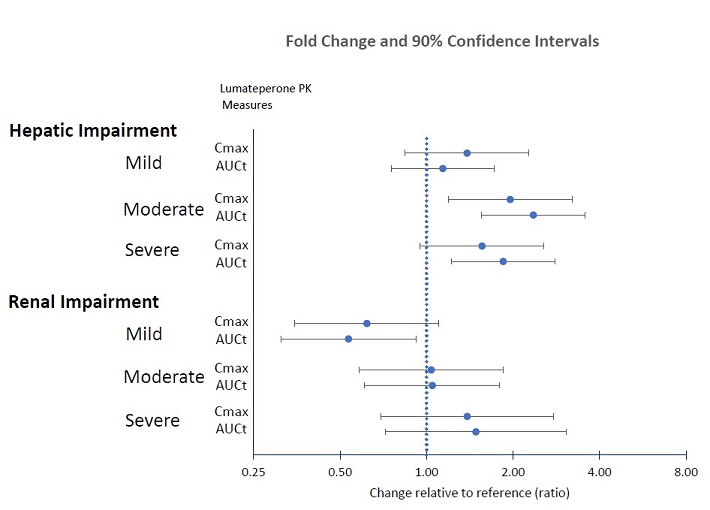
Patients with Hepatic Impairment: The pharmacokinetics of lumateperone were evaluated in patients with varying degrees of hepatic impairment (HI) and in those with normal hepatic function following administration of a single 14 mg dose of CAPLYTA. Table 7 displays the geometric mean ratios (90% confidence intervals) of lumateperone pharmacokinetic parameters in CAPLYTA-treated patients with varying degrees of HI to those with normal hepatic function [see Use in Specific Populations (8.6) ] .
| AUC t | C max | |
| Mild HI (Child-Pugh Class A) | 1.1 (0.8, 1.7) | 1.4 (0.8, 2.3) |
| Moderate HI (Child-Pugh Class B) | 2.4 (1.6, 3.6) | 2 (1.2, 3.2) |
| Severe HI (Child-Pugh Class C) | 1.8 (1.2, 2.8) | 1.6 (0.9, 2.6) |
Patients with Renal Impairment: The pharmacokinetics of lumateperone were evaluated in patients with varying degrees of renal impairment (RI) and in those with normal renal function following administration of a single 14 mg dose of CAPLYTA. Table 8 displays the geometric mean ratios (90% confidence intervals) of lumateperone pharmacokinetic parameters in CAPLYTA-treated patients with varying degrees of RI to those with normal renal function (eGFR > 90 mL/minute/1.73 m 2 ).
| AUC t | C max | |
| Mild RI (eGFR of 60 - 89 mL/minute/1.73 m 2 ) | 0.54 (0.31, 0.92) | 0.62 (0.35, 1.10) |
| Moderate RI (eGFR of 30 - 59 mL/minute/1.73 m 2 ) | 1 (0.6, 1.8)• | 1 (0.6, 1.9)• |
| Severe RI (eGFR ≤ 29 mL/minute/1.73 m 2 ) | 1.5 (0.8, 2.6) | 1.4 (0.8, 2.4) |
• The pharmacokinetics were unchanged.
Drug Interaction Studies
Clinical Studies:
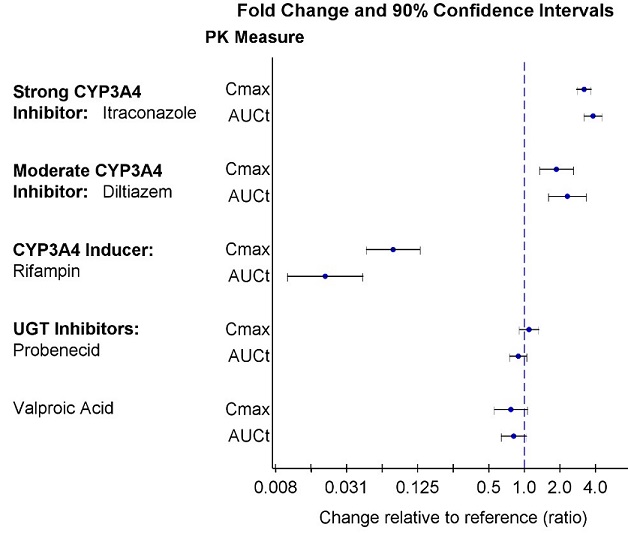
Strong CYP3A4 Inhibitors : Itraconazole (strong CYP3A4 inhibitor) increased geometric mean (90% confidence interval) of lumateperone AUC t by 3.8-fold (3.2, 4.5) and lumateperone C max by 3.2-fold (2.8 – 3.7) [see Drug Interactions (7.1) ]
Moderate CYP3A4 Inhibitors : Diltiazem (moderate CYP3A4 inhibitor) increased geometric mean (90% confidence interval) of lumateperone AUC t by 2.3-fold (1.6, 3.3) and lumateperone C max by 1.9-fold (1.3, 2.6) [see Drug Interactions (7.1) ]
Strong CYP3A4 Inducers : Rifampin (strong CYP3A4 inducer) decreased geometric mean (90% confidence interval) of lumateperone AUC t by 98% (96%, 99%) and lumateperone C max by 92% (87%, 95%) [see Drug Interactions (7.1) ]
UGT inhibitors : There were no clinically significant drug interactions with the UGT inhibitors probenecid and valproic acid.
CYP3A4 substrates : No clinically significant differences in the pharmacokinetics of midazolam (CYP3A4 substrate) or its metabolite 1-hydroxymidazolam were observed when used concomitantly with single or multiple doses of lumateperone in patients with schizophrenia.
In Vitro Studies:
Lumateperone showed little to no inhibition of CYP1A2, CYP2C9, CYP2C19, CYP2D6, or CYP3A4/5. It showed no induction of CYP1A2, CYP2B6, or CYP3A4.
Lumateperone did not appear to be a P-gp or BCRP substrate. It showed little to no inhibition of OCT2, OAT1, OAT3, OATP1B3, or OATP1B1.
NONCLINICAL TOXICOLOGY
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Lifetime carcinogenicity studies were conducted in rats and mice, and results showed no carcinogenic potential in either species.
In Sprague-Dawley rats, males were administered lumateperone (free base) at oral doses of 3.5, 7 or 14 mg/kg/day and females were administered lumateperone at oral doses of 3.5, 10.5, or 21 mg/kg/day for the first 385 days, then doses were reduced for the two higher dose groups so that the females were administered 3.5, 7 or 14 mg/kg/day, respectively, for the duration of the study. In this study the no adverse effect level for neoplastic lesions was determined to be 14 mg/kg/day (84 mg/m 2 /day) for males and 10.5/7 mg/kg/day (42 mg/m 2 /day) for females, which are 1.6 times (females) to 3.2 times (males) the MRHD on a mg/m 2 basis.
Male and female CD-1 mice were administered lumateperone at oral doses of 3.5, 10.5 or 21 mg/kg/day for the first 35 days, then doses were reduced to 1.4, 4.9, and 14 mg/kg/day, respectively, for the duration of the study. In this study, the no adverse effect level for neoplastic lesions was determined to be 10.5/4.9 mg/kg/day (15 mg/m 2 /day) for each sex which is 0.6 times the MRHD on a mg/m 2 basis.
Mutagenesis
No evidence of mutagenic potential was found in the in vitro bacterial reverse mutation assay (Ames test) and the mouse lymphoma test without metabolic activation. Lumateperone was positive in the Ames test only in the presence of metabolic activation and only in the TA1537 strain and was positive in the mouse lymphoma test only in the presence of metabolic activation and only at high concentrations that inhibited cell growth; together these results were thought to be related to solubility limits and/or nonspecific effects on cellular function. Lumateperone was negative for clastogenic activity in the in vivo micronucleus assay in rats and was not genotoxic in the in vivo Comet assay in rats.
Impairment of Fertility
Female rats were treated with oral doses of 3.5, 10.5, 21 or 42 mg/kg/day lumateperone (free base) (0.8, 2.4, 4.9, and 9.7 times the MRHD on a mg/m 2 basis) prior to mating and continuing through conception and implantation. Estrus cycle irregularities were observed at doses ≥10.5 mg/kg/day. Decreases in the median number of corpora lutea and implantation sites, and increases in the number of non-gravid uteruses, were recorded at 42 mg/kg/day. Decreased gestation body weight and body weight gain, and increases in time to mating, were observed at 21 and 42 mg/kg/day.
Male rats were treated with oral doses of 3.5, 10.5, 21 or 42 mg/kg/day lumateperone (0.8, 2.4, 4.9, and 9.7 times the MRHD on a mg/m 2 basis) for 9 weeks prior to mating and throughout 14 days of mating. Decreased sperm motility, changes in sperm morphology, reduced epididymal counts, and adverse histopathology changes in testes and epididymides were observed at 21 and 42 mg/kg/day.
Animal Toxicology and/or Pharmacology
Oral administration of lumateperone caused systemic intracytoplasmic accumulation of pigmented material in dogs, rats, and mice at clinically relevant exposures (AUC). Intracytoplasmic pigmentation appeared to be localized in lysosomes. Accumulation of pigmented material persisted without reversal at the end of 1- to 2-month drug-free periods. Pigmented material was observed in the brain and spinal cord of all three species, and in the heart and eye of rats. Although the composition of the pigmented material was not established, the material is likely polymers or protein adducts formed from aniline metabolites of lumateperone.
In the dog, accumulation of pigmented material in the brain and spinal cord was associated with neuronal degeneration and necrosis, followed by axonal degeneration and histiocytic inflammation after oral administration of lumateperone for up to 9 months. In the rat, accumulation of pigmented material was associated with degenerative changes and signs of an inflammatory response in the spinal cord, peripheral nervous system, eye, and heart after oral administration of lumateperone for up to 2 years. Although overt degenerative changes were not observed in the rat brain, the presence of pigment-containing infiltrating macrophages is consistent with an inflammatory response.
The role of intracytoplasmic pigmented material in causing these lesions was not definitively established; however, the colocalization of pigmented material in tissues with degenerative changes and signs of inflammation is supportive. Alternatively, the aniline metabolites of lumateperone may undergo metabolic activation forming reactive metabolites that contribute to the observed toxicities. The role of intracellular accumulation of lumateperone or its non-aniline metabolites in these toxicities could not be ruled out.
The aniline metabolites thought to be responsible for these toxicities were detected in dogs and rats but were not present in humans at quantifiable levels. Based on all the available evidence, these toxicities do not appear to be relevant to humans.
CLINICAL STUDIES
Schizophrenia
CAPLYTA was evaluated for the treatment of schizophrenia in adults in two placebo-controlled trials (i.e., Studies 1 and 2).
Study 1 (Adults with Schizophrenia)
Study 1 Design: Study 1 (NCT01499563) was a four-week, randomized, double-blind, placebo-controlled, multi-center study in adult patients with a diagnosis of schizophrenia according to the DSM-IV-TR criteria. The primary efficacy measure was change from baseline in the Positive and Negative Syndrome Scale (PANSS) total score at Week 4. The PANSS is a 30-item scale used to measure symptoms of schizophrenia. Each item is rated by a clinician on a seven-point scale. A score of 1 indicates the absence of symptoms, and a score of 7 indicates extremely severe symptoms. The PANSS total score ranges from 30 to 210 with higher scores reflecting greater overall symptom severity.
A total of 335 patients were randomized to receive oral CAPLYTA 42 mg, CAPLYTA 84 mg (two times the recommended daily dose), an active comparator, or placebo once daily. The study was not designed to allow for efficacy comparison of CAPLYTA and the active comparator.
Study 1 Baseline Demographics: Demographic and baseline disease characteristics were similar for the CAPLYTA, active comparator, and placebo groups. Median age was 42 years (range 20 to 55 years). 17% were female, 19% were White, and 78% were Black.
Study 1: Summary of Primary Efficacy Results: Compared to the placebo group, patients randomized to CAPLYTA 42 mg once daily showed a statistically significant reduction from baseline to Day 28 in the PANSS total score. The treatment effect in the CAPLYTA 84 mg once daily treatment group (vs. placebo group) was not statistically significant. The results of Study 1 are shown in Table 9.
Study 2 (Adults with Schizophrenia)
Study 2 Design: Study 2 (NCT02282761) was a four-week, randomized, double-blind, placebo-controlled, multi-center study in adult patients with a diagnosis of schizophrenia according to the DSM-5 criteria. The primary efficacy measure was change from baseline in the PANSS total score at Week 4.
A total of 450 patients were randomized to receive oral CAPLYTA 28 mg (two-thirds the recommended daily dose), CAPLYTA 42 mg, or placebo once daily.
Study 2 Baseline Demographics: Demographic and baseline disease characteristics were similar for the CAPLYTA and placebo groups. Median age was 44 years (range 19 to 60 years); 23% were female, 26% were White and 66% were Black.
Study 2: Summary of Primary Efficacy Results: Compared to the placebo group, patients in the CAPLYTA 42 mg group showed a statistically significant reduction from baseline to Day 28 in the PANSS total score. The treatment effect in the CAPLYTA 28 mg group (vs. placebo group) was not statistically significant. The results of Study 2 are shown in Table 9.
Efficacy Results in Studies 1 and 2 (Adults with Schizophrenia)
Studies 1 and 2 did not include any patients aged 65 or older. Examination of subgroups by sex and race did not suggest differences in response in either study.
| Primary Efficacy Endpoint: PANSS Total Score | |||||
Study Number | Treatment Group | N | Mean Baseline Score (SD) | LS Mean Change from Baseline (SE) | Placebo-subtracted Difference (95% CI) |
| 1 | CAPLYTA (42 mg once daily)• | 84 | 88.1 (11.0) | -13.2 (1.7) | -5.8 (-10.5, -1.1) a |
| Placebo | 85 | 86.3 (13.1) | -7.4 (1.7) | -- | |
| 2 | CAPLYTA (42 mg once daily)• | 150 | 90.0 (9.6) | -14.5 (1.3) | -4.2 (-7.8, -0.6) |
| Placebo | 150 | 89.0 (10.3) | -10.3 (1.3) | -- | |
The PANSS total score may range from 30 to 210; higher scores reflect greater symptom severity. SD: standard deviation; SE: standard error; LS Mean: least squares mean; CI: unadjusted confidence interval. a Difference (CAPLYTA minus placebo) in LS mean change from baseline not adjusted for sample size increase after unblinded interim analysis. • CAPLYTA statistically significantly superior to placebo.
Figure 1: Change from Baseline in PANSS Total Score by Time (Weeks) in Adult Patients with Schizophrenia in Study 2 .
Depressive Episodes Associated with Bipolar I or II Disorder (Bipolar Depression)
Clinical Study of CAPLYTA Monotherapy for Bipolar I or II Disorder
Study 3 Design: The efficacy of CAPLYTA, as monotherapy, for the treatment of depressive episodes associated with bipolar I or II disorder (bipolar depression) in adults was established in a 6-week, randomized, double-blind, placebo-controlled, multi-center study in adult patients who met DSM-5 criteria for depressive episodes associated with bipolar I or bipolar II disorder (Study 3; NCT03249376). The primary efficacy measure was the change from baseline in Montgomery-Asberg Depression Rating Scale (MADRS) total score at Week 6. The MADRS is a 10-item clinician-rated scale with total scores ranging from 0 (no depressive features) to 60 (maximum score). The secondary endpoint was the change from baseline in Clinical Global Impression-Bipolar-Severity of Illness scale (CGI-BP-S) total score at Week 6. The CGI-BP-S total score is a clinician-rated scale that measures the patient’s current illness state on a 21-point scale that assesses depression, mania, and overall illness, where a higher score is associated with greater illness severity.
A total of 381 patients were randomized to receive oral CAPLYTA 42 mg or placebo once daily.
Study 3 Baseline Demographic Characteristics: Demographic and baseline characteristics were similar for the CAPLYTA and placebo groups. Median age was 45 (range 18 to 72). 58% were female, 91% were White, and 8% were Black.
Study 3 Efficacy Results: Compared to the placebo group, patients in the CAPLYTA 42 mg group showed a statistically significant improvement from baseline to Day 43 in the MADRS total score and CGI-BP-S total score. The results of Study 3 are shown in Table 10.
Examination of subgroups by age, sex, and race did not suggest differences in response in the study.
Clinical Study of CAPLYTA Adjunctive Therapy with Lithium or Valproate for Bipolar I or II Disorder
Study 4 Design: The efficacy of CAPLYTA, as adjunctive therapy with lithium or valproate, for the treatment of depressive episodes associated with bipolar I or II disorder in adults was established in a 6-week, randomized, double-blind, placebo-controlled, multi-center study in adult patients who met DSM-5 criteria for depressive episodes associated with bipolar I or bipolar II disorder (Study 4; NCT02600507). The primary efficacy measure was the change from baseline in MADRS total score at Week 6. The secondary endpoint was the change from baseline in CGI-BP-S depression score at Week 6. The CGI-BP-S depression score is a clinician-rated scale that measures the patient’s current illness state on a 7-point scale, where a higher score is associated with greater illness severity.
A total of 529 patients were randomized to receive oral CAPLYTA 28 mg (two-thirds the recommended daily dosage), CAPLYTA 42 mg, or placebo once daily. Patients in all treatment groups continued treatment with lithium or valproate.
Study 4 Baseline Demographic Characteristics: Demographic and baseline characteristics were similar for the CAPLYTA and placebo groups. Median age was 46 (range 18 to 74). 58% were female, 88% were White, and 11% were Black.
Study 4 Efficacy Results: Compared to the placebo + lithium or valproate group, patients in the CAPLYTA 42 mg + lithium or valproate group showed a statistically significant improvement from baseline to Day 43 in the MADRS total score and CGI-BP-S depression score. The treatment effect in the CAPLYTA 28 mg + lithium or valproate group (vs. placebo + lithium or valproate group) was not statistically significant. The results of Study 4 are shown in Table 10.
Examination of subgroups by age, sex, and race did not suggest differences in response in the study.
| Primary Efficacy Endpoint: MADRS Total Score | ||||||
Study Number | Treatment Group | N | Mean Baseline Score (SD) | LS Mean Change from Baseline (SE) | Placebo-subtracted Difference a (95% CI) | |
| Monotherapy | ||||||
| 3 | CAPLYTA (42 mg) once daily• | 188 | 30.8 (4.9) | -16.7 (0.7) | -4.6 (-6.3, -2.8) | |
| Placebo | 188 | 30.3 (4.6) | -12.1 (0.7) | |||
| Adjunctive Therapy | ||||||
| 4 | CAPLYTA (42 mg) once daily• + lithium or valproate | 174 | 32.2 (5.0) | -16.9 (0.8) | -2.4 (-4.4, -0.4) | |
| Placebo + lithium or valproate | 174 | 32.1 (5.2) | -14.5 (0.8) | |||
The MADRS total score ranges from 0 to 60; higher scores reflect greater symptom severity SD: standard deviation; SE: standard error; LS Mean: least squares mean; CI: confidence interval a Difference (CAPLYTA minus placebo) in LS mean change from baseline • CAPLYTA statistically significantly superior to placebo.
Figure 2. Change from Baseline in MADRS Total Score by Visits (Study 3) in Adult Patients with Depressive Episodes Associated with Bipolar I or II Disorder (Monotherapy)
14.3 Adjunctive Treatment of Major Depressive Disorder in Adults
Studies 5 and 6 Designs: The effectiveness of CAPLYTA, as adjunctive therapy with antidepressants, for the treatment of major depressive disorder (MDD) in adults was established in two 6-week, randomized, double-blind, placebo-controlled, multi-center trials in adult patients who met DSM-5 criteria for MDD, with or without symptoms of anxiety who had inadequate response to one to two courses of prior antidepressant therapy (ADT) (Study 5 NCT04985942 and Study 6 NCT05061706). Inadequate response to ADT was defined as having less than 50% improvement after at least six weeks of treatment with selective serotonin or serotonin norepinephrine reuptake inhibitors or bupropion at the minimum effective ADT dosage or greater. Patients were randomized to receive oral CAPLYTA 42 mg or placebo once daily and all patients continued their background ADT.
Studies 5 and 6 Endpoints: In Study 5 and 6, the primary endpoint was change from baseline to Week 6 in the Montgomery-Asberg Depression Rating Scale (MADRS) total score [see Clinical Studies (14.2) ] in the CAPLYTA + ADT group compared to the placebo + ADT group. The key secondary endpoint was the change from baseline to Week 6 in the Clinical Global Impression Scale-Severity (CGI-S) score. The CGI-S is a validated clinician-rated scale that measures the patient’s current illness state on a 1 (normal, not at all ill) to 7-point (extremely ill) scale.
Studies 5 and 6 Baseline Demographics:
- In Study 5, a total of 485 patients were randomized to receive CAPLYTA 42 mg + ADT or placebo + ADT. Demographic and baseline characteristics were similar for the CAPLYTA and placebo groups. Median age was 46 (range 18 to 65). 66% were female, 77% were White, 15% were Asian, and 7% were Black.
- In Study 6, a total of 480 patients were randomized to receive CAPLYTA 42 mg + ADT or placebo + ADT. Demographic and baseline characteristics were similar for the CAPLYTA and placebo groups. Median age was 48 (range 18 to 65). 70% were female, 95% were White.
Studies 5 and 6 Efficacy Results: In Studies 5 and 6, patients randomized to CAPLYTA 42 mg + ADT showed a statistically significant improvement from baseline to day 43 in the MADRS total score and CGI-S score compared to the placebo + ADT group. The results of Study 5 and Study 6 are shown in Table 11.
Examination of subgroups by age, sex, race, and ADT class did not suggest differences in response in these studies.
| Primary Efficacy Endpoint: MADRS Total Score | |||||
| Study Number | Treatment Group | N | Mean Baseline Score (SD) | LS Mean Change from Baseline (SE) | Placebo-subtracted Difference a (95% CI) |
| 5 | CAPLYTA (42 mg) + ADT• | 239 | 30.4 (3.75) | -14.7 (0.54) | -4.9 (-6.38, -3.44) |
| Placebo + ADT | 242 | 30.1 (3.50) | -9.8 (0.53) | ||
| 6 | CAPLYTA (42 mg) + ADT• | 232 | 30.8 (3.88) | -14.7 (0.56) | -4.5 (-6.03, -3.02) |
| Placebo + ADT | 237 | 31.5 (3.97) | -10.2 (0.54) | ||
The MADRS total score ranges from 0 to 60; higher scores reflect greater symptom severity SD: standard deviation; SE: standard error; LS Mean: least squares mean; CI: confidence interval a Difference (CAPLYTA + ADT minus placebo + ADT) in LS mean change from baseline • CAPLYTA + ADT statistically significantly superior to placebo + ADT.
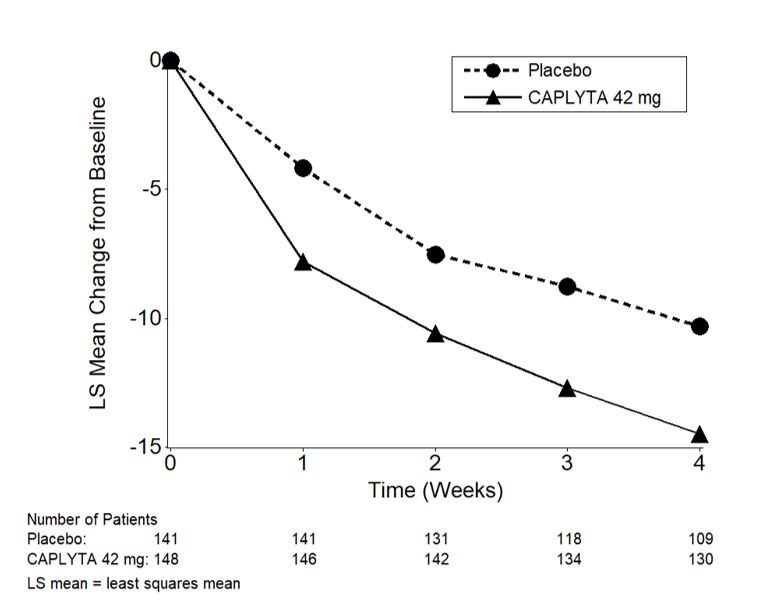
The change from baseline in MADRS total score over time in adult patients who received adjunctive treatment for MDD in Study 5 is displayed in Figure 3.
Figure 3. Change from Baseline in MADRS Total Score by Time (Weeks) in Patients with MDD (Adjunctive Treatment) in Study 5
HOW SUPPLIED/ STORAGE AND HANDLING
CAPLYTA (lumateperone) capsules are supplied as follows:
| Capsule Strength | Capsule Color | Imprint Codes | Package Configuration | NDC Code | |
| 42 mg | Blue cap and opaque white body | ITI-007 42 mg | Bottle of 30 | 72060-142-40 | |
| 21 mg | Opaque white cap and body | ITI-007 21 mg | Bottle of 30 | 72060-121-40 | |
| 10.5 mg | Opaque light pink cap and body | ITI-007 10.5 mg | Bottle of 30 | 72060-110-40 | |
Store at controlled room temperature 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature] .
Mechanism of Action
The mechanism of action of lumateperone for the treatment of schizophrenia in adults, for the treatment of depressive episodes associated with bipolar depression (as monotherapy or as adjunctive therapy with lithium or valproate), and as adjunctive therapy with antidepressants for the treatment of MDD is unknown. However, the mechanism of action of lumateperone for these uses could be mediated through a combination of antagonist activity at central serotonin 5-HT 2A receptors, and partial agonist activity at central dopamine D 2 receptors.