Get your patient on Cosentyx (Secukinumab)
Cosentyx prior authorization resources
Most recent state uniform prior authorization forms
Brand Resources
Cosentyx patient education
Patient toolkit
Patient Support Program
Dosage & administration
2 DOSAGE AND ADMINISTRATION
2.1 Testing and Procedures Prior to Treatment Initiation
Perform the following evaluations prior to COSENTYX initiation:
- Evaluate for active or latent tuberculosis (TB). COSENTYX initiation is not recommended in patients with active TB infection. Initiate treatment of latent TB prior to initiation of COSENTYX [see Warnings and Precautions (5.3)] .
- Complete all age-appropriate vaccinations as recommended by current immunization guidelines prior to initiating treatment with COSENTYX [see Warnings and Precautions (5.7)] .
2.2 Important Administration Instructions
- COSENTYX is for use under the guidance and supervision of a healthcare provider.
- UnoReady pens, Sensoready pens, and prefilled syringes are for subcutaneous use only.
- Solution in vials is for intravenous use in adult patients only.
Important Subcutaneous Administration Instructions
Adult patients may self-administer COSENTYX or be injected by a caregiver after proper training in subcutaneous injection technique.
Pediatric patients should not self-administer COSENTYX. An adult caregiver should prepare and inject COSENTYX after proper training in subcutaneous injection technique.
Administer each subcutaneous injection at a different anatomic location (such as upper arms, thighs, or any quadrant of abdomen) than the previous injection, and not into areas where the skin is tender, bruised, erythematous, indurated, or affected by psoriasis. Administration of subcutaneous COSENTYX in the upper, outer arm may be performed by a caregiver or healthcare provider.
The COSENTYX “Instructions for Use” for each presentation and strength contains more detailed instructions on the preparation and administration of COSENTYX for patients and caregivers [see Instructions for Use] .
Important Intravenous Infusion Instructions
Intravenous infusion is only for use by a healthcare professional in a healthcare setting. Prepare COSENTYX intravenous infusion by diluting COSENTYX injection in vial(s) and administering based on patient body weight [see Dosage and Administration (2.11)] . Intravenous infusion may be administered only in adults with PsA, AS, and nr-axSPA.
2.3 Recommended Dosage in Plaque Psoriasis
Recommended Subcutaneous Dosage in Adults with PsO
The recommended dosage in adults with PsO is 300 mg by subcutaneous injection at Weeks 0, 1, 2, 3, and 4 and every 4 weeks thereafter. Each 300 mg dosage is given as one subcutaneous injection of 300 mg or as two subcutaneous injections of 150 mg.
For some patients, a dosage of 150 mg by subcutaneous injection at Weeks 0, 1, 2, 3, and 4 and every 4 weeks thereafter may be acceptable.
Recommended Subcutaneous Dosage in Pediatric Patients 6 Years of Age and Older with PsO
The recommended weight-based dosage in pediatric patients 6 years of age and older with PsO is administered by subcutaneous injection at Weeks 0, 1, 2, 3, and 4 and every 4 weeks thereafter.
- For patients < 50 kg (at the time of dosing), the recommended dose is 75 mg.
- For patients ≥ 50 kg (at the time of dosing), the recommended dose is 150 mg.
2.4 Recommended Dosage in Adults with Psoriatic Arthritis
COSENTYX may be administered with or without methotrexate.
Recommended Subcutaneous Dosage
For adult patients with PsA and with coexistent moderate to severe PsO, use the dosage and administration recommendations for adults with PsO [see Dosage and Administration (2.3)] .
For other adult patients with PsA, administer COSENTYX with or without a loading dosage by subcutaneous injection.
The recommended dosage in adults with PsA:
- With a loading dosage is 150 mg at Weeks 0, 1, 2, 3, and 4 and every 4 weeks thereafter.
- Without a loading dosage is 150 mg every 4 weeks.
- If a patient continues to have active PsA, consider increasing the dosage to 300 mg by subcutaneous injection every 4 weeks. Each 300 mg dosage is given as one subcutaneous injection of 300 mg or as two subcutaneous injections of 150 mg.
Recommended Intravenous Dosage
COSENTYX injection for intravenous use (solution in vials) requires dilution prior to intravenous administration. The recommended intravenous dosage regimen in adults with PsA:
- With a loading dosage is 6 mg/kg loading dose given at Week 0, followed by 1.75 mg/kg every 4 weeks thereafter (maintenance dosage).
- Without a loading dosage is 1.75 mg/kg every 4 weeks.
Administer as an intravenous infusion over a period of 30 minutes [see Dosage and Administration (2.11)] .
Total doses exceeding 300 mg per infusion are not recommended for the 1.75 mg/kg maintenance dose in adults with PsA [see Dosage and Administration (2.11)] .
2.5 Recommended Dosage in Pediatric Patients 2 Years of Age and Older with Juvenile Psoriatic Arthritis
COSENTYX may be administered with or without methotrexate.
The recommended weight-based subcutaneous dosage in pediatric patients 2 years of age and older with PsA at Weeks 0, 1, 2, 3, and 4 and every 4 weeks thereafter is as follows:
- For patients ≥ 15 kg and < 50 kg, the recommended dose is 75 mg.
- For patients ≥ 50 kg, the recommended dose is 150 mg.
2.6 Recommended Dosage in Adults with Ankylosing Spondylitis
Recommended Subcutaneous Dosage
Administer COSENTYX with or without a loading dosage by subcutaneous injection in adult patients with active AS. The recommended dosage:
- With a loading dosage is 150 mg at Weeks 0, 1, 2, 3, and 4 and every 4 weeks thereafter.
- Without a loading dosage is 150 mg every 4 weeks.
- If a patient continues to have active AS, consider increasing the dosage to 300 mg every 4 weeks by subcutaneous injection. Each 300 mg dosage is given as one subcutaneous injection of 300 mg or as two subcutaneous injections of 150 mg.
Recommended Intravenous Dosage
COSENTYX injection for intravenous use (solution in vials) requires dilution prior to intravenous administration. The recommended intravenous dosage regimen in adult patients with active AS:
- With a loading dosage is 6 mg/kg loading dose given at Week 0, followed by 1.75 mg/kg every 4 weeks thereafter (maintenance dosage).
- Without a loading dosage is 1.75 mg/kg every 4 weeks.
Administer as an intravenous infusion over a period of 30 minutes [see Dosage and Administration (2.11)] .
Total doses exceeding 300 mg per infusion are not recommended for the 1.75 mg/kg maintenance dose in patients with AS [see Dosage and Administration (2.11)] .
2.7 Recommended Dosage in Adults with Non-Radiographic Axial Spondyloarthritis
Recommended Subcutaneous Dosage
Administer COSENTYX with or without a loading dosage by subcutaneous injection in adult patients with active nr-axSpA.
The recommended dosage:
- With a loading dosage is 150 mg at Weeks 0, 1, 2, 3, and 4 and every 4 weeks thereafter.
- Without a loading dosage is 150 mg every 4 weeks.
Recommended Intravenous Dosage
COSENTYX injection for intravenous use (solution in vials) requires dilution prior to intravenous administration. The recommended intravenous dosage regimen in adult patients with active nr-axSpA:
- With a loading dosage is 6 mg/kg loading dose given at Week 0, followed by 1.75 mg/kg every 4 weeks thereafter (maintenance dosage).
- Without a loading dosage is 1.75 mg/kg every 4 weeks.
Administer as an intravenous infusion over a period of 30 minutes [see Dosage and Administration (2.11)] .
Total doses exceeding 300 mg per infusion are not recommended for the 1.75 mg/kg maintenance dose in patients with nr-axSpA [see Dosage and Administration (2.11)] .
2.8 Recommended Dosage in Enthesitis-Related Arthritis
COSENTYX may only be administered as a subcutaneous injection in pediatric patients aged 4 years and older with active ERA.
The recommended weight-based dosage in pediatric patients 4 years of age and older with ERA is administered by subcutaneous injection at Weeks 0, 1, 2, 3, and 4 and every 4 weeks thereafter:
- For patients ≥ 15 kg and < 50 kg, the recommended dose is 75 mg.
- For patients ≥ 50 kg, the recommended dose is 150 mg.
2.9 Recommended Dosage in Hidradenitis Suppurativa
The recommended dose in adult patients with moderate to severe HS is 300 mg by subcutaneous injection at Weeks 0, 1, 2, 3 and 4 and every 4 weeks thereafter.
If a patient does not adequately respond, consider increasing the dosage to 300 mg every 2 weeks. Each 300 mg dosage is given as one subcutaneous injection of 300 mg or as two subcutaneous injections of 150 mg.
2.10 Preparation for Use of COSENTYX UnoReady Pen, Sensoready Pen and Prefilled Syringes
COSENTYX UnoReady pens, Sensoready pens and prefilled syringes are for subcutaneous injection only.
Before subcutaneous injection, remove COSENTYX from the refrigerator and allow COSENTYX to reach room temperature (15 to 30 minutes for the Sensoready pen, the 150 mg/mL and 75 mg/0.5 mL prefilled syringes; 30 to 45 minutes for the UnoReady pen and the 300 mg/2 mL prefilled syringe) without removing the needle cap.
The removable cap of the COSENTYX 150 mg/mL Sensoready pen and the COSENTYX prefilled syringes (150 mg/mL, 75 mg/0.5 mL) contain natural rubber latex and should not be handled by latex-sensitive individuals [see Warnings and Precautions (5.6)] .
Inspect COSENTYX visually for particulate matter and discoloration prior to administration. COSENTYX injection is a clear to slightly opalescent, colorless to slightly yellow solution. Do not use if the liquid contains visible particles, is discolored or cloudy. Discard any unused product.
2.11 Preparation and Administration of COSENTYX for Intravenous Use
COSENTYX (for intravenous use) must be diluted prior to infusion. Using aseptic technique, prepare COSENTYX (for intravenous use) as follows:
Step 1. Volume Calculation
- Calculate the total volume of COSENTYX for intravenous use solution (in mL) required based on the patient’s actual body weight as follows:
- Loading dose (6 mg/kg) is 0.24 mL/kg
- Maintenance dose (1.75 mg/kg) is 0.07 mL/kg
- Use the number of vials based on total volume needed (one vial contains 5 mL of COSENTYX solution).
Step 2. Dilution
- Parenteral drug product should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use if particulates or discolorations are noted.
- Follow Table 1 for recommended infusion bag size based on patient's body weight.
| • If a 50 mL infusion bag is unavailable, then use a 100 mL infusion bag and withdraw and discard 50 mL of saline using aseptic technique and continue to follow the preparation and administration steps. | ||
| Body weight at time of dosing | For the loading dose (6 mg/kg) recommended infusion bag | For maintenance dose (1.75 mg/kg) recommended infusion bag |
| Greater than 52 kg | 100 mL | 100 mL |
| Less than or equal to 52 kg | 100 mL | 50 mL • |
- From the infusion bag, withdraw and discard a volume of 0.9% Sodium Chloride Injection, USP, equal to the calculated volume of the COSENTYX solution required for the patient’s dose [see Dosage and Administration (2.4, 2.6, 2.7)] .
- From the vial(s), withdraw the calculated volume (mL) of COSENTYX solution and add slowly into the 0.9% Sodium Chloride Injection, USP infusion bag. To mix the solution, gently invert the bag to avoid foaming. Do not shake.
- Discard unused COSENTYX product in vials because it does not contain preservatives.
Allow the diluted COSENTYX solution for infusion to warm to room temperature prior to the start of the intravenous infusion. Administer the diluted COSENTYX solution for infusion as soon as possible. If not administered immediately, store the diluted solution either:
- At room temperature 20ºC to 25ºC (68ºF to 77ºF) for no more than 4.5 hours from the start of the preparation (piercing the first vial) to the completion of infusion.
- Under refrigeration at 2°C to 8°C (36°F to 46°F) for no more than 24 hours, from the start of the time of the preparation (piercing the first vial) to the completion of infusion. This time includes the refrigeration of the diluted solution and the time to allow the diluted solution to warm to room temperature. Protect the diluted solution from light during storage under refrigeration.
Step 3. Administration
- Use only an infusion set with an in-line, sterile, non-pyrogenic, low protein-binding filter (pore size 0.2 micrometer).
- Administer the infusion at a flow rate of about 3.3 mL/minute for a 100 mL bag or 1.7 mL/min for a 50 mL bag (total administration time: 30 minutes).
- When administration is complete, flush the line with 0.9% Sodium Chloride Injection, USP to guarantee that all the COSENTYX solution for infusion in the line has been administered.
- Do not infuse COSENTYX concomitantly in the same intravenous line with other drugs. No physical or biochemical compatibility studies have been conducted to evaluate the IV coadministration of COSENTYX with other drugs.

By using PrescriberAI, you agree to the AI Terms of Use.
Cosentyx prescribing information
1 INDICATIONS AND USAGE
1.1 Plaque Psoriasis
COSENTYX ® is indicated for the treatment of moderate to severe plaque psoriasis (PsO) in patients 6 years and older who are candidates for systemic therapy or phototherapy.
1.2 Psoriatic Arthritis
COSENTYX is indicated for the treatment of active psoriatic arthritis (PsA) in patients 2 years of age and older.
1.3 Ankylosing Spondylitis
COSENTYX is indicated for the treatment of adult patients with active ankylosing spondylitis (AS).
1.4 Non-Radiographic Axial Spondyloarthritis
COSENTYX is indicated for the treatment of adult patients with active non-radiographic axial spondyloarthritis (nr-axSpA) with objective signs of inflammation.
1.5 Enthesitis-Related Arthritis
COSENTYX is indicated for the treatment of active enthesitis-related arthritis (ERA) in pediatric patients 4 years of age and older.
1.6 Hidradenitis Suppurativa
COSENTYX is indicated for the treatment of adult patients with moderate to severe hidradenitis suppurativa (HS).
2 DOSAGE AND ADMINISTRATION
2.1 Testing and Procedures Prior to Treatment Initiation
Perform the following evaluations prior to COSENTYX initiation:
- Evaluate for active or latent tuberculosis (TB). COSENTYX initiation is not recommended in patients with active TB infection. Initiate treatment of latent TB prior to initiation of COSENTYX [see Warnings and Precautions (5.3)] .
- Complete all age-appropriate vaccinations as recommended by current immunization guidelines prior to initiating treatment with COSENTYX [see Warnings and Precautions (5.7)] .
2.2 Important Administration Instructions
- COSENTYX is for use under the guidance and supervision of a healthcare provider.
- UnoReady pens, Sensoready pens, and prefilled syringes are for subcutaneous use only.
- Solution in vials is for intravenous use in adult patients only.
Important Subcutaneous Administration Instructions
Adult patients may self-administer COSENTYX or be injected by a caregiver after proper training in subcutaneous injection technique.
Pediatric patients should not self-administer COSENTYX. An adult caregiver should prepare and inject COSENTYX after proper training in subcutaneous injection technique.
Administer each subcutaneous injection at a different anatomic location (such as upper arms, thighs, or any quadrant of abdomen) than the previous injection, and not into areas where the skin is tender, bruised, erythematous, indurated, or affected by psoriasis. Administration of subcutaneous COSENTYX in the upper, outer arm may be performed by a caregiver or healthcare provider.
The COSENTYX “Instructions for Use” for each presentation and strength contains more detailed instructions on the preparation and administration of COSENTYX for patients and caregivers [see Instructions for Use] .
Important Intravenous Infusion Instructions
Intravenous infusion is only for use by a healthcare professional in a healthcare setting. Prepare COSENTYX intravenous infusion by diluting COSENTYX injection in vial(s) and administering based on patient body weight [see Dosage and Administration (2.11)] . Intravenous infusion may be administered only in adults with PsA, AS, and nr-axSPA.
2.3 Recommended Dosage in Plaque Psoriasis
Recommended Subcutaneous Dosage in Adults with PsO
The recommended dosage in adults with PsO is 300 mg by subcutaneous injection at Weeks 0, 1, 2, 3, and 4 and every 4 weeks thereafter. Each 300 mg dosage is given as one subcutaneous injection of 300 mg or as two subcutaneous injections of 150 mg.
For some patients, a dosage of 150 mg by subcutaneous injection at Weeks 0, 1, 2, 3, and 4 and every 4 weeks thereafter may be acceptable.
Recommended Subcutaneous Dosage in Pediatric Patients 6 Years of Age and Older with PsO
The recommended weight-based dosage in pediatric patients 6 years of age and older with PsO is administered by subcutaneous injection at Weeks 0, 1, 2, 3, and 4 and every 4 weeks thereafter.
- For patients < 50 kg (at the time of dosing), the recommended dose is 75 mg.
- For patients ≥ 50 kg (at the time of dosing), the recommended dose is 150 mg.
2.4 Recommended Dosage in Adults with Psoriatic Arthritis
COSENTYX may be administered with or without methotrexate.
Recommended Subcutaneous Dosage
For adult patients with PsA and with coexistent moderate to severe PsO, use the dosage and administration recommendations for adults with PsO [see Dosage and Administration (2.3)] .
For other adult patients with PsA, administer COSENTYX with or without a loading dosage by subcutaneous injection.
The recommended dosage in adults with PsA:
- With a loading dosage is 150 mg at Weeks 0, 1, 2, 3, and 4 and every 4 weeks thereafter.
- Without a loading dosage is 150 mg every 4 weeks.
- If a patient continues to have active PsA, consider increasing the dosage to 300 mg by subcutaneous injection every 4 weeks. Each 300 mg dosage is given as one subcutaneous injection of 300 mg or as two subcutaneous injections of 150 mg.
Recommended Intravenous Dosage
COSENTYX injection for intravenous use (solution in vials) requires dilution prior to intravenous administration. The recommended intravenous dosage regimen in adults with PsA:
- With a loading dosage is 6 mg/kg loading dose given at Week 0, followed by 1.75 mg/kg every 4 weeks thereafter (maintenance dosage).
- Without a loading dosage is 1.75 mg/kg every 4 weeks.
Administer as an intravenous infusion over a period of 30 minutes [see Dosage and Administration (2.11)] .
Total doses exceeding 300 mg per infusion are not recommended for the 1.75 mg/kg maintenance dose in adults with PsA [see Dosage and Administration (2.11)] .
2.5 Recommended Dosage in Pediatric Patients 2 Years of Age and Older with Juvenile Psoriatic Arthritis
COSENTYX may be administered with or without methotrexate.
The recommended weight-based subcutaneous dosage in pediatric patients 2 years of age and older with PsA at Weeks 0, 1, 2, 3, and 4 and every 4 weeks thereafter is as follows:
- For patients ≥ 15 kg and < 50 kg, the recommended dose is 75 mg.
- For patients ≥ 50 kg, the recommended dose is 150 mg.
2.6 Recommended Dosage in Adults with Ankylosing Spondylitis
Recommended Subcutaneous Dosage
Administer COSENTYX with or without a loading dosage by subcutaneous injection in adult patients with active AS. The recommended dosage:
- With a loading dosage is 150 mg at Weeks 0, 1, 2, 3, and 4 and every 4 weeks thereafter.
- Without a loading dosage is 150 mg every 4 weeks.
- If a patient continues to have active AS, consider increasing the dosage to 300 mg every 4 weeks by subcutaneous injection. Each 300 mg dosage is given as one subcutaneous injection of 300 mg or as two subcutaneous injections of 150 mg.
Recommended Intravenous Dosage
COSENTYX injection for intravenous use (solution in vials) requires dilution prior to intravenous administration. The recommended intravenous dosage regimen in adult patients with active AS:
- With a loading dosage is 6 mg/kg loading dose given at Week 0, followed by 1.75 mg/kg every 4 weeks thereafter (maintenance dosage).
- Without a loading dosage is 1.75 mg/kg every 4 weeks.
Administer as an intravenous infusion over a period of 30 minutes [see Dosage and Administration (2.11)] .
Total doses exceeding 300 mg per infusion are not recommended for the 1.75 mg/kg maintenance dose in patients with AS [see Dosage and Administration (2.11)] .
2.7 Recommended Dosage in Adults with Non-Radiographic Axial Spondyloarthritis
Recommended Subcutaneous Dosage
Administer COSENTYX with or without a loading dosage by subcutaneous injection in adult patients with active nr-axSpA.
The recommended dosage:
- With a loading dosage is 150 mg at Weeks 0, 1, 2, 3, and 4 and every 4 weeks thereafter.
- Without a loading dosage is 150 mg every 4 weeks.
Recommended Intravenous Dosage
COSENTYX injection for intravenous use (solution in vials) requires dilution prior to intravenous administration. The recommended intravenous dosage regimen in adult patients with active nr-axSpA:
- With a loading dosage is 6 mg/kg loading dose given at Week 0, followed by 1.75 mg/kg every 4 weeks thereafter (maintenance dosage).
- Without a loading dosage is 1.75 mg/kg every 4 weeks.
Administer as an intravenous infusion over a period of 30 minutes [see Dosage and Administration (2.11)] .
Total doses exceeding 300 mg per infusion are not recommended for the 1.75 mg/kg maintenance dose in patients with nr-axSpA [see Dosage and Administration (2.11)] .
2.8 Recommended Dosage in Enthesitis-Related Arthritis
COSENTYX may only be administered as a subcutaneous injection in pediatric patients aged 4 years and older with active ERA.
The recommended weight-based dosage in pediatric patients 4 years of age and older with ERA is administered by subcutaneous injection at Weeks 0, 1, 2, 3, and 4 and every 4 weeks thereafter:
- For patients ≥ 15 kg and < 50 kg, the recommended dose is 75 mg.
- For patients ≥ 50 kg, the recommended dose is 150 mg.
2.9 Recommended Dosage in Hidradenitis Suppurativa
The recommended dose in adult patients with moderate to severe HS is 300 mg by subcutaneous injection at Weeks 0, 1, 2, 3 and 4 and every 4 weeks thereafter.
If a patient does not adequately respond, consider increasing the dosage to 300 mg every 2 weeks. Each 300 mg dosage is given as one subcutaneous injection of 300 mg or as two subcutaneous injections of 150 mg.
2.10 Preparation for Use of COSENTYX UnoReady Pen, Sensoready Pen and Prefilled Syringes
COSENTYX UnoReady pens, Sensoready pens and prefilled syringes are for subcutaneous injection only.
Before subcutaneous injection, remove COSENTYX from the refrigerator and allow COSENTYX to reach room temperature (15 to 30 minutes for the Sensoready pen, the 150 mg/mL and 75 mg/0.5 mL prefilled syringes; 30 to 45 minutes for the UnoReady pen and the 300 mg/2 mL prefilled syringe) without removing the needle cap.
The removable cap of the COSENTYX 150 mg/mL Sensoready pen and the COSENTYX prefilled syringes (150 mg/mL, 75 mg/0.5 mL) contain natural rubber latex and should not be handled by latex-sensitive individuals [see Warnings and Precautions (5.6)] .
Inspect COSENTYX visually for particulate matter and discoloration prior to administration. COSENTYX injection is a clear to slightly opalescent, colorless to slightly yellow solution. Do not use if the liquid contains visible particles, is discolored or cloudy. Discard any unused product.
2.11 Preparation and Administration of COSENTYX for Intravenous Use
COSENTYX (for intravenous use) must be diluted prior to infusion. Using aseptic technique, prepare COSENTYX (for intravenous use) as follows:
Step 1. Volume Calculation
- Calculate the total volume of COSENTYX for intravenous use solution (in mL) required based on the patient’s actual body weight as follows:
- Loading dose (6 mg/kg) is 0.24 mL/kg
- Maintenance dose (1.75 mg/kg) is 0.07 mL/kg
- Use the number of vials based on total volume needed (one vial contains 5 mL of COSENTYX solution).
Step 2. Dilution
- Parenteral drug product should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use if particulates or discolorations are noted.
- Follow Table 1 for recommended infusion bag size based on patient's body weight.
| • If a 50 mL infusion bag is unavailable, then use a 100 mL infusion bag and withdraw and discard 50 mL of saline using aseptic technique and continue to follow the preparation and administration steps. | ||
| Body weight at time of dosing | For the loading dose (6 mg/kg) recommended infusion bag | For maintenance dose (1.75 mg/kg) recommended infusion bag |
| Greater than 52 kg | 100 mL | 100 mL |
| Less than or equal to 52 kg | 100 mL | 50 mL • |
- From the infusion bag, withdraw and discard a volume of 0.9% Sodium Chloride Injection, USP, equal to the calculated volume of the COSENTYX solution required for the patient’s dose [see Dosage and Administration (2.4, 2.6, 2.7)] .
- From the vial(s), withdraw the calculated volume (mL) of COSENTYX solution and add slowly into the 0.9% Sodium Chloride Injection, USP infusion bag. To mix the solution, gently invert the bag to avoid foaming. Do not shake.
- Discard unused COSENTYX product in vials because it does not contain preservatives.
Allow the diluted COSENTYX solution for infusion to warm to room temperature prior to the start of the intravenous infusion. Administer the diluted COSENTYX solution for infusion as soon as possible. If not administered immediately, store the diluted solution either:
- At room temperature 20ºC to 25ºC (68ºF to 77ºF) for no more than 4.5 hours from the start of the preparation (piercing the first vial) to the completion of infusion.
- Under refrigeration at 2°C to 8°C (36°F to 46°F) for no more than 24 hours, from the start of the time of the preparation (piercing the first vial) to the completion of infusion. This time includes the refrigeration of the diluted solution and the time to allow the diluted solution to warm to room temperature. Protect the diluted solution from light during storage under refrigeration.
Step 3. Administration
- Use only an infusion set with an in-line, sterile, non-pyrogenic, low protein-binding filter (pore size 0.2 micrometer).
- Administer the infusion at a flow rate of about 3.3 mL/minute for a 100 mL bag or 1.7 mL/min for a 50 mL bag (total administration time: 30 minutes).
- When administration is complete, flush the line with 0.9% Sodium Chloride Injection, USP to guarantee that all the COSENTYX solution for infusion in the line has been administered.
- Do not infuse COSENTYX concomitantly in the same intravenous line with other drugs. No physical or biochemical compatibility studies have been conducted to evaluate the IV coadministration of COSENTYX with other drugs.
3 DOSAGE FORMS AND STRENGTHS
Injection for subcutaneous use:
- 300 mg/2 mL as a clear to opalescent, colorless to slightly yellowish solution in a single-dose UnoReady pen
- 300 mg/2 mL as a clear to opalescent, colorless to slightly yellowish solution in a single-dose prefilled syringe
- 150 mg/mL as a clear to opalescent, colorless to slightly yellowish solution in a single-dose Sensoready pen
- 150 mg/mL as a clear to opalescent, colorless to slightly yellowish solution in a single-dose prefilled syringe
- 75 mg/0.5 mL as a clear to opalescent, colorless to slightly yellowish solution in a single-dose prefilled syringe (for pediatric patients less than 50 kg)
Injection for intravenous use:
- 125 mg/5 mL as a clear to opalescent, colorless to slightly yellowish solution in a single-dose vial for dilution prior to intravenous infusion (for healthcare professional use only).
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Limited available human data with COSENTYX use in pregnant women are insufficient to inform a drug-associated risk of adverse developmental outcomes. In an embryo-fetal development study, no adverse developmental effects were observed in infants born to pregnant monkeys after subcutaneous administration of secukinumab during organogenesis at doses up to 30 times the maximum recommended human dose (MRHD) (see Data) .
The background risk of major birth defects and miscarriage for the indicated population is unknown; however, the background risk in the U.S. general population of major birth defects is 2% to 4% and of miscarriage is 15% to 20% of clinically recognized pregnancies.
Data
Animal Data
An embryo-fetal development study was performed in cynomolgus monkeys with secukinumab. No malformations or embryo-fetal toxicity were observed in fetuses from pregnant monkeys that were administered secukinumab weekly by the subcutaneous route during the period of organogenesis at doses up to 30 times the MRHD (on a mg/kg basis at a maternal dose of 150 mg/kg).
A pre- and post-natal development toxicity study was performed in mice with a murine analog of secukinumab. No treatment-related effects on functional, morphological, or immunological development were observed in fetuses from pregnant mice that were administered the murine analog of secukinumab on gestation days 6, 11, and 17 and on postpartum days 4, 10, and 16 at doses up to 150 mg/kg/dose.
8.2 Lactation
Risk Summary
It is not known whether secukinumab is excreted in human milk or absorbed systemically after ingestion. There are no data on the effects of COSENTYX on the breastfed child or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for COSENTYX and any potential adverse effects on the breastfed child from COSENTYX or from the underlying maternal condition.
8.4 Pediatric Use
Subcutaneous Administration
Pediatric Plaque Psoriasis
The safety and effectiveness of COSENTYX have been established for the treatment of moderate to severe PsO in pediatric patients aged 6 years and older who are candidates for systemic therapy or phototherapy [see Adverse Reactions (6.1) and Clinical Studies (14.2)] .
Safety and effectiveness of COSENTYX in pediatric patients with PsO below the age of 6 years have not been established.
Juvenile Psoriatic Arthritis
The safety and effectiveness of COSENTYX have been established for the treatment of active JPsA in pediatric patients aged 2 years and older who weigh 15 kg or more [see Adverse Reactions (6.1) and Clinical Studies (14.6)] .
The safety and effectiveness of COSENTYX in pediatric patients less than 2 years of age with JPsA or with a body weight less than 15 kg has not been established.
Enthesitis-Related Arthritis
The safety and effectiveness of COSENTYX for the treatment of active ERA in pediatric patients aged 4 years and older who weigh 15 kg or more has been established [see Adverse Reactions (6.1) and Clinical Studies (14.6)] .
The safety and effectiveness of COSENTYX in pediatric patients below the age of 4 years old or with body weight less than 15 kg have not been established.
Hidradenitis Suppurativa
The safety and effectiveness of COSENTYX in pediatric patients with HS have not been established.
Intravenous Administration
The safety and effectiveness of intravenous COSENTYX in pediatric patients have not been established.
8.5 Geriatric Use
Of the 3,430 PsO subjects exposed to subcutaneous COSENTYX in clinical trials, a total of 230 (7%) were 65 years of age or older, and 32 (1%) subjects were 75 years of age or older. Although no differences in safety or efficacy were observed between subjects 65 years of age or older and younger adult subjects, the number of subjects 65 years of age and older was not sufficient to determine whether they respond differently from younger adult subjects.
Of the 1,060 subjects with HS exposed to COSENTYX in clinical trials, a total of 14 (1.3%) were 65 years of age and older. Clinical trials in HS did not include sufficient numbers of subjects 65 years of age and older to determine whether they respond differently from younger adult subjects.
4 CONTRAINDICATIONS
COSENTYX is contraindicated in patients with a previous serious hypersensitivity reaction to secukinumab or to any of the excipients in COSENTYX. Cases of anaphylaxis and angioedema have been reported during treatment with COSENTYX [see Warnings and Precautions (5.2)] .
5 WARNINGS AND PRECAUTIONS
- Infections : Serious infections have occurred. Exercise caution when considering the use of COSENTYX in patients with a chronic infection or a history of recurrent infection. If a serious infection develops, discontinue COSENTYX until the infection resolves. (5.1 )
- Hypersensitivity Reactions : If an anaphylactic reaction or other serious allergic reaction occurs, discontinue COSENTYX immediately and initiate appropriate therapy. (5.2 )
- Tuberculosis (TB) : Prior to initiating treatment with COSENTYX, evaluate for TB. (5.3 )
- Inflammatory Bowel Disease (IBD) : Cases of IBD were observed in clinical trials. Exercise caution when prescribing COSENTYX to patients with IBD. (5.4 )
- Eczematous Eruptions : Cases of severe eczematous eruptions have occurred in patients receiving COSENTYX. (5.5 )
- Immunizations : Avoid use of live vaccines in patients treated with COSENTYX. (5.7 )
5.1 Infections
COSENTYX may increase the risk of infections. In clinical trials, a higher rate of infections was observed in COSENTYX treated subjects compared to placebo-treated subjects. In placebo-controlled clinical trials in subjects with moderate to severe PsO, higher rates of common infections, such as nasopharyngitis (11.4% versus 8.6%), upper respiratory tract infection (2.5% versus 0.7%) and mucocutaneous infections with candida (1.2% versus 0.3%) were observed in subjects treated with COSENTYX compared to placebo-treated subjects. A similar increase in risk of infection in subjects treated with COSENTYX was seen in placebo-controlled trials in subjects with PsA, AS and nr-axSpA. The incidence of some types of infections, including fungal infections, appeared to be dose-dependent in clinical trials [see Adverse Reactions (6.1)] .
In the postmarketing setting, serious bacterial, viral, and fungal opportunistic infections, and some fatal infections have been reported in patients receiving IL-17 inhibitors including COSENTYX. Cases of Hepatitis B virus reactivation have been reported [see Adverse Reactions (6.2)] .
Exercise caution when considering the use of COSENTYX in patients with a chronic infection or a history of recurrent infection. Instruct patients to seek medical advice if signs or symptoms suggestive of an infection occur. If a patient develops a serious infection, monitor the patient closely and discontinue COSENTYX until the infection resolves.
If signs of Hepatitis B virus reactivation occur, consult a hepatitis specialist. COSENTYX is not recommended for use in patients with active viral hepatitis.
5.2 Hypersensitivity Reactions
Serious hypersensitivity reactions including anaphylaxis, angioedema, and urticaria have been reported in COSENTYX treated subjects in clinical trials and in the post-marketing setting [see Adverse Reactions (6.1, 6.2)] . If an anaphylactic or other serious allergic reaction occurs, immediately discontinue administration of COSENTYX and initiate appropriate therapy [see Contraindications (4)] .
5.3 Pre-Treatment Evaluation for Tuberculosis
Evaluate patients for active or latent TB infection prior to initiating treatment with COSENTYX. Avoid administration of COSENTYX to patients with active TB infection. Initiate treatment of latent TB prior to administering COSENTYX. Consider anti-TB therapy prior to initiation of COSENTYX in patients with a past history of latent or active TB in whom an adequate course of treatment cannot be confirmed. Monitor patients closely for signs and symptoms of active TB during and after treatment.
In the postmarketing setting, cases were reported where patients with a history of latent tuberculosis (TB) who were treated with COSENTYX developed active TB.
5.4 Inflammatory Bowel Disease
Inflammatory Bowel Disease (IBD) exacerbations, in some cases serious and/or leading to discontinuation of COSENTYX, occurred in COSENTYX treated subjects during clinical trials in PsO, PsA, AS, nr-axSpA, and HS. In adult subjects with HS, the incidence of IBD was higher in subjects who received COSENTYX 300 mg every 2 weeks (Ulcerative Colitis [UC] 1 case, EAIR 0.2/100 subject-years; Crohn`s Disease [CD] 1 case, EAIR 0.2/100 subject-years) compared to subjects who received COSENTYX 300 mg every 4 weeks (IBD 1 case, EAIR 0.2/100 subject-years). In addition, new onset IBD cases occurred in subjects treated with COSENTYX in clinical trials. In an exploratory trial in 59 subjects with active Crohn’s disease [COSENTYX is not approved for the treatment of Crohn`s disease], there were trends toward greater disease activity and increased adverse reactions in subjects treated with COSENTYX as compared to placebo-treated subjects.
Exercise caution when prescribing COSENTYX to patients with IBD. Patients treated with COSENTYX should be monitored for signs and symptoms of IBD [see Adverse Reactions (6.1)] .
5.5 Eczematous Eruptions
In postmarketing reports, cases of severe eczematous eruptions, including atopic dermatitis-like eruptions, dyshidrotic eczema, and erythroderma, were reported in patients receiving COSENTYX; some cases resulted in hospitalization. The onset of eczematous eruptions was variable, ranging from days to months after the first dose of COSENTYX.
Treatment may need to be discontinued to resolve the eczematous eruption. Some patients were successfully treated for eczematous eruptions while continuing COSENTYX.
5.6 Risk of Hypersensitivity in Latex-Sensitive Individuals
The removable caps of the COSENTYX 150 mg/mL Sensoready pen and the COSENTYX 1 mL and 0.5 mL prefilled syringes contain natural rubber latex, which may cause a hypersensitivity reaction in latex-sensitive individuals. The safe use of COSENTYX 150 mg/mL Sensoready pen or 1 mL and 0.5 mL prefilled syringes in latex-sensitive individuals has not been studied.
5.7 Immunizations
Prior to initiating therapy with COSENTYX, consider completion of all age-appropriate immunizations according to current immunization guidelines. COSENTYX may alter a patient's immune response to live vaccines. Avoid use of live vaccines in patients treated with COSENTYX [see Clinical Pharmacology (12.2)] .
6 ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail elsewhere in the labeling:
- Infections [see Warnings and Precautions (5.1)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.2)]
- Inflammatory Bowel Disease [see Warnings and Precautions (5.4)]
- Eczematous Eruptions [see Warnings and Precautions (5.5)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adverse Reactions in Clinical Trials of Subcutaneous COSENTYX
Adverse Reactions from Clinical Trials in Adults with PsO
A total of 3,430 adult subjects with PsO were treated with COSENTYX in controlled and uncontrolled clinical trials. Of these, 1,641 subjects were treated with COSENTYX for at least 1 year.
Four placebo-controlled Phase 3 trials in PsO subjects (Trials PsO1, PsO2, PsO3, and PsO4) were pooled to evaluate the safety of COSENTYX in comparison to placebo up to 12 weeks after treatment initiation. In total, 2,077 subjects were evaluated (691 in the COSENTYX 300 mg group, 692 in the COSENTYX 150 mg group, and 694 in the placebo group). Subjects randomized to COSENTYX received 300 mg or 150 mg doses subcutaneously at Weeks 0, 1, 2, 3, and 4 followed by the same dose every 4 weeks [see Clinical Studies (14)] .
Table 2 summarizes the adverse reactions that occurred at a rate of at least 1% and at a higher rate in the COSENTYX groups than the placebo group during the 12-week placebo-controlled period of these trials.
| COSENTYX | |||
| Adverse reactions | 300 mg (N = 691) n (%) | 150 mg (N = 692) n (%) | Placebo (N = 694) n (%) |
| Nasopharyngitis | 79 (11.4) | 85 (12.3) | 60 (8.6) |
| Diarrhea | 28 (4.1) | 18 (2.6) | 10 (1.4) |
| Upper respiratory tract infection | 17 (2.5) | 22 (3.2) | 5 (0.7) |
| Rhinitis | 10 (1.4) | 10 (1.4) | 5 (0.7) |
| Oral herpes | 9 (1.3) | 1 (0.1) | 2 (0.3) |
| Pharyngitis | 8 (1.2) | 7 (1.0) | 0 (0) |
| Urticaria | 4 (0.6) | 8 (1.2) | 1 (0.1) |
| Rhinorrhea | 8 (1.2) | 2 (0.3) | 1 (0.1) |
Adverse reactions that occurred in subjects treated with COSENTYX at rates less than 1% in the placebo-controlled period of Trials PsO1, PsO2, PsO3, and PsO4 through Week 12 included: sinusitis, tinea pedis, conjunctivitis, tonsillitis, oral candidiasis, impetigo, otitis media, otitis externa, IBD, increased liver transaminases, and neutropenia.
Infections
In the placebo-controlled period of the clinical trials in PsO (a total of 1,382 subjects treated with COSENTYX and 694 subjects treated with placebo up to 12 weeks), infections were reported in 28.7% of subjects treated with COSENTYX compared with 18.9% of subjects treated with placebo.
Over the entire treatment period (a total of 3,430 PsO subjects treated with COSENTYX for up to 52 weeks for the majority of subjects), infections were reported in 47.5% of subjects treated with COSENTYX (0.9 per subject-year of follow-up) and serious infections were reported in 1.2% of subjects treated with COSENTYX (0.015 per subject-year of follow-up).
Phase 3 data showed an increasing trend for some types of infection with increasing serum secukinumab concentrations. Candida infections, herpes viral infections, staphylococcal skin infections, and infections requiring treatment increased as serum secukinumab concentration increased.
In the PsO open-label extension of Trials PsO1 and PsO2 (median follow-up of 3.9 years), representing 3,582 subject-years of exposure, 74% of COSENTYX treated subjects reported infections (55 per 100 subject-years) and serious infections were reported in 4.5% of COSENTYX treated subjects (1.4 per 100 subject-years). Sepsis was reported in 5 COSENTYX treated subjects (0.2 per 100 subject-years).
Neutropenia was observed in controlled portion of clinical trials. Most cases of COSENTYX associated neutropenia were transient and reversible. No serious infections were associated with cases of neutropenia.
In the open-label extension of Trials PsO1 and PsO2, neutropenia (ANC < 1 x 10 9 /L) was reported in 1% of COSENTYX treated subjects (0.3 per 100 subject-years). Some cases of serious infections were associated with neutropenia; however, the causal relationship was not established.
Inflammatory Bowel Disease
Cases of IBD, in some cases serious, were observed in subjects treated with COSENTYX in clinical trials. In the PsO program, with 3,430 subjects exposed to COSENTYX over the entire treatment period for up to 52 weeks (2,725 subject-years), there were 3 cases (0.11 per 100 subject-years) of exacerbation of CD, 2 cases (0.08 per 100 subject-years) of exacerbation of UC, and 2 cases (0.08 per 100 subject-years) of new onset UC. There were no IBD cases in placebo-treated subjects (N = 793; 176 subject-years) during the 12-week placebo-controlled period.
One case of exacerbation of Crohn’s disease in a subject treated with COSENTYX subject was reported in open-label portions of clinical trials in PsO.
Adverse Reactions from Clinical Trials in Pediatric Subjects with PsO
The safety of COSENTYX was assessed in two Phase 3 trials in pediatric subjects with PsO.
- The first was a randomized, double-blind, placebo and active-controlled, 236-week trial (Trial PsO8) that enrolled 162 pediatric subjects 6 years of age and older, with severe PsO (defined by PASI score ≥ 20, an IGA modified 2011 score of 4, and involving ≥ 10% of the body surface area [BSA]) who were candidates for systemic therapy. The 162 subjects were randomized to receive placebo, a biologic active control, or COSENTYX. In the COSENTYX groups, subjects with body weight (BW) less than 25 kg received 75 mg, subjects with BW 25 to less than 50 kg received either 75 mg or 150 mg (2 times the recommended dose), and subjects with BW of at least 50 kg received either 150 mg or 300 mg (2 times the recommended dose). Subjects were dosed at Weeks 0, 1, 2, 3, and 4 and every 4 weeks thereafter.
- The second trial was a randomized, open-label, 208-week trial (Trial PsO9; NCT03668613) of 84 pediatric subjects 6 years of age and older with moderate to severe PsO (defined by a PASI score ≥ 12, IGA mod 2011 score of ≥ 3, and BSA involvement of ≥ 10% at randomization) who were randomized into two COSENTYX arms [Arm 1: 75 mg for BW < 50 kg or 150 mg for ≥ 50 kg; and Arm 2: 75 mg for BW < 25 kg, 150 mg for BW ≥ 25 kg and < 50 kg, or 300 mg for BW ≥ 50 kg]. Subjects were dosed at Weeks 0, 1, 2, 3, and 4 and every 4 weeks thereafter.
The safety profile of COSENTYX reported in these trials was consistent with the safety profile reported in adult PsO trials.
Infections
One case of methicillin-resistant Staphylococcus aureus (MRSA) toxic shock syndrome (TSS) was reported in a COSENTYX treated pediatric subject during the placebo-controlled period.
In the pediatric safety pool, which includes all subjects who took at least one dose of COSENTYX during the treatment periods [198 subjects (287 subject-years)], 22 (11%) subjects reported ≥ Common Terminology Criteria for Adverse Events (CTCAE) Grade 2 neutropenia (≥ 1,000 to < 1,500 cells/mm 3 ) with 57% of subjects followed for one year or more and 30% of subjects followed for two years or more. During the placebo-controlled period, which included a total of 80 pediatric subjects treated with COSENTYX and 41 subjects treated with placebo up to 12 weeks, ≥ CTCAE Grade 2 neutropenia was reported in 3 (4%) of the subjects treated with COSENTYX compared with no subjects treated with placebo. No serious infections were associated with cases of neutropenia.
Adverse Reactions from Clinical Trials in Adults with PsA
COSENTYX was studied in two placebo-controlled PsA trials with 1,003 adult patients (703 patients on COSENTYX and 300 patients on placebo). Of the 703 patients who received COSENTYX, 299 patients received a subcutaneous loading dose of COSENTYX (PsA1) and 404 patients received an intravenous loading dose of secukinumab (PsA2) followed by COSENTYX administered by subcutaneous injection every four weeks. During the 16-week placebo-controlled period of the trials in patients with PsA, the overall proportion of patients with adverse events was similar in the secukinumab and placebo-treatment groups (59% and 58%, respectively). The adverse events that occurred at a proportion of at least 2% and at a higher proportion in the COSENTYX groups than the placebo groups during the 16-week placebo-controlled period were nasopharyngitis, upper respiratory tract infection, headache, nausea, and hypercholesterolemia. The safety profile observed in adult patients with PsA treated with COSENTYX is consistent with the safety profile in the PsO trials in adults.
Similar to the clinical trials in patients with PsO, there was an increased proportion of patients with infections in the COSENTYX groups (29%) compared to placebo group (26%).
There were cases of CD and UC in the secukinumab group that included patients who experienced either exacerbations or the development of new disease. There were three cases of IBD, of which two patients received secukinumab and one received placebo.
Adverse Reactions from Clinical Trials in Adults with AS
COSENTYX was studied in two placebo-controlled AS trials with 590 adult patients (394 patients on COSENTYX and 196 patients on placebo). Of the 394 patients who received COSENTYX, 145 patients received a subcutaneous load of COSENTYX (study AS1), and 249 received an intravenous loading dose of secukinumab (study AS2) followed by COSENTYX administered by subcutaneous injection every four weeks. During the 16-week placebo-controlled period of the trials in patients with AS, the overall proportion of patients with adverse events was higher in the secukinumab groups than the placebo-treatment groups (66% and 59%, respectively). The adverse events that occurred at a proportion of at least 2% and at a higher proportion in the COSENTYX groups than the placebo groups during the 16-week placebo-controlled period were nasopharyngitis, nausea, and upper respiratory tract infection. The safety profile observed in patients with AS treated with COSENTYX is consistent with the safety profile in PsO clinical trials. In a third controlled trial of AS (study AS3), the safety profile of the 300 mg dose of COSENTYX was consistent with the safety profile of the 150 mg dose of COSENTYX.
Similar to clinical trials in patients with PsO, there was an increased proportion of patients with infections in the COSENTYX groups (31%) compared to the placebo group (18%).
In the original AS program, with 571 patients exposed to COSENTYX, there were 8 cases of IBD during the entire treatment period [5 cases of Crohn’s (0.7 per 100 patient-years) and 3 cases of UC (0.4 per 100 patient-years)]. During the placebo-controlled 16-week period, there were 2 Crohn’s disease exacerbations and 1 new onset UC case that was a serious adverse event in patients treated with COSENTYX compared to none of the patients treated with placebo. During the remainder of the trial when all patients received COSENTYX, 1 patient developed Crohn’s disease, 2 patients had Crohn’s exacerbations, 1 patient developed UC, and 1 patient had an UC exacerbation.
Adverse Reactions from Clinical Trials in Adults with nr-axSpA
COSENTYX was studied in one randomized, double-blind, placebo-controlled nr-axSpA trial with 555 adult patients (185 patients received a loading COSENTYX dose, 184 patients did not receive a loading COSENTYX dose, and 186 patients received placebo). The safety profile for patients with nr-axSpA treated with COSENTYX was overall similar to the safety profile seen in patients with AS and other previous experience with COSENTYX. Patients in nr-axSpA1 trial who received the loading dosing regimen compared to those without the loading regimen, had higher incidence of infections and infestations (92 per 100 patient-years versus 72 per 100 patient-years), including nasopharyngitis, upper respiratory tract infection and urinary tract infection, and gastrointestinal disorders (27 per 100 patient-years versus 22 per 100 patient-years), including gastritis, lower abdominal pain, colitis, diarrhea, and hematochezia.
Adverse Reactions from Clinical Trials in Pediatric Patients with Juvenile Psoriatic Arthritis (JPsA) and ERA
COSENTYX was studied in one double-blind, placebo-controlled, event-driven, randomized trial in 86 pediatric patients aged 2 to less than 18 years old with JPsA and ERA. The safety profile reported in this trial was consistent with the safety profile of secukinumab.
Adverse Reactions from Clinical Trials in Adults with HS
COSENTYX was studied in two 52-week, randomized, double-blind, placebo-controlled HS trials with 1,084 adult subjects (361 subjects received COSENTYX 300 mg every 2 weeks, 360 subjects received COSENTYX 300 mg every 4 weeks, and 363 subjects received placebo) with a total of 901 subject-years of COSENTYX exposure (the median duration of exposure for subjects treated with COSENTYX was 360 days). The safety profile of COSENTYX observed in these HS trials was consistent with the known safety profile of COSENTYX observed in the PsO trials.
Infections
During the 16-week placebo-controlled period, subjects who received COSENTYX 300 mg every 2 weeks had the highest incidence of fungal infections (5.3%), compared to subjects who received COSENTYX 300 mg every 4 weeks (4.2%) and subjects who received placebo (2.8%). With longer exposure, the rate of fungal infections remained higher for subjects who received COSENTYX 300 mg every 2 weeks (14.7/100 subject-years) compared to subjects who received COSENTYX 300 mg every 4 weeks (10.1/100 subject-years). The majority of the cases were reported as non-serious, non-severe, and resolved with anti-fungal treatment.
Inflammatory Bowel Disease
In the open-labeled portion of HS clinical trials, five (0.7%) IBD adverse reactions were reported, all of which were serious and led to withdrawal of trial drug, and occurred only in subjects treated with COSENTYX 300 mg every 2 weeks. There were no IBD cases in subjects treated with COSENTYX 300 mg every 4 weeks.
Adverse Reactions of Intravenous COSENTYX
The safety of intravenous COSENTYX is based on the pharmacokinetic exposure and extrapolation of the established safety of subcutaneous COSENTYX in PsA, AS and nr-axSpA patients [see Clinical Pharmacology (12.3)] .
6.2 Postmarketing Experience
The following adverse reactions have been reported during post-approval use of COSENTYX. Because they are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Immune system disorders : anaphylaxis, angioedema, systemic vasculitis
Skin and subcutaneous tissue disorders : eczematous eruptions (atopic dermatitis-like eruptions, dyshidrotic eczema, and erythroderma), cutaneous vasculitis, pyoderma gangrenosum
Infections : bacterial, viral, and fungal opportunistic infections, including esophageal candidiasis, tracheobronchial candidiasis, cutaneous aspergillosis, cytomegalovirus gastroenteritis/colitis, herpes simplex encephalitis, herpes simplex keratitis, Pneumocystis jiroveci pneumonia, Hepatitis B virus reactivation, histoplasmosis, toxoplasmosis
7 DRUG INTERACTIONS
Certain CYP450 Substrates
Increased concentrations of cytokines (e.g., IL-17) during chronic inflammation associated with certain diseases including PsO, PsA, AS, nr-axSpA, ERA, and HS may suppress the formation of CYP enzymes.
Upon initiation or discontinuation of COSENTYX in patients who are receiving concomitant CYP450 substrates, particularly those where minimal decreases in the concentration may reduce CYP substrate effectiveness or minimal increases in the concentration may increase CYP substrate adverse reactions, consider monitoring for therapeutic effect or concentration of the CYP substrate and consider dosage adjustment of the CYP substrate as needed [see Clinical Pharmacology (12.3)] .
11 DESCRIPTION
Secukinumab, a recombinant human monoclonal IgG1/κ antibody, is an interleukin-17A antagonist. It is expressed in a recombinant Chinese Hamster Ovary (CHO) cell line. Secukinumab has a molecular mass of approximately 151 kDa; both heavy chains of secukinumab contain oligosaccharide chains.
COSENTYX Injection for Subcutaneous Use
COSENTYX injection is a sterile, preservative-free, clear to slightly opalescent, colorless to slightly yellow solution for subcutaneous use. COSENTYX injection is supplied in a single-dose 300 mg/2 mL UnoReady pen with a 27-gauge fixed ½-inch needle, a single-dose 150 mg/mL Sensoready pen with a 27-gauge fixed ½-inch needle, or a single-dose prefilled syringe (300 mg/2 mL, 150 mg/mL, 75 mg/0.5 mL) with a 27-gauge fixed ½-inch needle. The removable cap of the COSENTYX 150 mg/mL Sensoready pen or 1 mL and 0.5 mL prefilled syringes contains natural rubber latex.
Each COSENTYX 300 mg/2 mL UnoReady pen or 300 mg/2 mL prefilled syringe contains 300 mg of secukinumab formulated in: L-histidine/histidine hydrochloride monohydrate (6.206 mg), L-methionine (1.492 mg), polysorbate 80 (0.4 mg), trehalose dihydrate (151.34 mg), and Sterile Water for Injection, USP, at pH of 5.8.
Each COSENTYX 150 mg/mL Sensoready pen or 150 mg/mL prefilled syringe contains 150 mg of secukinumab formulated in: L-histidine/histidine hydrochloride monohydrate (3.103 mg), L-methionine (0.746 mg), polysorbate 80 (0.2 mg), trehalose dihydrate (75.67 mg), and Sterile Water for Injection, USP, at pH of 5.8.
Each COSENTYX 75 mg/0.5 mL prefilled syringe contains 75 mg of secukinumab formulated in: L-histidine/histidine hydrochloride monohydrate (1.552 mg), L-methionine (0.373 mg), polysorbate 80 (0.1 mg), trehalose dihydrate (37.83 mg), and Sterile Water for Injection, USP, at pH of 5.8.
COSENTYX Injection for Intravenous Use
COSENTYX solution is supplied as a sterile, preservative free, clear to opalescent, colorless to slightly yellowish solution in single-dose vials for intravenous infusion after dilution.
Each COSENTYX vial contains 125 mg of secukinumab formulated in: L-histidine (5.67 mg), L-histidine hydrochloride monohydrate (13.3 mg), L-methionine (3.73 mg), polysorbate 80 (1 mg), trehalose dihydrate (426 mg), and Sterile Water for Injection, USP, at pH of 5.8.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Secukinumab is a human IgG1 monoclonal antibody that selectively binds to the interleukin-17A (IL-17A) cytokine and inhibits its interaction with the IL-17 receptor. IL-17A is a naturally occurring cytokine that is involved in normal inflammatory and immune responses. Secukinumab inhibits the release of proinflammatory cytokines and chemokines.
12.2 Pharmacodynamics
Elevated levels of IL-17A are found in psoriatic plaques and in HS lesions. Treatment with COSENTYX may reduce epidermal neutrophils and IL-17A levels in psoriatic plaques. Serum levels of total IL-17A (free and secukinumab-bound IL-17A) measured at Week 4 and Week 12 were increased following secukinumab treatment. These pharmacodynamic activities are based on small exploratory trials. The relationship between these pharmacodynamic activities and the mechanism(s) by which secukinumab exerts its clinical effects is unknown.
Increased numbers of IL-17A producing lymphocytes and innate immune cells and increased levels of IL-17A have been found in the blood of patients with PsA and AS. Increased numbers of IL-17A producing lymphocytes have also been found in patients with nr-axSpA.
Immune Response to Non-Live Vaccines During Treatment
Healthy individuals who received a single 150 mg dose of COSENTYX 2 weeks prior to vaccination with a non-U.S.-approved group C meningococcal polysaccharide conjugate vaccine and a non-U.S.-approved inactivated seasonal influenza vaccine had similar antibody responses compared to individuals who did not receive COSENTYX prior to vaccination. The clinical effectiveness of meningococcal and influenza vaccines has not been assessed in patients undergoing treatment with COSENTYX [see Warnings and Precautions (5.7)] .
12.3 Pharmacokinetics
Pharmacokinetics Following Subcutaneous Administration
The observed pharmacokinetics (PK) of secukinumab administered subcutaneously in patients with PsO, PsA, AS and nr-axSpA were similar. The secukinumab PK is also similar in pediatric patients with ERA and PsO for the same weight tiered dosing regimen.
The mean steady-state trough concentration of secukinumab was approximately 26% lower in HS subjects than that of PsO subjects.
Absorption
Following a single subcutaneous dose of either 150 mg or 300 mg (administered as two injections of 150 mg) of COSENTYX in PsO subjects, secukinumab reached peak mean (± SD) serum concentrations (C max ) of 13.7 ± 4.8 mcg/mL and 27.3 ± 9.5 mcg/mL, respectively, by approximately 6 days post dose.
Following multiple subcutaneous doses of COSENTYX (administered as one or two injections of 150 mg), the mean (± SD) serum trough concentrations of secukinumab ranged from 22.8 ± 10.2 mcg/mL (150 mg) to 45.4 ± 21.2 mcg/mL (300 mg) at Week 12. At the 300 mg dose at Week 4 and Week 12, the mean trough concentrations resulted from the Sensoready pen were approximately 30% higher than those from the prefilled syringe. Following multiple subcutaneous doses of 300 mg administered via the 300 mg/2 mL UnoReady pen, the mean serum trough concentrations of secukinumab were generally consistent with those in the previous Sensoready pen study used to deliver 300 mg.
Steady-state concentrations of secukinumab were achieved by Week 24 following the every 4-week COSENTYX dosing regimens. The mean (± SD) steady-state trough concentrations ranged from 16.7 ± 8.2 mcg/mL (150 mg) to 34.4 ± 16.6 mcg/mL (300 mg administered as two injections of 150 mg).
In healthy subjects and subjects with PsO, secukinumab bioavailability ranged from 55% to 77% following subcutaneous COSENTYX dose of 150 mg or 300 mg (administered as two injections of 150 mg).
Following subcutaneous administrations of 300 mg of COSENTYX at Weeks 0, 1, 2, 3, and 4 and then every 2 weeks thereafter, steady-state concentrations of secukinumab were achieved by Week 24 in both HS trials. The mean (± SD) steady-state trough concentrations were 55.7 ± 28.9 mcg/mL and 50.5 ± 28.2 mcg/mL in HS Trial 1 and HS Trial 2, respectively.
Distribution
The mean volume of distribution during the terminal phase (Vz) following a single intravenous administration ranged from 7.10 to 8.60 L in PsO subjects.
Secukinumab concentrations in interstitial fluid in lesional and non-lesional skin of PsO subjects ranged from 27% to 40% of those in serum at 1 and 2 weeks after a single subcutaneous dose of COSENTYX 300 mg (administered as two injections of 150 mg).
Elimination
Metabolism
The metabolic pathway of secukinumab has not been characterized. As a human IgG1κ monoclonal antibody secukinumab is expected to be degraded into small peptides and amino acids via catabolic pathways in the same manner as endogenous IgG.
Excretion
The mean systemic clearance (CL) ranged from 0.14 L/day to 0.22 L/day and the mean half-life ranged from 22 to 31 days in PsO subjects following intravenous and subcutaneous administration across all PsO trials.
In a population PK analysis, the mean systemic CL in subjects with HS was 0.26 L/day. The mean elimination half-life, as estimated from population PK analysis, was 23 days in HS subjects.
Dose Linearity
Secukinumab exhibited dose-proportional PK in subjects with PsO over a dose range from 25 mg (approximately 0.083 times the recommended dose) to 300 mg following subcutaneous administrations.
Weight
Secukinumab clearance and volume of distribution increase as body weight increases.
Specific Populations
Patients with Hepatic or Renal Impairment
No formal trial of the effect of hepatic or renal impairment on the PK of secukinumab was conducted.
Geriatric Patients
Population PK analysis indicated that the clearance of secukinumab was not significantly influenced by age in adult subjects with PsO, PsA and AS. Subjects who are 65 years or older had apparent clearance of secukinumab similar to subjects less than 65 years old.
Pediatric Patients
In a pool of the two pediatric trials, subjects with moderate to severe PsO (6 years of age and older) were administered subcutaneous COSENTYX at the recommended pediatric dosing regimen. At Week 24, secukinumab steady state mean ± SD serum trough concentrations were 32.6 ± 10.8 mcg/mL (n = 8), 19.8 ± 6.96 mcg/mL (n = 24), and 27.3 ± 10.1 mcg/mL (n = 36), in subjects who weighed less than 25 kg and received 75 mg of subcutaneous COSENTYX, subjects who weighed at least 25 kg and less than 50 kg and received 75 mg of subcutaneous COSENTYX, and subjects who weighed at least 50 kg and received 150 mg of subcutaneous COSENTYX, respectively.
In a pediatric trial, JPsA and ERA patients (2 to less than 18 years of age) were administered subcutaneous COSENTYX at the recommended pediatric dosing regimen. At Week 24, patients who weighed at least 15 kg and less than 50 kg, and patients who weighed at least 50 kg had a mean ± SD steady-state trough concentration of 25.2 ± 5.45 mcg/mL (n = 10) and 27.9 ± 9.57 mcg/mL (n = 19), respectively.
Drug Interactions
Cytochrome P450 Substrates
In adult subjects with PsO, midazolam (CYP3A4 substrate) PK was similar when administered alone, or when administered following either a single or five weekly subcutaneous administrations of 300 mg of COSENTYX [see Drug Interactions (7)] .
Pharmacokinetics Following Intravenous Administration
Following an intravenous administration of 1.75 mg/kg maintenance dose every four weeks, with or without a loading dose of 6 mg/kg at Day 0, the secukinumab concentrations [steady state trough secukinumab concentrations (C min,ss ), mean secukinumab concentrations (C avg,ss ), and maximum secukinumab concentrations (C max,ss )] are estimated to be within the range of the steady state concentrations following subcutaneous administration of 150 mg and 300 mg doses of COSENTYX administered every four weeks.
12.6 Immunogenicity
The observed incidence of anti-drug antibodies is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of anti-drug antibodies (ADA) in the trials described below with the incidence of ADA in other trials, including those of COSENTYX (secukinumab).
The immunogenicity of COSENTYX was evaluated using an electrochemiluminescence-based bridging immunoassay. Less than 1% of subjects treated with COSENTYX developed antibodies to secukinumab in up to 52 weeks of treatment. However, this assay has limitations in detecting anti-secukinumab antibodies in the presence of secukinumab; therefore, the incidence of antibody development might not have been reliably determined.
In up to 52 weeks of treatment in controlled trials in patients with PsO, PsA, AS, nr-axSpA, HS, pediatric PsO, JPsA and ERA [see Clinical Studies (14)] , the incidence of anti-secukinumab antibodies (referred as ADA) formation was less than 1% (25 of 6268 total of subjects treated with COSENTYX). Of the subjects treated with COSENTYX who developed ADA, approximately 8% developed neutralizing antibodies. Because of the low occurrence of ADA, the effect of these antibodies on pharmacokinetics, pharmacodynamics, safety, or effectiveness of COSENTYX is unknown.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Animal studies have not been conducted to evaluate the carcinogenic or mutagenic potential of COSENTYX. Some published literature suggests that IL-17A directly promotes cancer cell invasion in vitro, whereas other reports indicate IL-17A promotes T-cell mediated tumor rejection. Depletion of IL-17A with a neutralizing antibody inhibited tumor development in mice. The relevance of experimental findings in mouse models for malignancy risk in humans is unknown.
No effects on fertility were observed in male and female mice that were administered a murine analog of secukinumab at subcutaneous doses up to 150 mg/kg once weekly prior to and during the mating period.
14 CLINICAL STUDIES
14.1 Adult Plaque Psoriasis
Four multicenter, randomized, double-blind, placebo-controlled trials of subcutaneous COSENTYX (Trials PsO1, PsO2, PsO3, and PsO4) enrolled 2,403 subjects (691 randomized to COSENTYX 300 mg, 692 to COSENTYX 150 mg, 694 to placebo, and 323 to a biologic active control) 18 years of age and older with PsO who had a minimum BSA involvement of 10%, and Psoriasis Area and Severity Index (PASI) score greater than or equal to 12, and who were candidates for phototherapy or systemic therapy. In these studies, each 300 mg dose was administered as two injections of 150 mg.
- Trial PsO1 (NCT01365455) enrolled 738 subjects (245 randomized to COSENTYX 300 mg, 245 to COSENTYX 150 mg, and 248 to placebo). Subjects received subcutaneous treatment at Weeks 0, 1, 2, 3, and 4 followed by dosing every 4 weeks. Subjects randomized to receive placebo that were non-responders at Week 12 were crossed over to receive COSENTYX (either 300 mg or 150 mg) at Weeks 12, 13, 14, 15, and 16 followed by the same dose every 4 weeks. All subjects were followed for up to 52 weeks following first administration of trial treatment.
- Trial PsO2 (NCT01358578) enrolled 1306 subjects (327 randomized to COSENTYX 300 mg, 327 to COSENTYX 150 mg, 326 to placebo, and 323 to a biologic active control). Subjects received subcutaneous treatment at Weeks 0, 1, 2, 3, and 4 followed by dosing every 4 weeks. Subjects randomized to receive placebo that were non-responders at Week 12 crossed over to receive COSENTYX (either 300 mg or 150 mg) at Weeks 12, 13, 14, 15, and 16 followed by the same dose every 4 weeks. All subjects were followed for up to 52 weeks following first administration of trial treatment.
- Trial PsO3 (NCT01555125) enrolled 177 subjects (59 randomized to COSENTYX 300 mg, 59 to COSENTYX 150 mg, and 59 to placebo) and assessed safety, tolerability, and usability of COSENTYX self-administration via prefilled syringe for 12 weeks. Subjects received subcutaneous treatment at Weeks 0, 1, 2, 3, and 4, followed by the same dose every 4 weeks for up to 12 weeks total.
- Trial PsO4 (NCT01636687) enrolled 182 subjects (60 randomized to COSENTYX 300 mg, 61 to COSENTYX 150 mg, and 61 to placebo) and assessed safety, tolerability, and usability of COSENTYX self-administration via Sensoready pen for 12 weeks. Subjects received subcutaneous treatment at Weeks 0, 1, 2, 3, and 4, followed by the same dose every 4 weeks for up to 12 weeks total.
Endpoints
In all trials, the endpoints were the proportion of subjects who achieved a reduction in PASI score of at least 75% (PASI 75) from baseline to Week 12 and treatment success (clear or almost clear) on the Investigator’s Global Assessment modified 2011 (IGA). Other evaluated outcomes included the proportion of subjects who achieved a reduction in PASI score of at least 90% (PASI 90) from baseline at Week 12, maintenance of efficacy to Week 52, and improvements in itching, pain, and scaling at Week 12 based on the Psoriasis Symptom Diary © .
The PASI is a composite score that takes into consideration both the percentage of BSA affected and the nature and severity of psoriatic changes within the affected regions (induration, erythema, and scaling). The IGA is a 5-category scale, including “0 = clear”, “1 = almost clear”, “2 = mild”, “3 = moderate” or “4 = severe” indicating the physician’s overall assessment of the psoriasis severity focusing on induration, erythema, and scaling. Treatment success of “clear” or “almost clear” consisted of no signs of psoriasis or normal to pink coloration of lesions, no thickening of the plaque, and none to minimal focal scaling.
Baseline Disease Characteristics
Across all treatment groups, the baseline PASI score ranged from 11 to 72 with a median of 20 and the baseline IGA score ranged from “moderate” (62%) to “severe” (38%). Of the 2,077 PsO subjects who were included in the placebo-controlled trials, 79% were biologic-naïve (have never received a prior treatment with biologics) and 45% were non-biologic failures (failed to respond to a prior treatment with non-biologic therapies). Of the subjects who received a prior treatment with biologics, over one-third were biologic failures. Approximately 15% to 25% of trial subjects had a history of psoriatic arthritis.
Clinical Response
The results of Trials PsO1 and PsO2 are presented in Table 3.
| Trial PsO1 | Trial PsO2 | |||||
| COSENTYX 300 mg (N = 245) n (%) | COSENTYX 150 mg (N = 245) n (%) | Placebo (N = 248) n (%) | COSENTYX 300 mg (N = 327) n (%) | COSENTYX 150 mg (N = 327) n (%) | Placebo (N = 326) n (%) | |
| PASI 75 response | 200 (82) | 174 (71) | 11 (4) | 249 (76) | 219 (67) | 16 (5) |
| IGA of clear or almost clear | 160 (65) | 125 (51) | 6 (2) | 202 (62) | 167 (51) | 9 (3) |
The results of Trials PsO3 and PsO4 are presented in Table 4.
| Trial PsO3 | Trial PsO4 | |||||
| COSENTYX 300 mg (N = 59) n (%) | COSENTYX 150 mg (N = 59) n (%) | Placebo (N = 59) n (%) | COSENTYX 300 mg (N = 60) n (%) | COSENTYX 150 mg (N = 61) n (%) | Placebo (N = 61) n (%) | |
| PASI 75 response | 44 (75) | 41 (69) | 0 (0) | 52 (87) | 43 (70) | 2 (3) |
| IGA of clear or almost clear | 40 (68) | 31 (53) | 0 (0) | 44 (73) | 32 (52) | 0 (0) |
Examination of age, sex, and racial subgroups did not identify differences in response to COSENTYX among these subgroups. Based on post-hoc subgroup analyses in subjects with moderate to severe PsO, subjects with lower body weight and lower disease severity may achieve an acceptable response with COSENTYX 150 mg.
PASI 90 response at Week 12 was achieved with COSENTYX 300 mg and 150 mg compared to placebo in 59% (145/245) and 39% (95/245) versus 1% (3/248) of subjects, respectively (Trial PsO1) and 54% (175/327) and 42% (137/327) versus 2% (5/326) of subjects, respectively (Trial PsO2). Similar results were seen in Trials PsO3 and PsO4.
- With continued treatment over 52 weeks, subjects in Trial PsO1 who were PASI 75 responders at Week 12 maintained their responses in 81% (161/200) of the subjects treated with COSENTYX 300 mg and in 72% (126/174) of subjects treated with COSENTYX 150 mg. Trial PsO1 subjects who were clear or almost clear on the IGA at Week 12 also maintained their responses in 74% (119/160) of subjects treated with COSENTYX 300 mg and in 59% (74/125) of subjects treated with COSENTYX 150 mg.
- Similarly in Trial PsO2, PASI 75 responders maintained their responses in 84% (210/249) of subjects treated with COSENTYX 300 mg and in 82% (180/219) of subjects treated with COSENTYX 150 mg. Trial PsO2 subjects who were clear or almost clear on the IGA also maintained their responses in 80% (161/202) of subjects treated with COSENTYX 300 mg and in 68% (113/167) of subjects treated with COSENTYX 150 mg.
Among the subjects who chose to participate (39%) in assessments of patient reported outcomes, improvements in signs and symptoms related to itching, pain, and scaling at Week 12 compared to placebo (Trials PsO1 and PsO2) were observed using the Psoriasis Symptom Diary © .
Psoriasis Lesions of Scalp
A randomized, placebo-controlled trial (Trial PsO5; NCT02267135) enrolled 102 subjects with moderate to severe psoriasis lesions of scalp, defined as having a Psoriasis Scalp Severity Index (PSSI) score of greater than or equal to 12, an IGA scalp only score of 3 or greater, and at least 30% of the scalp affected. In this trial, 62% of subjects had at least 50% of scalp surface area affected. In this study, each 300 mg dose was administered as two injections of 150 mg. The proportions of subjects achieving an IGA scalp only score of 0 or 1 (clear or almost clear) were 56.9% and 5.9% for the COSENTYX 300 mg and the placebo groups, respectively.
300 mg/2 mL Pre-filled Syringe and 300 mg/2 mL UnoReady Pen
Two randomized, double-blind, placebo-controlled, 52-week trials (PsO6 and PsO7) enrolled 336 subjects at least 18 years of age with moderate to severe PsO who are candidates for systemic therapy or phototherapy to evaluate the safety and efficacy of COSENTYX 300 mg subcutaneously administered with a single 300 mg/2 mL prefilled syringe (Trial PsO6, NCT02748863, 214 patients) or with a single 300 mg/2 mL UnoReady pen (Trial PsO7, NCT03589885, 122 patients) compared to two subcutaneous injections using a 150 mg/1 mL prefilled syringe. The co-primary endpoints for both trials were the proportion of subjects who achieved a PASI 75 response and IGA mod 2011 ‘clear’ or ‘almost clear’ response with at least a two-grade reduction from baseline at Week 12.
| Abbreviation: PFS, prefilled syringe. Missing data was imputed using multiple imputation. | ||||||
| Trial PsO6 | Trial PsO7 | |||||
| COSENTYX 300 mg | COSENTYX 300 mg | |||||
| 2 mL PFS (N = 72) % | Two 1 mL PFS (N = 71) % | Placebo (N = 71) % | 2 mL Pen (N = 41) % | Two 1 mL PFS (N = 41) % | Placebo (N = 40) % | |
| IGA of clear or almost clear | 76 | 69 | 1 | 76 | 68 | 8 |
| PASI 75 response | 89 | 82 | 2 | 95 | 83 | 10 |
| PASI 90 response | 67 | 70 | 2 | 76 | 62 | 5 |
14.2 Pediatric Plaque Psoriasis
A 52-week, multicenter randomized, double-blind, placebo and active-controlled trial (Trial PsO8; NCT02471144) enrolled 162 pediatric subjects 6 years of age and older, with severe plaque psoriasis (as defined by a PASI score ≥ 20, an IGA modified 2011 score of 4, and involving ≥ 10% of the BSA) who were candidates for systemic therapy.
Subjects were randomized to receive subcutaneous placebo, COSENTYX, or a biologic active control. In the COSENTYX groups, subjects with BW less than 25 kg received 75 mg, subjects with BW 25 to less than 50 kg received either 75 mg or 150 mg (2 times the recommended dose), and subjects with BW at least 50 kg received either 150 mg or 300 mg (2 times the recommended dose). In this study, each 300 mg dose was administered as two subcutaneous injections of 150 mg. Subjects in the COSENTYX and placebo groups received subcutaneous treatment at Weeks 0, 1, 2, 3, and 4 followed by dosing every 4 weeks. At Week 12, subjects randomized to placebo who were non-responders were switched to COSENTYX (dose based on body weight) and received COSENTYX at Weeks 12, 13, 14, and 15, followed by the same dose every 4 weeks starting at Week 16.
Baseline Characteristics
Overall, 60% of the subjects were female, 83% were White, the median BW was 50.6 kg, and the mean age was 13.5 years with 23% of the subjects less than 12 years. At baseline, the median PASI score was 26 (ranged from 17 to 60), and 99% of the subjects had an IGA modified 2011 score of 4 (‘severe’). Approximately 43% of the subjects had prior exposure to phototherapy, 53% to conventional systemic therapy, 3% to biologics, and 9% had concomitant psoriatic arthritis.
Endpoints
The co-primary endpoints were the proportion of subjects who achieved a reduction in PASI score of at least 75% (PASI 75) from baseline to Week 12 and the proportion of subjects who achieved an IGA modified 2011 score of ‘clear’ or ‘almost clear’ (0 or 1) with at least a 2-point improvement from baseline to Week 12. The key secondary endpoint was the proportion of subjects who achieved a reduction in PASI score of at least 90% (PASI 90) from baseline to Week 12.
Clinical Response
Table 6 presents the efficacy results at Week 12 by baseline weight strata for the approved dosage in Trial PsO8.
| Non-responder imputation was used to handle missing values. a COSENTYX treated subjects received 75 mg for subjects less than 50 kg and 150 mg for subjects at least 50 kg body weight. | ||||||
| Body weight < 50 kg | Body weight ≥ 50 kg | Total | ||||
| COSENTYX 75 mg (N = 22) n (%) | Placebo (N = 20) n (%) | COSENTYX 150 mg (N = 21) n (%) | Placebo (N = 21) n (%) | COSENTYX a (N = 43) n (%) | Placebo (N = 41) n (%) | |
| IGA of clear or almost clear | 7 (32) | 1 (5) | 17 (81) | 1 (5) | 24 (56) | 2 (5) |
| PASI 75 response | 12 (55) | 2 (10) | 18 (86) | 4 (19) | 30 (70) | 6 (15) |
| PASI 90 response | 9 (41) | 1 (5) | 17 (81) | 0 (0) | 26 (60) | 1 (2) |
14.3 Adult Psoriatic Arthritis
The safety and efficacy of COSENTYX were assessed in 1,999 patients, in 3 randomized, double-blind, placebo-controlled trials (PsA1, PsA2, and PsA3) in adult patients, age 18 years and older with active PsA (greater than or equal to 3 swollen and greater than or equal to 3 tender joints) despite non-steroidal anti-inflammatory drug (NSAID), corticosteroid or disease modifying anti-rheumatic drug (DMARD) therapy. Patients in these trials had a diagnosis of PsA of at least 5 years across all trials.
- PsA1 Study (NCT 01752634) evaluated 397 patients, who were treated with 75 mg, 150 mg or 300 mg of COSENTYX (administered as two subcutaneous injections of 150 mg) at Weeks 0, 1, 2, 3, and 4, followed by the same subcutaneous dose every 4 weeks. Patients who received placebo were re-randomized to receive subcutaneous COSENTYX (either 150 mg or 300 mg every 4 weeks) at Week 16 or Week 24 based on responder status. The primary endpoint was the percentage of patients achieving an ACR20 response at Week 24.
- PsA2 Study (NCT 01392326) evaluated 606 patients, who were treated with intravenous secukinumab 10 mg/kg, or placebo at Weeks 0, 2, and 4, followed by either 75 mg or 150 mg of subcutaneous COSENTYX treatment (or placebo) every 4 weeks. Patients who received placebo were re-randomized to receive subcutaneous COSENTYX (either 75 mg or 150 mg every 4 weeks) at Week 16 or Week 24 based on responder status.
- PsA3 Study (NCT 02404350) evaluated 996 patients, who were treated with 150 mg or 300 mg of COSENTYX (administered as two subcutaneous injections of 150 mg) at Weeks 0, 1, 2, 3, and 4 followed by the same subcutaneous dose every 4 weeks, or once every 4 weeks of COSENTYX 150 mg. Patients treated with placebo received subcutaneous COSENTYX, either 150 mg or 300 mg, per baseline randomization, at Week 16 or Week 24 based upon responder status. The primary endpoint was ACR20 response at Week 16 with the key secondary endpoint the change from baseline in modified Total Sharp Score (mTSS) at Week 24.
Baseline Disease Characteristics
At baseline, over 61% and 42% of the patients had enthesitis and dactylitis, respectively. Overall, 31% of patients discontinued previous treatment with anti-TNFα agents due to either lack of efficacy or intolerance. In addition, approximately 53% of patients from both studies had concomitant methotrexate (MTX) use. Patients with different subtypes of PsA were enrolled, including polyarticular arthritis with no evidence of rheumatoid nodules (80%), asymmetric peripheral arthritis (63%), distal interphalangeal involvement (58%), spondylitis with peripheral arthritis (20%), and arthritis mutilans (7%).
Clinical Response
In PsA1, patients treated with 150 mg or 300 mg COSENTYX demonstrated a greater clinical response, including ACR20, ACR50, and ACR70 compared to patients treated with placebo at Week 24 (Table 7). Responses were similar in patients regardless of concomitant MTX treatment. Responses were seen regardless of prior anti-TNFα exposure.
In patients with coexistent PsO receiving COSENTYX (n = 99), the skin lesions of psoriasis improved with treatment, relative to placebo, as measured by the Psoriasis Area Severity Index (PASI).
| a Patients who met escape criteria (less than 20% improvement in tender or swollen joint counts) at Week 16 were considered non-responders. | |||||
| COSENTYX | COSENTYX | Placebo | Difference from placebo (95% CI) | ||
| 150 mg (N = 100) | 300 mg (N = 100) | (N = 98) | COSENTYX 150 mg | COSENTYX 300 mg | |
| ACR20 response | |||||
| Week 16 (%) | 60 | 57 | 18 | 42 (30, 54) | 38 (26, 51) |
| Week 24 (%) | 51 | 54 | 15 | 36 (24, 48) | 39 (27, 51) |
| ACR50 response | |||||
| Week 16 (%) | 37 | 35 | 6 | 31 (21, 42) | 28 (18, 39) |
| Week 24 (%) | 35 | 35 | 7 | 28 (18, 38) | 28 (17, 38) |
| ACR70 response | |||||
| Week 16 (%) | 17 | 15 | 2 | 15 (7, 23) | 13 (5, 20) |
| Week 24 (%) | 21 | 20 | 1 | 20 (12, 28) | 19 (11, 27) |
The percentage of patients who achieved a ACR20 response by visit is shown in Figure 1. Patients on placebo who received COSENTYX without a loading regimen achieved similar ACR20 responses over time (data not shown).
Figure 1: Percent of Adult Patients Who Achieved ACR 20 Response a in PsA1 Study Through Week 24 (Subcutaneous Treatment)

a Patients who met escape criteria (less than 20% improvement in tender or swollen joint counts) at Week 16 were considered non-responders.
The improvements in the components of the ACR response criteria in the PsA1 study are shown in Table 8.
| a Week 16 rather than Week 24 data are displayed to provide comparison between arms prior to placebo escape to COSENTYX. b Mean change based upon observed data. | |||
| COSENTYX 150 mg (N = 100) | COSENTYX 300 mg (N = 100) | Placebo (N = 98) | |
| Number of swollen joints | |||
| Baseline | 12.0 | 11.2 | 12.1 |
| Mean change at Week 16 | -4.86 | -5.83 | -3.22 |
| Number of tender joints | |||
| Baseline | 24.1 | 20.2 | 23.5 |
| Mean change at Week 16 | -10.70 | -10.01 | -1.77 |
| Patient’s assessment of pain | |||
| Baseline | 58.9 | 57.7 | 55.4 |
| Mean change at Week 16 | -22.91 | -23.97 | -7.98 |
| Patient global assessment | |||
| Baseline | 62.0 | 60.7 | 57.6 |
| Mean change at Week 16 | -25.47 | -25.40 | -8.25 |
| Physician global assessment | |||
| Baseline | 56.7 | 55.0 | 55.0 |
| Mean change at Week 16 | -29.24 | -34.71 | -14.95 |
| Disability index (HAQ) | |||
| Baseline | 1.2200 | 1.2828 | 1.1684 |
| Mean change at Week 16 | -0.45 | -0.55 | -0.23 |
| CRP (mg/L) | |||
| Baseline | 14.15 | 10.88 | 7.87 |
| Mean change at Week 16 b | -8.41 | -7.21 | 0.79 |
Improvements in enthesitis and dactylitis scores were observed in each COSENTYX group compared to placebo at Week 24.
Radiographic Response
In PsA3 Study, inhibition of progression of structural damage was assessed radiographically and expressed by the modified mTSS and its components, the Erosion Score (ES) and Joint Space Narrowing Score (JSN), at Week 24 compared to baseline. Radiographs of hands, wrists, and feet were obtained at baseline, Week 16 and/or Week 24 and scored independently by at least two readers who were blinded to treatment group and visit number. Treatment with subcutaneous COSENTYX 150 mg without a loading dose, 150 mg with a loading dose and 300 mg with a loading dose significantly inhibited progression of peripheral joint damage compared with treatment with placebo as measured by change from baseline in mTSS at Week 24. The percentage of patients with no disease progression (defined as a change from baseline in mTSS of less than or equal to 0.0) from randomization to Week 24 was 75.7%, 70.9%, and 76.5% for COSENTYX 150 mg without a loading dose, 150 mg, 300 mg, respectively versus 68.2% for placebo.
| Results from a linear mixed effects model that excluded data after escape for placebo subjects who received escape therapy at Week 16. The model assumes approximately linear progression over time and estimates a difference in rates (slopes) of progression over 24 weeks to compare treatment arms. | |||
| Treatment | N | Rate of change per 24 weeks | Difference from placebo (95% CI) |
| COSENTYX 150 mg without a loading dose | 210 | -0.10 | -0.61 (-0.95, -0.26) |
| COSENTYX 150 mg with a loading dose | 213 | 0.14 | -0.37 (-0.71, -0.03) |
| COSENTYX 300 mg with a loading dose | 217 | 0.03 | -0.48 (-0.82, -0.14) |
| Placebo | 296 | 0.51 | -- |
Physical Function
Improvement in physical function as assessed by Health Assessment Questionnaire-Disability Index (HAQ-DI) demonstrated that the proportion of patients who achieved at least -0.3 improvement in HAQ-DI score from baseline was greater in the subcutaneous COSENTYX 150 mg and 300 mg groups compared to the placebo group at Weeks 16 and 24. At Week 16 in PsA1 study, estimated mean change from baseline was -0.23 in the placebo group compared with -0.45 in the COSENTYX 150 mg group and -0.55 in the COSENTYX 300 mg group.
Treatment of Adult Patients with Active Psoriatic Arthritis with Intravenous COSENTYX
The effectiveness of intravenous COSENTYX in the treatment of adult patients with active PsA was extrapolated from the established effectiveness of subcutaneous COSENTYX in adult patients with active PsA based on pharmacokinetic exposure [see Clinical Pharmacology (12.3)] .
14.4 Ankylosing Spondylitis
The safety and efficacy of subcutaneous COSENTYX were assessed in 816 adult patients (18 years of age and older) with active AS in three randomized, double-blind, placebo-controlled trials (AS1, AS2, and AS3). Patients had active disease as defined by the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) greater or equal to 4 despite non-steroidal anti-inflammatory drug (NSAID), corticosteroid or disease modifying anti-rheumatic drug (DMARD) therapy.
- AS1 Study (NCT01649375) evaluated 219 patients, who were treated with 75 mg or 150 mg of subcutaneous COSENTYX treatment at Weeks 0, 1, 2, 3, and 4, followed by the same dose every 4 weeks. At Week 16, patients who received placebo were re-randomized to either 75 mg or 150 mg of subcutaneous COSENTYX every 4 weeks. The primary endpoint was the percentage of patients who achieved an ASAS20 response at Week 16.
- AS2 Study (NCT01358175) evaluated 371 patients, who were treated with intravenous secukinumab 10 mg/kg at Weeks 0, 2, and 4 (for both treatment arms) or placebo, followed by either 75 mg or 150 mg subcutaneous COSENTYX treatment every 4 weeks or placebo. Patients who received placebo were re-randomized to receive subcutaneous COSENTYX (either 75 mg or 150 mg every 4 weeks) at Week 16 or Week 24 based on responder status.
- AS3 Study (NCT02008916) evaluated 226 patients, who were treated with intravenous secukinumab 10 mg/kg at Weeks 0, 2, and 4 (for both treatment arms) or placebo, followed by either 150 mg or 300 mg subcutaneous COSENTYX treatment every 4 weeks or placebo. Patients who received placebo were re-randomized to receive subcutaneous COSENTYX (either 150 mg or 300 mg every 4 weeks) at Week 16. The primary endpoint was the percentage of patients who achieved an ASAS20 response at Week 16. Patients were blinded to the treatment regimen up to Week 52, and the trial continued to Week 156. In this study, each 300 mg dose was administered as two injections of 150 mg.
Baseline Disease Characteristics
At baseline, approximately 13% and 25% used concomitant MTX or sulfasalazine, respectively. Overall, 29% of patients discontinued previous treatment with anti-TNFα agents due to either lack of efficacy or intolerance.
Clinical Response
In AS1, patients treated with 150 mg COSENTYX demonstrated greater improvements in ASAS20 and ASAS40 responses compared to patients treated with placebo at Week 16 (Table 10). Responses were similar in patients regardless of concomitant therapies.
| COSENTYX 150 mg (n = 72) | Placebo (n = 74) | Difference from placebo (95% CI) | |
| ASAS20 response, % | 61 | 28 | 33 (18, 48) |
| ASAS40 response, % | 36 | 11 | 25 (12, 38) |
The improvements in the main components of the ASAS20 response criteria and other measures of disease activity are shown in Table 11.
| ||||
| COSENTYX 150 mg (N = 72) | Placebo (N = 74) | |||
| Baseline | Week 16 change from baseline | Baseline | Week 16 change from baseline | |
| ASAS20 response criteria | ||||
| -Patient Global Assessment of Disease Activity (0-100 mm) 1 | 67.5 | -27.7 | 70.5 | -12.9 |
| -Total spinal pain (0-100 mm) | 66.2 | -28.5 | 69.2 | -10.9 |
| -BASFI (0-10) 2 | 6.2 | -2.2 | 6.1 | -0.7 |
| -Inflammation (0-10) 3 | 6.5 | -2.5 | 6.5 | -0.8 |
| BASDAI score 4 | 6.6 | -2.2 | 6.8 | -0.9 |
| BASMI 5 | 3.6 | -0.51 | 3.9 | -0.22 |
| hsCRP 6 (mg/L) mean change at Week 16 | 27.0 | -17.2 | 15.9 | 0.8 |
The percentage of patients who achieved ASAS20 responses by visit is shown in Figure 2. Patients on placebo who received COSENTYX without a loading regimen achieved similar ASAS20 responses over time (data not shown).
Figure 2: ASAS20 Responses in All AS1 Study Patients Over Time Up to Week 16 (Subcutaneous Treatment)

In AS3 Study, patients treated with subcutaneous COSENTYX (150 mg and 300 mg) demonstrated improved signs and symptoms, and had comparable efficacy responses, regardless of dose, that were superior to placebo at Week 16 for the primary and most secondary endpoints. At Week 16, the ASAS20 and ASAS40 responses were 58.1% and 40.5% for 150 mg and 60.5% and 42.1% for 300 mg, respectively. The percent of patients achieving ASAS20 responses by visit is shown in Figure 3.
COSENTYX treated patients showed improvement compared to placebo-treated patients in health-related quality of life as assessed by ASQoL at Week 16.
Figure 3: ASAS20 Responses in All AS3 Study Patients Over Time Up to Week 16 (Subcutaneous Treatment)

Treatment of Adult Patients with Active Ankylosing Spondylitis with Intravenous COSENTYX
The effectiveness of intravenous COSENTYX in the treatment of adult patients with active AS was extrapolated from the established effectiveness of subcutaneous COSENTYX in adult patients with active AS based on pharmacokinetic exposure [see Clinical Pharmacology (12.3)] .
14.5 Non-Radiographic Axial Spondyloarthritis
The safety and efficacy of COSENTYX were assessed in 555 adult patients (18 years of age and older) with active nr-axSpA in one randomized, double-blind, placebo-controlled Phase 3 study (nr-axSpA1, NCT02696031). Patients met ASAS criteria for axSpA with objective signs of inflammation and had active disease as defined by a BASDAI greater or equal to 4, a Visual Analogue Scale (VAS) for total back pain greater or equal to 40 (on a scale of 0-100 mm) despite NSAID therapy and no evidence of radiographic changes in the sacroiliac joints that would meet the modified New York criteria for AS. Patients also had to have objective signs of inflammation with a C-reactive protein (CRP) level above the upper limit of normal and/or evidence of sacroiliitis on Magnetic Resonance Imaging (MRI).
Patients were treated with 150 mg of subcutaneous COSENTYX treatment with a loading dosage (Weeks 0, 1, 2, 3, and 4) or without a loading dosage (Weeks 0 and 4) followed by the same dose every 4 weeks or placebo. In the double-blind period, patients (n = 555) received either placebo or COSENTYX for 52 weeks. Starting Week 16, dosage adjustment or addition of concomitant NSAIDs and DMARDs was permitted. Starting at Week 20, patients were allowed to switch to open-label 150 mg of subcutaneous COSENTYX monthly or other biologic at the discretion of the investigator and patient. The primary endpoint was at least 40% improvement in Assessment of Spondyloarthritis International Society (ASAS40) at Week 52.
Baseline Disease Characteristics
Approximately 10% and 15% of patients used concomitant MTX or sulfasalazine, respectively. Overall, 10% of patients had received previous treatment with anti-TNFα agents and discontinued these due to either lack of efficacy or intolerance.
Clinical Response
In nr-axSpA1 Study, treatment with COSENTYX 150 mg resulted in significant improvements in the measure of disease activity compared to treatment with placebo at Week 16 and Week 52 (Table 12).
| Difference in proportions with 95% CI based on normal approximation. | |||||
| Number of subjects with ASAS40 response (%) | COSENTYX 150 mg without load (n = 184) | COSENTYX 150 mg with load (n = 185) | Difference from placebo (95% CI) | ||
| Placebo (n = 186) | COSENTYX 150 mg without load | COSENTYX 150 mg with load | |||
| Week 16 | 75 (41) | 74 (40) | 52 (28) | 13 (3, 22) | 12 (2, 22) |
| Week 52 | 70 (38) | 62 (34) | 36 (19) | 19 (10, 28) | 14 (5, 23) |
The results of the main components of the ASAS40 response criteria in the nr-axSpA1 Study are shown in Table 13.
| COSENTYX 150 mg without loading dosage (N = 184) | COSENTYX 150 mg with a loading dosage (N = 185) | Placebo (N = 186) | ||||
| Baseline | Week 16 change from baseline | Baseline | Week 16 change from baseline | Baseline | Week 16 change from baseline | |
| ASAS40 response criteria | ||||||
| -Patient Global Assessment of Disease Activity (0-100 mm) | 71.0 | -26.2 | 72.6 | -24.1 | 68.8 | -13.8 |
| -Total back pain (0-100 mm) | 72.0 | -25.5 | 73.3 | -25.0 | 70.9 | -15.6 |
| -BASFI (0-10) | 5.9 | -1.6 | 6.2 | -1.8 | 5.9 | -1.0 |
| -Inflammation (0-10) | 6.8 | -2.8 | 7.2 | -2.8 | 6.6 | -1.7 |
| hsCRP (mg/L) mean change at Week 16 | 9.8 | -4.7 | 13.4 | -7.9 | 9.2 | -2.4 |
| BASDAI (0-10) | 6.9 | -2.4 | 7.1 | -2.4 | 6.8 | -1.5 |
| -Spinal pain | 7.6 | -3.0 | 7.8 | -3.0 | 7.5 | -2.0 |
| -Peripheral pain and swelling (0-10) | 6.6 | -2.4 | 6.3 | -2.3 | 6.1 | -1.6 |
| BASMI | 2.8 | -0.3 | 2.9 | -0.3 | 2.8 | -0.1 |
The percentage of patients achieving an ASAS40 response by visit is shown in Figure 4.
Figure 4: ASAS40 Responses in nr-axSpA1 Study Over Time up to Week 16 (Subcutaneous Treatment)

Health-Related Quality of Life
COSENTYX treated patients showed improvement in both loading and without loading dosage arms compared to placebo-treated patients at Week 16 in health-related quality of life as measured by ASQoL (LS mean change: Week 16: -3.5 and -3.6 versus -1.8, respectively).
Treatment of Adult Patients with Active Non-radiographic Axial Spondyloarthritis with Intravenous COSENTYX
The effectiveness of intravenous COSENTYX in the treatment of adult patients with active nr-axSpA was extrapolated from the established effectiveness of subcutaneous COSENTYX in adult patients with active nr-axSpA based on pharmacokinetic exposure [see Clinical Pharmacology (12.3)] .
14.6 Juvenile Psoriatic Arthritis and Enthesitis-Related Arthritis
The efficacy and safety of subcutaneous secukinumab were assessed in a two-year, 3-part, double-blind, placebo-controlled, event-driven, randomized, Phase 3 study (NCT03031782) in 86 pediatric patients (2 to less than 18 years of age) with active ERA or JPsA as diagnosed based on a modified International League of Associations for Rheumatology (ILAR) Juvenile Idiopathic Arthritis (JIA) classification criteria. The trial consisted of an open-label portion (Part 1) followed by randomized withdrawal (Part 2) followed by open-label treatment (Part 3). Patients were given subcutaneous doses of 75 mg if they weighed less than 50 kg, or subcutaneous doses of 150 mg if they weighed at least 50 kg or greater, administered by subcutaneous injection at Weeks 0, 1, 2, 3, and 4, and every 4 weeks thereafter.
The primary endpoint was time to flare in Part 2. Disease flare was defined as at least 30% worsening in at least three of the six JIA ACR response criteria and at least 30% improvement in not more than one of the six JIA ACR response criteria and a minimum of two active joints.
In open-label Part 1, all patients received subcutaneous secukinumab until Week 12. Patients classified as responders (achieving JIA ACR30 response) at Week 12 entered into the Part 2 double-blind phase and were randomized 1:1 to continue treatment with secukinumab or begin treatment with placebo.
Baseline Disease Characteristics
The JIA patient subtypes at study entry were: 60.5% ERA and 39.5% JPsA. In the study, 67.6% of patients with JPsA, and 63.5% of patients with ERA, were treated concomitantly with MTX.
Clinical Response
Similar responses were seen in each JIA subtype (JPsA and ERA). The JIA ACR 30, 50, 70, and 90 responses for patients with JPsA and ERA at Week 12 are presented below in Table 14.
| Number of subjects with response (%) | JIA ACR 30 | JIA ACR 50 | JIA ACR 70 | JIA ACR 90 |
| JPsA (N = 34) | 31 (91) | 31 (91) | 24 (71) | 16 (47) |
| ERA (N = 52) | 44 (85) | 41 (79) | 34 (65) | 17 (33) |
Juvenile Psoriatic Arthritis Results
During Part 2, a total of 11 JPsA patients in the placebo group experienced a flare event compared with 4 JPsA patients in the secukinumab group. The risk of flare was reduced by 85% for patients who received secukinumab compared with patients who received placebo (Hazard Ratio = 0.15, 95% CI: 0.04 to 0.56) (Figure 5).
Figure 5: Kaplan-Meier Estimates of the Time to Disease Flare in Part 2 for JPsA Patients (Subcutaneous Treatment)

Enthesitis-Related Arthritis Results
During Part 2, a total of 10 ERA patients in the placebo group experienced a flare event compared with 6 ERA patients in the secukinumab group. The risk of flare was reduced by 53% for patients who received secukinumab compared with patients who received placebo (Hazard Ratio = 0.47, 95% CI: 0.17 to 1.32) (Figure 6). Supplementary analyses provided confirmatory evidence of the treatment effect in ERA.
Figure 6: Kaplan-Meier Estimates of the Time to Disease Flare in Part 2 for ERA Patients (Subcutaneous Treatment)

14.7 Hidradenitis Suppurativa
Two randomized, double-blind, placebo-controlled 52-week Phase 3 trials (i.e., HS Trial 1 [NCT03713619] and HS Trial 2 [NCT03713632]) assessed the efficacy and safety of COSENTYX in the treatment of adult patients with moderate to severe hidradenitis suppurativa (HS). In both trials, subjects were randomized to placebo or COSENTYX 300 mg by subcutaneous injection at Weeks 0, 1, 2, 3 and 4, followed by 300 mg every 2 weeks or every 4 weeks. At Week 16, subjects who were randomized to placebo were reassigned to receive COSENTYX 300 mg at Weeks 16, 17, 18, 19, and 20 followed by either COSENTYX 300 mg every 2 weeks (Q2W) or COSENTYX 300 mg every 4 weeks (Q4W).
Baseline Demographics and Disease Characteristics
HS Trials 1 and 2 included 1,084 adult subjects with moderate to severe HS. HS Trial 1 evaluated 541 subjects and HS Trial 2 evaluated 543 subjects, of whom 13% and 11%, respectively, received concomitant stable dose of systemic antibiotics. In HS Trial 1 and HS Trial 2, 24% and 23% of patients, respectively, were previously treated with a biologic (biologic-exposed patients) and discontinued the biologic agent for either lack of efficacy or intolerance.
Endpoints
The primary endpoint in both trials was the proportion of subjects who achieved a Hidradenitis Suppurativa Clinical Response (HiSCR50) defined as at least a 50% decrease in abscesses and inflammatory nodules (AN) count with no increase in the number of abscesses and/or in the number of draining fistulae relative to baseline at Week 16.
Clinical Response
In HS Trial 1 and HS Trial 2, a statistically significantly higher proportion of subjects treated with COSENTYX 300 mg every 2 weeks (after the first four weeks) achieved a HiSCR50 response at Week 16 compared to patients treated with placebo (see Table 15). In both HS trials, a higher proportion of subjects treated with COSENTYX 300 mg every 4 weeks (after the first four weeks) achieved HiSCR50 at Week 16 compared to subjects treated with placebo (see Table 15), where statistical significance was reached in HS Trial 2. In both trials, the onset of action of COSENTYX occurred as early as Week 2 and the efficacy progressively increased up to Week 16.
For the primary endpoint, HiSCR50, subjects who received any rescue medication or lesion intervention were considered treatment failures and handled as non-responders (n = 20 in Placebo, 11 in Q4W, and 8 in Q2W in HS Trial 1; n = 23 in Placebo, 17 in Q4W, and 13 in Q2W in HS Trial 2).
Improvements were seen for the primary endpoint in HS subjects regardless of previous or concomitant antibiotic treatment or previous biologic exposure.
| 1 Multiple imputation was implemented for missing data. 2 Subjects received COSENTYX 300 mg by subcutaneous injection at Weeks 0, 1, 2, 3 and 4, followed by 300 mg every 4 weeks (Q4W) or every 2 weeks (Q2W). • Statistically significant versus placebo based on the pre-defined hierarchy with overall alpha = 0.05 (two-sided). | ||||||
| HS Trial 1 | HS Trial 2 | |||||
| Placebo (n = 180) | COSENTYX 300 mg every 4 weeks 2 (n = 180) | COSENTYX 300 mg every 2 weeks 2 (n = 181) | Placebo (n = 183) | COSENTYX 300 mg every 4 weeks 2 (n = 180) | COSENTYX 300 mg every 2 weeks 2 (n = 180) | |
| HiSCR50 | 29.4% | 41.3% | 44.5% • | 26.1% | 42.5% • | 38.3% • |
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
COSENTYX (secukinumab) injection is a clear to opalescent, colorless to slightly yellowish solution available as follows:
COSENTYX injection for subcutaneous use
COSENTYX 300 mg/2 mL UnoReady pen
- NDC 0078-1070-68: Carton of one 300 mg/2 mL (300 mg dose) single-dose UnoReady pen (injection)
COSENTYX 300 mg/2 mL (150 mg/mL) prefilled syringe
- NDC 0078-1070-97: Carton of one 300 mg/2 mL (150 mg/mL) single-dose prefilled syringe (injection)
COSENTYX 150 mg/mL Sensoready pen
- NDC 0078-0639-41: Carton of two 150 mg/mL (300 mg dose) single-dose Sensoready pens (injection)
- NDC 0078-0639-68: Carton of one 150 mg/mL single-dose Sensoready pen (injection)
COSENTYX 150 mg/mL prefilled syringe
- NDC 0078-0639-98: Carton of two 150 mg/mL (300 mg dose) single-dose prefilled syringes (injection)
- NDC 0078-0639-97: Carton of one 150 mg/mL single-dose prefilled syringe (injection)
COSENTYX 75 mg/0.5 mL prefilled syringe (for pediatric patients less than 50 kg)
- NDC 0078-1056-97: Carton of one 75 mg/0.5 mL single-dose prefilled syringe (injection)
The removable cap of the COSENTYX 150 mg/mL Sensoready pen and prefilled syringe, and 75 mg/0.5 mL prefilled syringe contains natural rubber latex. Each 300 mg/2 mL UnoReady pen, 150 mg/mL Sensoready pen and 300 mg/2 mL, 150 mg/mL, and 75 mg/0.5 mL prefilled syringe is equipped with a needle safety guard.
COSENTYX injection for intravenous use
- NDC 0078-1168-61: Carton containing one 125 mg/5 mL (25 mg/mL) solution in a single-dose vial for dilution prior to intravenous infusion.
Storage and Handling
Refrigerate COSENTYX injection for subcutaneous use (300 mg/2 mL UnoReady Pen, 150 mg/mL Sensoready Pens, and 150 mg/mL and 75 mg/0.5 mL Prefilled Syringes), and COSENTYX injection for intravenous use at 2ºC to 8ºC (36ºF to 46ºF). Keep the products in the original carton to protect from light until the time of use. Do not freeze. To avoid foaming, do not shake. COSENTYX does not contain a preservative; discard any unused portion.
If removed from refrigeration, COSENTYX 150 mg/mL Sensoready Pens, and 150 mg/mL and 75 mg/0.5 mL Prefilled Syringes:
- May be stored for up to 4 days at room temperature not to exceed 30°C (86°F).
- Write the date COSENTYX is removed from and returned to the refrigerator in the space provided on the carton.
- Discard if stored outside of the refrigerator over 4 days.
- May be returned to the refrigerator only one time and must be stored at 2ºC to 8ºC (36ºF to 46ºF) until used or expired.
INSTRUCTIONS FOR USE
COSENTYX ® [koe-sen-tix]
(secukinumab)
injection, for subcutaneous use
300 mg/2 mL single-dose prefilled syringe
Be sure that you read, understand, and follow this Instructions for Use before injecting COSENTYX. Your healthcare provider should show you how to prepare and inject COSENTYX properly using the prefilled syringe before you use it for the first time. Talk to your healthcare provider if you have any questions.
Important Information You Need to Know Before Injecting COSENTYX:
- Do not use the COSENTYX prefilled syringe if either the seal on the outside carton or the seal of the blister are broken. Keep the COSENTYX prefilled syringe in the sealed carton until you are ready to use it.
- Do not use the COSENTYX prefilled syringe if the syringe has been dropped onto a hard surface or dropped after removing the needle cap.
- Do not shake the COSENTYX prefilled syringe.
- The prefilled syringe has a needle guard that will be activated to cover the needle after the injection is finished. The needle guard will help to prevent needle stick injuries to anyone who handles the prefilled syringe.
- Do not remove the needle cap until just before you give the injection.
- Avoid touching the syringe guard wings before use. Touching them may cause the syringe guard to be activated too early.
- Throw away (dispose of) the used COSENTYX prefilled syringe right away after use. Do not re-use the COSENTYX prefilled syringe . See “How should I dispose of the used COSENTYX prefilled syringe?” at the end of this Instructions for Use.
How should I store COSENTYX?
- Store your carton of COSENTYX prefilled syringe in a refrigerator, between 36°F to 46°F (2°C to 8°C).
- Keep the COSENTYX prefilled syringe in the original carton until ready to use to protect from light.
- Do not freeze the COSENTYX prefilled syringe.
- Throw away (dispose of) any expired or unused COSENTYX prefilled syringes.
Keep COSENTYX and all medicines out of the reach of children. COSENTYX prefilled syringe parts (see Figure A):
| This Instructions for Use has been approved by the U.S. Food and Drug Administration. | Issued: July 2023 |
| Figure A | |
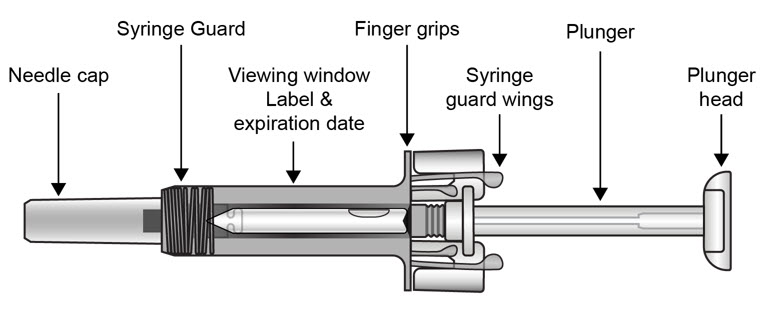 | |
| What you need for your injection: | |
| Included in the carton: | |
| A new COSENTYX prefilled syringe. | |
| Each COSENTYX prefilled syringe contains 300 mg of COSENTYX. Check to make sure that you have the correct medicine and dose. | |
| Not included in the carton (see Figure B): • 1 Alcohol wipe • 1 Cotton ball or gauze • Sharps disposal container See “ How should I dispose of the used COSENTYX prefilled syringe ?” at the end of this Instructions for Use. | Figure B  |
| Prepare the COSENTYX 300 mg prefilled syringe | |
| Step 1. Find a clean, well-lit, flat work surface. | |
| Step 2. Take the carton containing the COSENTYX prefilled syringe out of the refrigerator and leave it unopened on your work surface for about 30 to 45 minutes so that it reaches room temperature. | |
| Step 3. Wash your hands well with soap and water. | |
| Step 4. Remove the COSENTYX prefilled syringe from the outer carton and take it out of the blister. | |
| Step 5. Look through the viewing window on the COSENTYX prefilled syringe. The liquid inside should be clear. The color may be colorless to slightly yellow. You may see a small air bubble in the liquid. This is normal. Do not use the prefilled syringe if the liquid contains visible particles, or if the liquid is cloudy or discolored. | |
| Step 6. Do not use the COSENTYX prefilled syringe if it is broken. Return the prefilled syringe and the package it came in to the pharmacy. | |
| Step 7. Do not use the COSENTYX prefilled syringe if the expiration date has passed. | |
| Choose and clean the injection site | |
| Figure C 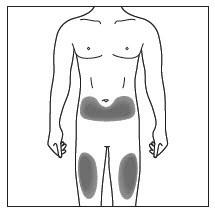 |
| Step 8. Using a circular motion, clean the injection site with the alcohol wipe. Leave it to dry before injecting. Do not touch the cleaned area again before injecting. | Figure D 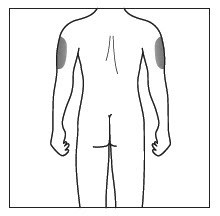 |
| Giving the injection | |
| Step 9. Carefully remove the needle cap from the COSENTYX prefilled syringe (see Figure E) . Throw away the needle cap. You may see a drop of liquid at the end of the needle. This is normal. | Figure E 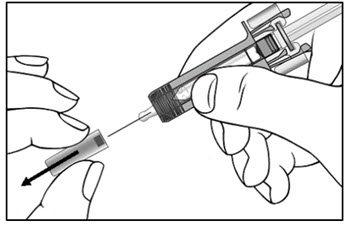 |
| Step 10. With one hand gently pinch the skin at the injection site. With your other hand insert the needle into your skin at a 45-degree angle as shown (see Figure F) . Push the needle all the way in to make sure that you inject your full dose. | Figure F 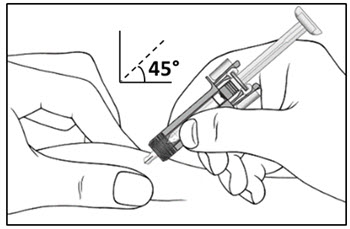 |
| Step 11. Hold the COSENTYX prefilled syringe finger grips as shown (see Figure G) . Slowly press down on the plunger as far as it will go, so that the plunger head is completely between the syringe guard wings. This will make sure that the syringe guard has been activated. Step 12. Continue to press fully on the plunger for an additional 5 seconds. Hold the syringe in place for the full 5 seconds. | Figure G 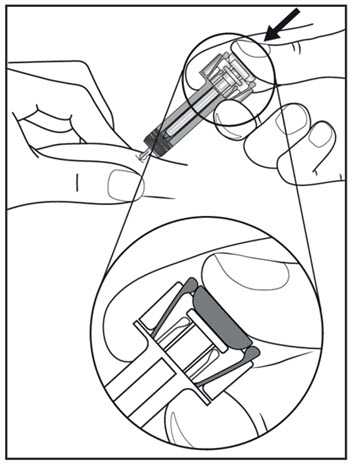 |
| Step 13. Keep the plunger fully depressed while you carefully pull the needle straight out from the injection site (see Figure H) . | Figure H 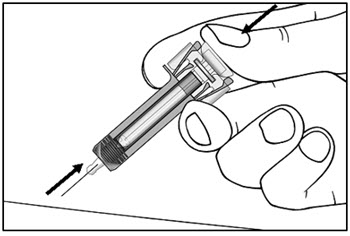 |
| Step 14. Slowly release the plunger and allow the syringe guard to automatically cover the exposed needle (see Figure I) . Step 15. There may be a small amount of blood at the injection site. You can press a cotton ball or gauze over the injection site and hold it for 10 seconds. Do not rub the injection site. You may cover the injection site with a small adhesive bandage, if needed. | Figure I 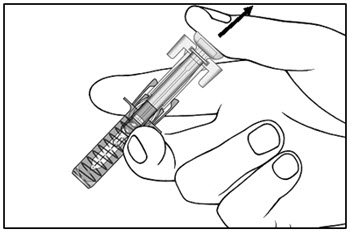 |
| How should I dispose of the used COSENTYX prefilled syringe? | |
Step 16. Put your used prefilled syringe in an FDA-cleared sharps disposal container right away after use (see Figure J). Do not throw away (dispose of) the prefilled syringe in your household trash. If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
| Figure J  |
| Manufactured by: Novartis Pharmaceuticals Corporation East Hanover, New Jersey 07936 US License Number 1244 | |
| © Novartis | |
T2023-42
12.1 Mechanism of Action
Secukinumab is a human IgG1 monoclonal antibody that selectively binds to the interleukin-17A (IL-17A) cytokine and inhibits its interaction with the IL-17 receptor. IL-17A is a naturally occurring cytokine that is involved in normal inflammatory and immune responses. Secukinumab inhibits the release of proinflammatory cytokines and chemokines.