Enbrel prior authorization resources
Most recent state uniform prior authorization forms
Brand Resources
Patient education
Administration guides
Patient education materials
Patient support program
Dosing resources
Clinical information
Insurance resources
Prior authorization & coverage support
Financial assistance & copay programs
Specialty pharmacy coordination
Other resources
Dosage & administration
DOSAGE AND ADMINISTRATION
Enbrel is administered by subcutaneous injection.
| Patient Population | Recommended Dose and Frequency |
|---|---|
| Adult RA and PsA (2.3 ) | 50 mg once weekly with or without methotrexate (MTX) |
| AS (2.3 ) | 50 mg once weekly |
| Adult PsO (2.3 ) | 50 mg twice weekly for 3 months, followed by 50 mg once weekly |
| pJIA, Pediatric PsO and JPsA (2.4 ) | 0.8 mg/kg weekly, with a maximum of 50 mg per week |
Testing and Procedures Prior to Treatment Initiation
Perform the following evaluations and procedures prior to initiating treatment with Enbrel:
- Prior to initiating Enbrel and periodically during therapy, evaluate patients for active tuberculosis and test for latent infection [see Warnings and Precautions (5.1) ].
- Complete all age-appropriate vaccinations as recommended by current immunization guidelines prior to initiating treatment with Enbrel [see Warnings and Precautions (5.8) ].
Important Administration Instructions
Administration of one 50 mg Enbrel single - dose prefilled syringe, one single - dose prefilled Enbrel SureClick autoinjector, or one Enbrel Mini single - dose prefilled cartridge (for use with the AutoTouch reusable autoinjector only), provides a dose equivalent to two 25 mg Enbrel single-dose prefilled syringes, two 25 mg single - dose vials, or two multiple-dose vials of lyophilized Enbrel, when multiple - dose vials are reconstituted and administered as recommended.
Recommended Dosage in Adult Patients with Rheumatoid Arthritis, Ankylosing Spondylitis, Psoriatic Arthritis, and Plaque Psoriasis
Enbrel is administered by subcutaneous injection (Table 1).
| Patient Population | Recommended Dosage |
|---|---|
| Adult RA, AS, and PsA | 50 mg weekly |
| Adult PsO | Starting Dose : 50 mg twice weekly for 3 months Maintenance Dose : 50 mg once weekly |
See the Enbrel (etanercept) "Instructions for Use" insert for detailed information on injection site selection and dose administration [see Dosage and Administration (2.3) and Patient Counseling Information (17) ] .
Adult Rheumatoid Arthritis, Ankylosing Spondylitis, and Psoriatic Arthritis Patients
Methotrexate, glucocorticoids, salicylates, nonsteroidal anti-inflammatory drugs (NSAIDs), or analgesics may be continued during treatment with Enbrel.
Based on a study of 50 mg Enbrel twice weekly in patients with RA that suggested higher incidence of adverse reactions but similar American College of Rheumatology (ACR) response rates, doses higher than 50 mg per week are not recommended.
Adult Plaque Psoriasis Patients
In addition to the 50 mg twice weekly recommended starting dose, starting doses of 25 mg or 50 mg per week were shown to be efficacious. The proportion of responders was related to Enbrel dosage [see Clinical Studies (14.5) ] .
Recommended Dosage for Pediatric Patients with Polyarticular Juvenile Idiopathic Arthritis, Plaque Psoriasis, and Juvenile Psoriatic Arthritis
The recommended weight-based dosage for pediatric patients is administered by subcutaneous injection (Table 2).
| Body Weight | Recommended Dosage |
|---|---|
| 63 kg (138 pounds) or more | 50 mg weekly |
| Less than 63 kg (138 pounds) | 0.8 mg/kg weekly |
To achieve pediatric doses other than 25 mg or 50 mg, use Enbrel solution in a single-dose vial or reconstituted lyophilized powder in a multiple-dose vial.
Dosages of Enbrel higher than those described in Table 2 have not been studied in pediatric patients.
In pJIA patients, glucocorticoids, NSAIDs, or analgesics may be continued during treatment with Enbrel.
Preparation Instructions for Enbrel
Enbrel is intended for use under the guidance and supervision of a physician. Patients may self-inject when deemed appropriate and if they receive medical follow-up, as necessary. Patients should not self-administer until they receive proper training in how to prepare and administer the correct dose. Administer injections subcutaneously in the thigh, abdomen or outer area of the upper arm.
The Enbrel devices are not made with natural rubber latex.
The Enbrel (etanercept) "Instructions for Use" insert for each presentation contains more detailed instructions on injection site selection and the preparation of Enbrel.
Preparation of Enbrel Single-dose Prefilled Syringe
For a more comfortable injection, leave Enbrel prefilled syringes at room temperature for about 15 to 30 minutes before injecting. DO NOT remove the needle cover while allowing the prefilled syringe to reach room temperature.
Inspect visually for particulate matter and discoloration prior to administration. There may be small white particles of protein in the solution. This is not unusual for proteinaceous solutions. The solution should not be used if discolored or cloudy, or if foreign particulate matter is present.
When using the Enbrel single-dose prefilled syringe, check to see if the amount of liquid in the prefilled syringe falls between the two purple fill level indicator lines on the syringe. If the syringe does not have the right amount of liquid, DO NOT USE THAT SYRINGE.
Preparation of Enbrel Single-dose Prefilled SureClick Autoinjector
Leave the autoinjector at room temperature for at least 30 minutes before injecting. DO NOT remove the needle cover while allowing the prefilled syringe to reach room temperature.
Inspect visually for particulate matter and discoloration prior to administration. There may be small white particles of protein in the solution. This is not unusual for proteinaceous solutions. The solution should not be used if discolored or cloudy, or if foreign particulate matter is present.
Preparation of Enbrel Single-dose Vial
For a more comfortable injection, leave Enbrel vial(s) at room temperature for at least 30 minutes before injecting. DO NOT remove the vial cap while allowing the vial to reach room temperature.
Inspect visually for particulate matter and discoloration prior to administration. There may be small white particles of protein in the solution. This is not unusual for proteinaceous solutions. The solution should not be used if discolored or cloudy, or if foreign particulate matter is present.
When using the Enbrel single-dose vial, administer the correct dose of solution using the following recommended materials:
- A 1 mL Luer-Lock syringe.
- A withdrawal needle with Luer-Lock connection, sterile, 22-gauge, length 1 ½ inch.
- An injection needle with Luer-Lock connection, sterile, 27-gauge, length ½ inch.
Two vials may be required to administer the total prescribed dose. Use the same syringe for each vial. The vial does not contain preservatives; therefore, discard unused portions.
Preparation of Enbrel Lyophilized Powder in a Multiple-dose Vial
Enbrel lyophilized powder should be reconstituted aseptically with 1 mL of the supplied Sterile Bacteriostatic Water for Injection, USP (0.9% benzyl alcohol), giving a solution of 1 mL containing 25 mg of Enbrel.
A vial adapter is supplied for use when reconstituting the lyophilized powder. However, the vial adapter should not be used if multiple doses are going to be withdrawn from the vial. If the vial will be used for multiple doses, a 25-gauge needle should be used for reconstituting and withdrawing Enbrel, and the supplied "Mixing Date:" sticker should be attached to the vial and the date of reconstitution entered. Reconstituted solution must be refrigerated at 36°F to 46°F (2°C to 8°C) and used within 14 days. Discard reconstituted solution after 14 days because product stability and sterility cannot be assured after 14 days. DO NOT store reconstituted Enbrel solution at room temperature.
For a more comfortable injection, leave the Enbrel dose tray at room temperature for about 15 to 30 minutes before injecting.
If using the vial adapter, twist the vial adapter onto the diluent syringe. Then, place the vial adapter over the Enbrel vial and insert the vial adapter into the vial stopper. Push down on the plunger to inject the diluent into the Enbrel vial. If using a 25-gauge needle to reconstitute and withdraw Enbrel, the diluent should be injected very slowly into the Enbrel vial. It is normal for some foaming to occur. Keeping the diluent syringe in place, gently swirl the contents of the Enbrel vial during dissolution. To avoid excessive foaming, do not shake or vigorously agitate.
Generally, dissolution of Enbrel takes less than 10 minutes. Do not use the solution if discolored or cloudy, or if particulate matter remains.
Withdraw the correct dose of reconstituted solution into the syringe. Some foam or bubbles may remain in the vial. Remove the syringe from the vial adapter or remove the 25-gauge needle from the syringe. Attach a 27-gauge needle to inject Enbrel.
The contents of one vial of Enbrel solution should not be mixed with, or transferred into, the contents of another vial of Enbrel. No other medications should be added to solutions containing Enbrel, and do not reconstitute Enbrel with other diluents. Do not filter reconstituted solution during preparation or administration.
Preparation of Enbrel Mini ® single-dose prefilled cartridge using the AutoTouch ® reusable autoinjector
Leave Enbrel Mini single-dose prefilled cartridge at room temperature for at least 30 minutes before injecting. DO NOT remove the purple cap while allowing the cartridge to reach room temperature.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration. There may be small white particles of protein in the solution. This is not unusual for proteinaceous solutions. The solution should not be used if discolored or cloudy, or if foreign particulate matter is present.
To use AutoTouch reusable autoinjector, open the door by pushing the door button and inserting Enbrel Mini single-dose prefilled cartridge into AutoTouch. When inserted correctly, Enbrel Mini single-dose prefilled cartridge will slide freely and completely into the door. Close the door and AutoTouch reusable autoinjector is ready for injection.

By using PrescriberAI, you agree to the AI Terms of Use.
Enbrel prescribing information
WARNING: SERIOUS INFECTIONS and MALIGNANCIES
WARNING: SERIOUS INFECTIONS and MALIGNANCIES
See full prescribing information for complete boxed warning.
SERIOUS INFECTIONS
- Increased risk of serious infections leading to hospitalization or death, including tuberculosis (TB), bacterial sepsis, invasive fungal infections (such as histoplasmosis), and infections due to other opportunistic pathogens. (5.1 )
- Enbrel should be discontinued if a patient develops a serious infection or sepsis during treatment. (5.1 )
- Perform test for latent TB; if positive, start treatment for TB prior to starting Enbrel. (5.1 )
- Monitor all patients for active TB during treatment, even if initial latent TB test is negative. (5.1 )
MALIGNANCIES
- Lymphoma and other malignancies, some fatal, have been reported in children and adolescent patients treated with TNF-blockers, including Enbrel. (5.3 )
SERIOUS INFECTIONS
Patients treated with Enbrel are at increased risk for developing serious infections that may lead to hospitalization or death [see Warnings and Precautions (5.1) and Adverse Reactions (6) ] . Most patients who developed these infections were taking concomitant immunosuppressants such as methotrexate or corticosteroids.
Enbrel should be discontinued if a patient develops a serious infection or sepsis.
Reported infections include:
- Active tuberculosis, including reactivation of latent tuberculosis. Patients with tuberculosis have frequently presented with disseminated or extrapulmonary disease. Test patients for latent tuberculosis before Enbrel use and during therapy. Initiate treatment for latent infection prior to Enbrel use.
- Invasive fungal infections, including histoplasmosis, coccidioidomycosis, candidiasis, aspergillosis, blastomycosis, and pneumocystosis. Patients with histoplasmosis or other invasive fungal infections may present with disseminated, rather than localized, disease. Antigen and antibody testing for histoplasmosis may be negative in some patients with active infection. Consider empiric anti-fungal therapy in patients at risk for invasive fungal infections who develop severe systemic illness.
- Bacterial, viral, and other infections due to opportunistic pathogens, including Legionella and Listeria.
The risks and benefits of treatment with Enbrel should be carefully considered prior to initiating therapy in patients with chronic or recurrent infection.
Monitor patients closely for the development of signs and symptoms of infection during and after treatment with Enbrel, including the possible development of tuberculosis in patients who tested negative for latent tuberculosis infection prior to initiating therapy.
MALIGNANCIES
Lymphoma and other malignancies, some fatal, have been reported in children and adolescent patients treated with TNF-blockers, including Enbrel.
INDICATIONS AND USAGE
Enbrel is a tumor necrosis factor (TNF) blocker indicated for the treatment of:
Adult patients with:
- Rheumatoid Arthritis (RA) (1.1 )
- Psoriatic Arthritis (PsA) (1.3 )
- Ankylosing Spondylitis (AS) (1.4 )
- Plaque Psoriasis (PsO) (1.5 )
Pediatric patients with:
Rheumatoid Arthritis
Enbrel is indicated for reducing signs and symptoms, inducing major clinical response, inhibiting the progression of structural damage, and improving physical function in patients with moderately to severely active rheumatoid arthritis (RA). Enbrel can be initiated in combination with methotrexate (MTX) or used alone.
Polyarticular Juvenile Idiopathic Arthritis
Enbrel is indicated for reducing signs and symptoms of moderately to severely active polyarticular juvenile idiopathic arthritis (pJIA) in patients 2 years of age and older.
Psoriatic Arthritis
Enbrel is indicated for reducing signs and symptoms, inhibiting the progression of structural damage of active arthritis, and improving physical function in adult patients with psoriatic arthritis (PsA). Enbrel can be used with or without methotrexate.
Ankylosing Spondylitis
Enbrel is indicated for reducing signs and symptoms in patients with active ankylosing spondylitis (AS).
Plaque Psoriasis
Enbrel is indicated for the treatment of patients 4 years or older with chronic moderate to severe plaque psoriasis (PsO) who are candidates for systemic therapy or phototherapy.
Juvenile Psoriatic Arthritis
Enbrel is indicated for the treatment of active juvenile psoriatic arthritis (JPsA) in pediatric patients 2 years of age and older.
DOSAGE AND ADMINISTRATION
Enbrel is administered by subcutaneous injection.
| Patient Population | Recommended Dose and Frequency |
|---|---|
| Adult RA and PsA (2.3 ) | 50 mg once weekly with or without methotrexate (MTX) |
| AS (2.3 ) | 50 mg once weekly |
| Adult PsO (2.3 ) | 50 mg twice weekly for 3 months, followed by 50 mg once weekly |
| pJIA, Pediatric PsO and JPsA (2.4 ) | 0.8 mg/kg weekly, with a maximum of 50 mg per week |
Testing and Procedures Prior to Treatment Initiation
Perform the following evaluations and procedures prior to initiating treatment with Enbrel:
- Prior to initiating Enbrel and periodically during therapy, evaluate patients for active tuberculosis and test for latent infection [see Warnings and Precautions (5.1) ].
- Complete all age-appropriate vaccinations as recommended by current immunization guidelines prior to initiating treatment with Enbrel [see Warnings and Precautions (5.8) ].
Important Administration Instructions
Administration of one 50 mg Enbrel single - dose prefilled syringe, one single - dose prefilled Enbrel SureClick autoinjector, or one Enbrel Mini single - dose prefilled cartridge (for use with the AutoTouch reusable autoinjector only), provides a dose equivalent to two 25 mg Enbrel single-dose prefilled syringes, two 25 mg single - dose vials, or two multiple-dose vials of lyophilized Enbrel, when multiple - dose vials are reconstituted and administered as recommended.
Recommended Dosage in Adult Patients with Rheumatoid Arthritis, Ankylosing Spondylitis, Psoriatic Arthritis, and Plaque Psoriasis
Enbrel is administered by subcutaneous injection (Table 1).
| Patient Population | Recommended Dosage |
|---|---|
| Adult RA, AS, and PsA | 50 mg weekly |
| Adult PsO | Starting Dose : 50 mg twice weekly for 3 months Maintenance Dose : 50 mg once weekly |
See the Enbrel (etanercept) "Instructions for Use" insert for detailed information on injection site selection and dose administration [see Dosage and Administration (2.3) and Patient Counseling Information (17) ] .
Adult Rheumatoid Arthritis, Ankylosing Spondylitis, and Psoriatic Arthritis Patients
Methotrexate, glucocorticoids, salicylates, nonsteroidal anti-inflammatory drugs (NSAIDs), or analgesics may be continued during treatment with Enbrel.
Based on a study of 50 mg Enbrel twice weekly in patients with RA that suggested higher incidence of adverse reactions but similar American College of Rheumatology (ACR) response rates, doses higher than 50 mg per week are not recommended.
Adult Plaque Psoriasis Patients
In addition to the 50 mg twice weekly recommended starting dose, starting doses of 25 mg or 50 mg per week were shown to be efficacious. The proportion of responders was related to Enbrel dosage [see Clinical Studies (14.5) ] .
Recommended Dosage for Pediatric Patients with Polyarticular Juvenile Idiopathic Arthritis, Plaque Psoriasis, and Juvenile Psoriatic Arthritis
The recommended weight-based dosage for pediatric patients is administered by subcutaneous injection (Table 2).
| Body Weight | Recommended Dosage |
|---|---|
| 63 kg (138 pounds) or more | 50 mg weekly |
| Less than 63 kg (138 pounds) | 0.8 mg/kg weekly |
To achieve pediatric doses other than 25 mg or 50 mg, use Enbrel solution in a single-dose vial or reconstituted lyophilized powder in a multiple-dose vial.
Dosages of Enbrel higher than those described in Table 2 have not been studied in pediatric patients.
In pJIA patients, glucocorticoids, NSAIDs, or analgesics may be continued during treatment with Enbrel.
Preparation Instructions for Enbrel
Enbrel is intended for use under the guidance and supervision of a physician. Patients may self-inject when deemed appropriate and if they receive medical follow-up, as necessary. Patients should not self-administer until they receive proper training in how to prepare and administer the correct dose. Administer injections subcutaneously in the thigh, abdomen or outer area of the upper arm.
The Enbrel devices are not made with natural rubber latex.
The Enbrel (etanercept) "Instructions for Use" insert for each presentation contains more detailed instructions on injection site selection and the preparation of Enbrel.
Preparation of Enbrel Single-dose Prefilled Syringe
For a more comfortable injection, leave Enbrel prefilled syringes at room temperature for about 15 to 30 minutes before injecting. DO NOT remove the needle cover while allowing the prefilled syringe to reach room temperature.
Inspect visually for particulate matter and discoloration prior to administration. There may be small white particles of protein in the solution. This is not unusual for proteinaceous solutions. The solution should not be used if discolored or cloudy, or if foreign particulate matter is present.
When using the Enbrel single-dose prefilled syringe, check to see if the amount of liquid in the prefilled syringe falls between the two purple fill level indicator lines on the syringe. If the syringe does not have the right amount of liquid, DO NOT USE THAT SYRINGE.
Preparation of Enbrel Single-dose Prefilled SureClick Autoinjector
Leave the autoinjector at room temperature for at least 30 minutes before injecting. DO NOT remove the needle cover while allowing the prefilled syringe to reach room temperature.
Inspect visually for particulate matter and discoloration prior to administration. There may be small white particles of protein in the solution. This is not unusual for proteinaceous solutions. The solution should not be used if discolored or cloudy, or if foreign particulate matter is present.
Preparation of Enbrel Single-dose Vial
For a more comfortable injection, leave Enbrel vial(s) at room temperature for at least 30 minutes before injecting. DO NOT remove the vial cap while allowing the vial to reach room temperature.
Inspect visually for particulate matter and discoloration prior to administration. There may be small white particles of protein in the solution. This is not unusual for proteinaceous solutions. The solution should not be used if discolored or cloudy, or if foreign particulate matter is present.
When using the Enbrel single-dose vial, administer the correct dose of solution using the following recommended materials:
- A 1 mL Luer-Lock syringe.
- A withdrawal needle with Luer-Lock connection, sterile, 22-gauge, length 1 ½ inch.
- An injection needle with Luer-Lock connection, sterile, 27-gauge, length ½ inch.
Two vials may be required to administer the total prescribed dose. Use the same syringe for each vial. The vial does not contain preservatives; therefore, discard unused portions.
Preparation of Enbrel Lyophilized Powder in a Multiple-dose Vial
Enbrel lyophilized powder should be reconstituted aseptically with 1 mL of the supplied Sterile Bacteriostatic Water for Injection, USP (0.9% benzyl alcohol), giving a solution of 1 mL containing 25 mg of Enbrel.
A vial adapter is supplied for use when reconstituting the lyophilized powder. However, the vial adapter should not be used if multiple doses are going to be withdrawn from the vial. If the vial will be used for multiple doses, a 25-gauge needle should be used for reconstituting and withdrawing Enbrel, and the supplied "Mixing Date:" sticker should be attached to the vial and the date of reconstitution entered. Reconstituted solution must be refrigerated at 36°F to 46°F (2°C to 8°C) and used within 14 days. Discard reconstituted solution after 14 days because product stability and sterility cannot be assured after 14 days. DO NOT store reconstituted Enbrel solution at room temperature.
For a more comfortable injection, leave the Enbrel dose tray at room temperature for about 15 to 30 minutes before injecting.
If using the vial adapter, twist the vial adapter onto the diluent syringe. Then, place the vial adapter over the Enbrel vial and insert the vial adapter into the vial stopper. Push down on the plunger to inject the diluent into the Enbrel vial. If using a 25-gauge needle to reconstitute and withdraw Enbrel, the diluent should be injected very slowly into the Enbrel vial. It is normal for some foaming to occur. Keeping the diluent syringe in place, gently swirl the contents of the Enbrel vial during dissolution. To avoid excessive foaming, do not shake or vigorously agitate.
Generally, dissolution of Enbrel takes less than 10 minutes. Do not use the solution if discolored or cloudy, or if particulate matter remains.
Withdraw the correct dose of reconstituted solution into the syringe. Some foam or bubbles may remain in the vial. Remove the syringe from the vial adapter or remove the 25-gauge needle from the syringe. Attach a 27-gauge needle to inject Enbrel.
The contents of one vial of Enbrel solution should not be mixed with, or transferred into, the contents of another vial of Enbrel. No other medications should be added to solutions containing Enbrel, and do not reconstitute Enbrel with other diluents. Do not filter reconstituted solution during preparation or administration.
Preparation of Enbrel Mini ® single-dose prefilled cartridge using the AutoTouch ® reusable autoinjector
Leave Enbrel Mini single-dose prefilled cartridge at room temperature for at least 30 minutes before injecting. DO NOT remove the purple cap while allowing the cartridge to reach room temperature.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration. There may be small white particles of protein in the solution. This is not unusual for proteinaceous solutions. The solution should not be used if discolored or cloudy, or if foreign particulate matter is present.
To use AutoTouch reusable autoinjector, open the door by pushing the door button and inserting Enbrel Mini single-dose prefilled cartridge into AutoTouch. When inserted correctly, Enbrel Mini single-dose prefilled cartridge will slide freely and completely into the door. Close the door and AutoTouch reusable autoinjector is ready for injection.
DOSAGE FORMS AND STRENGTHS
- Injection: 25 mg/0.5 mL and 50 mg/mL clear, colorless solution in a single-dose prefilled syringe
- Injection: 50 mg/mL clear, colorless solution in a single-dose prefilled SureClick autoinjector
- Injection: 25 mg/0.5 mL clear, colorless solution in a single-dose vial
- For Injection: 25 mg lyophilized powder in a multiple-dose vial for reconstitution
- Injection: 50 mg/mL clear, colorless solution in Enbrel Mini single-dose prefilled cartridge for use with the AutoTouch reusable autoinjector only
USE IN SPECIFIC POPULATIONS
Pregnancy
Risk Summary
Available studies with use of etanercept during pregnancy do not reliably support an association between etanercept and major birth defects. Clinical data are available from the Organization of Teratology Information Specialists (OTIS) Enbrel Pregnancy Registry in women with rheumatic diseases or psoriasis and a Scandinavian study in pregnant women with chronic inflammatory disease. Both the OTIS Registry and the Scandinavian study showed the proportion of liveborn infants with major birth defects was higher for women exposed to etanercept compared to diseased etanercept unexposed women. However, the lack of pattern of major birth defects is reassuring and differences between exposure groups (e.g., disease severity) may have impacted the occurrence of birth defects (see Data ).
Reports of etanercept use during the third trimester of pregnancy demonstrated that placental transfer of etanercept was low in infants at birth (see Data ) . There are risks to the mother and fetus associated with active rheumatoid arthritis. The theoretical risks of administration of live or live-attenuated vaccines to the infants exposed in utero to Enbrel should be weighed against the benefits of vaccinations (see Clinical Considerations ) .
In animal reproduction studies with pregnant rats and rabbits, no fetal harm or malformations were observed with subcutaneous administration of etanercept during the period of organogenesis at doses that achieved systemic exposures 48 to 58 times the exposure in patients treated with 50 mg Enbrel once weekly (see Data ) .
All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. The estimated background risk of major birth defects and miscarriage for the indicated populations is unknown. In the United States, about 2-4% of liveborn babies have a major birth defect and about 15-20% of pregnancies end in miscarriage, regardless of drug exposure.
Clinical Considerations
Disease-Associated Maternal and/or Embryo/Fetal Risk
Published data suggest that the risk of adverse pregnancy outcomes in women with rheumatoid arthritis is correlated with maternal disease activity and that active disease increases the risk of adverse pregnancy outcomes, including fetal loss, preterm delivery (before 37 weeks of gestation), low birth weight (less than 2500 g) and small for gestational age birth.
Fetal/Neonatal Adverse Reactions
The risk of fetal/neonatal adverse reactions with in utero exposure to Enbrel is unknown. Risks and benefits should be considered prior to administering live or live -attenuated vaccines to infants exposed to Enbrel in utero [see Warnings and Precautions (5.8) and Drug Interactions (7.1) ] .
Data
Human Data
A prospective cohort pregnancy registry conducted by OTIS in the US and Canada between 2000 and 2012 compared the risk of major birth defects in liveborn infants of women with rheumatic diseases or psoriasis exposed to etanercept in the first trimester. The proportion of major birth defects among liveborn infants in the etanercept-exposed (N = 319) and diseased etanercept unexposed cohorts (N = 144) was 9.4% and 3.5%, respectively. The findings showed no statistically significant increased risk of minor birth defects and no pattern of major or minor birth defects.
A Scandinavian study compared the risk of major birth defects in liveborn infants of women with chronic inflammatory disease (CID) exposed to TNF-inhibitors during early pregnancy. Women were identified from the Danish (2004-2012) and Swedish (2006-2012) population-based health registers. The proportion of major birth defects among liveborn infants in the etanercept-exposed (N = 344) and CID etanercept unexposed cohorts (N = 21,549) was 7.0% and 4.7%, respectively.
Overall, while both the OTIS Registry and Scandinavian study show a higher proportion of major birth defects in etanercept-exposed patients compared to diseased etanercept unexposed patients, the lack of pattern of birth defects is reassuring and differences between exposure groups (e.g., disease severity) may have impacted the occurrence of birth defects.
Reports from the literature showed that cord blood levels of etanercept at delivery, in infants born to women administered etanercept during pregnancy, varied from undetectable to 32% of the maternal serum level. In a cohort study of 30 pregnant women with RA, 29 were treated with etanercept until 30 weeks of gestation and 1 was treated until 36 weeks of gestation. Etanercept was not detected in the cord blood sample from any infant at delivery. In three published case reports, etanercept was detected in cord blood at levels of 3.3, 3.6, and 7.4% of the maternal concentration, when etanercept was administered at 50 mg every 7-12 days in pregnancy until 4 days prior to delivery, 25 mg twice weekly until 36 weeks of gestation, and 25 mg subcutaneous every week through the third trimester, respectively. There was one post-marketing safety report of a pregnant woman who received etanercept 25 mg once to twice weekly throughout pregnancy, and etanercept was detected in cord blood at 32% of the maternal concentration.
Animal Data
In embryofetal development studies with etanercept administered during the period of organogenesis to pregnant rats from gestation day (GD) 6 through 20 or pregnant rabbits from GD 6 through 18, there was no evidence of fetal malformations or embryotoxicity in rats or rabbits at respective doses that achieved systemic exposures 48 to 58 times the exposure in patients treated with 50 mg Enbrel once weekly (on an AUC basis with maternal subcutaneous doses up to 30 mg/kg/day in rats and 40 mg/kg/day in rabbits). In a peri-and post-natal development study with pregnant rats that received etanercept during organogenesis and the later gestational period from GD 6 through 21, development of pups through post-natal day 4 was unaffected at doses that achieved exposures 48 times the exposure in patients treated with 50 mg Enbrel once weekly (on an AUC basis with maternal subcutaneous doses up to 30 mg/kg/day).
Lactation
Risk Summary
Data from published literature show that etanercept is present in low levels in human milk but is not detected in the plasma of breastfed infants (see Data ) . There are no data on the effects of etanercept on milk production. There have been no consistent reports of adverse events in breastfed infants over decades of use. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for Enbrel and any potential adverse effects on the breastfed child from the drug or from the underlying maternal condition.
Data
In three case reports, etanercept was detected in breast milk at levels ranging from <2 to 7.5 ng/mL after lactating women had received doses of etanercept of 25 mg weekly or twice weekly. Although etanercept was detected in breast milk in these cases, etanercept was not detected in the serum of the breastfed infants.
Pediatric Use
Polyarticular Juvenile Idiopathic Arthritis
The safety and effectiveness of Enbrel have been established in pediatric patients 2 years of age and older with pJIA. Enbrel has been studied in 69 children with moderately to severely active polyarticular JIA 2 to 17 years of age.
The safety and effectiveness of Enbrel in pediatric patients less than 2 years of age with pJIA have not been established.
Juvenile Psoriatic Arthritis
The safety and effectiveness of Enbrel have been established in pediatric patients 2 years to 17 years old with JPsA. Use of Enbrel in JPsA is supported by evidence from adequate and well controlled studies of Enbrel in adults with PsA; pharmacokinetic data from adult patients with PsA, RA, and PsO; and pharmacokinetic data from pediatric patients with active JIA and PsO. Safety of Enbrel in JPsA is supported by a clinical study in 69 pediatric patients with moderately to severely active JIA aged 2 to 17 years; a clinical study in 211 pediatric patients with moderate to severe PsO aged 4 to 17 years; and an open-label extension study in 182 pediatric patients with moderate to severe PsO aged 4 to 17 years.
The observed pre-dose (trough) concentrations are generally comparable between adults with RA and PsA and pediatric patients with active JIA, as well as adults with PsO and pediatric patients with PsO. The PK exposure is expected to be comparable between adults with PsA and pediatric patients with JPsA [see Adverse Reactions (6.1) , Clinical Pharmacology (12.3) , and Clinical Studies (14.1 , 14.2 , 14.3 , 14.5 , 14.6) ] .
The safety and effectiveness in pediatric patients below the age of 2 years have not been established in JPsA.
Plaque Psoriasis
The safety and effectiveness of Enbrel for plaque psoriasis have been established in pediatric patients 4 years of age and older. Enbrel has been studied in 211 pediatric patients with moderate to severe PsO aged 4 to 17 years.
The safety and effectiveness of Enbrel in pediatric patients below the age of 4 years with PsO have not been established.
Malignancies in Pediatric Patients
Malignancies, some fatal, have been reported among children, adolescents, and young adults who received treatment with TNF-blocking agents (initiation of therapy at ≤ 18 years of age), including Enbrel [see Warnings and Precautions (5.3) ] .
Geriatric Use
A total of 480 RA patients ages 65 years or older have been studied in clinical trials. In PsO randomized clinical trials, a total of 138 out of 1965 patients treated with Enbrel or placebo were age 65 or older. No overall differences in safety or effectiveness were observed between these patients and younger patients, but the number of geriatric PsO patients is too small to determine whether they respond differently from younger patients. Because there is a higher incidence of infections in the elderly population in general, caution should be used in treating the elderly.
Use in Patients with Diabetes
There have been reports of hypoglycemia following initiation of Enbrel therapy in patients receiving medication for diabetes, necessitating a reduction in anti-diabetic medication in some of these patients.
CONTRAINDICATIONS
Enbrel is contraindicated in patients with sepsis.
WARNINGS AND PRECAUTIONS
- Do not start Enbrel during an active infection. If an infection develops, monitor carefully and stop Enbrel if infection becomes serious. (5.1 )
- Consider empiric anti-fungal therapy for patients at risk for invasive fungal infections who develop a severe systemic illness on Enbrel (those who reside or travel to regions where mycoses are endemic). (5.1 )
- Demyelinating disease, exacerbation or new onset, may occur. (5.2 )
- Cases of lymphoma have been observed in patients receiving TNF-blocking agents. (5.3 )
- Congestive heart failure, worsening or new onset, may occur. (5.4 )
- Advise patients to seek immediate medical attention if symptoms of pancytopenia or aplastic anemia develop, and consider stopping Enbrel. (5.5 )
- Monitor patients previously infected with hepatitis B virus for reactivation during and several months after therapy. If reactivation occurs, consider stopping Enbrel and beginning anti-viral therapy. (5.6 )
- Anaphylaxis or serious allergic reactions may occur. (5.7 )
- Stop Enbrel if lupus-like syndrome or autoimmune hepatitis develops. (5.9 )
Serious Infections
Patients treated with Enbrel are at increased risk for developing serious infections involving various organ systems and sites that may lead to hospitalization or death.
Opportunistic infections due to bacterial, mycobacterial, invasive fungal, viral, parasitic, or other opportunistic pathogens including aspergillosis, blastomycosis, candidiasis, coccidioidomycosis, histoplasmosis, legionellosis, listeriosis, pneumocystosis, and tuberculosis have been reported with TNF-blockers. Patients have frequently presented with disseminated rather than localized disease.
Treatment with Enbrel should not be initiated in patients with an active infection, including clinically important localized infections. Patients greater than 65 years of age, patients with co-morbid conditions, and/or patients taking concomitant immunosuppressants (such as corticosteroids or methotrexate), may be at greater risk of infection. The risks and benefits of treatment should be considered prior to initiating therapy in patients:
- With chronic or recurrent infection;
- Who have been exposed to tuberculosis;
- With a history of an opportunistic infection;
- Who have resided or traveled in areas of endemic tuberculosis or endemic mycoses, such as histoplasmosis, coccidioidomycosis, or blastomycosis; or
- With underlying conditions that may predispose them to infection, such as advanced or poorly controlled diabetes [see Adverse Reactions (6.1) ] .
Patients should be closely monitored for the development of signs and symptoms of infection during and after treatment with Enbrel.
Enbrel should be discontinued if a patient develops a serious infection or sepsis. A patient who develops a new infection during treatment with Enbrel should be closely monitored, undergo a prompt and complete diagnostic workup appropriate for an immunocompromised patient, and appropriate antimicrobial therapy should be initiated.
Tuberculosis
Cases of reactivation of tuberculosis or new tuberculosis infections have been observed in patients receiving Enbrel, including patients who have previously received treatment for latent or active tuberculosis. Data from clinical trials and preclinical studies suggest that the risk of reactivation of latent tuberculosis infection is lower with Enbrel than with TNF-blocking monoclonal antibodies. Nonetheless, postmarketing cases of tuberculosis reactivation have been reported for TNF-blockers, including Enbrel. Tuberculosis has developed in patients who tested negative for latent tuberculosis prior to initiation of therapy. Patients should be evaluated for tuberculosis risk factors and tested for latent infection prior to initiating Enbrel and periodically during therapy. Tests for latent tuberculosis infection may be falsely negative while on therapy with Enbrel.
Treatment of latent tuberculosis infection prior to therapy with TNF-blocking agents has been shown to reduce the risk of tuberculosis reactivation during therapy. Induration of 5 mm or greater with tuberculin skin testing should be considered a positive test result when assessing if treatment for latent tuberculosis is needed prior to initiating Enbrel, even for patients previously vaccinated with Bacillus Calmette-Guerin (BCG).
Anti-tuberculosis therapy should also be considered prior to initiation of Enbrel in patients with a past history of latent or active tuberculosis in whom an adequate course of treatment cannot be confirmed, and for patients with a negative test for latent tuberculosis but having risk factors for tuberculosis infection. Consultation with a physician with expertise in the treatment of tuberculosis is recommended to aid in the decision whether initiating anti-tuberculosis therapy is appropriate for an individual patient.
Tuberculosis should be strongly considered in patients who develop a new infection during Enbrel treatment, especially in patients who have previously or recently traveled to countries with a high prevalence of tuberculosis, or who have had close contact with a person with active tuberculosis.
Invasive Fungal Infections
Cases of serious and sometimes fatal fungal infections, including histoplasmosis, have been reported with TNF-blockers, including Enbrel. For patients who reside or travel in regions where mycoses are endemic, invasive fungal infection should be suspected if they develop a serious systemic illness. Appropriate empiric anti-fungal therapy should be considered while a diagnostic workup is being performed. Antigen and antibody testing for histoplasmosis may be negative in some patients with active infection. When feasible, the decision to administer empiric anti-fungal therapy in these patients should be made in consultation with a physician with expertise in the diagnosis and treatment of invasive fungal infections and should take into account both the risk for severe fungal infection and the risks of anti-fungal therapy. In 38 Enbrel clinical trials and 4 cohort studies in all approved indications representing 27,169 patient-years of exposure (17,696 patients) from the United States and Canada, no histoplasmosis infections were reported among patients treated with Enbrel.
Neurologic Reactions
Treatment with TNF-blocking agents, including Enbrel, has been associated with rare (< 0.1%) cases of new onset or exacerbation of central nervous system demyelinating disorders, some presenting with mental status changes and some associated with permanent disability, and with peripheral nervous system demyelinating disorders. Cases of transverse myelitis, optic neuritis, multiple sclerosis, Guillain-Barre syndromes, other peripheral demyelinating neuropathies, and new onset or exacerbation of seizure disorders have been reported in postmarketing experience with Enbrel therapy. Prescribers should exercise caution in considering the use of Enbrel in patients with preexisting or recent-onset central or peripheral nervous system demyelinating disorders [see Postmarketing Experience (6.3) ] .
Malignancies
Lymphomas
In the controlled portions of clinical trials of TNF-blocking agents, more cases of lymphoma have been observed among patients receiving a TNF-blocker compared to control patients. During the controlled portions of Enbrel trials in adult patients with RA, AS, and PsA, 2 lymphomas were observed among 3306 Enbrel-treated patients versus 0 among 1521 control patients (duration of controlled treatment ranged from 3 to 36 months).
Among 6543 adult rheumatology (RA, PsA, AS) patients treated with Enbrel in controlled and uncontrolled portions of clinical trials, representing approximately 12,845 patient-years of therapy, the observed rate of lymphoma was 0.10 cases per 100 patient-years. This was 3-fold higher than the rate of lymphoma expected in the general U.S. population based on the Surveillance, Epidemiology, and End Results (SEER) Database. An increased rate of lymphoma up to several-fold has been reported in the RA patient population, and may be further increased in patients with more severe disease activity.
Among 4410 adult PsO patients treated with Enbrel in clinical trials up to 36 months, representing approximately 4278 patient-years of therapy, the observed rate of lymphoma was 0.05 cases per 100 patient-years, which is comparable to the rate in the general population. No cases were observed in Enbrel- or placebo-treated patients during the controlled portions of these trials.
Leukemia
Cases of acute and chronic leukemia have been reported in association with postmarketing TNF-blocker use in rheumatoid arthritis and other indications. Even in the absence of TNF-blocker therapy, patients with rheumatoid arthritis may be at higher risk (approximately 2-fold) than the general population for the development of leukemia.
During the controlled portions of Enbrel trials, 2 cases of leukemia were observed among 5445 (0.06 cases per 100 patient-years) Enbrel-treated patients versus 0 among 2890 (0%) control patients (duration of controlled treatment ranged from 3 to 48 months).
Among 15,401 patients treated with Enbrel in controlled and open portions of clinical trials representing approximately 23,325 patient-years of therapy, the observed rate of leukemia was 0.03 cases per 100 patient-years.
Other Malignancies
Information is available from 10,953 adult patients with 17,123 patient-years and 696 pediatric patients with 1282 patient-years of experience across 45 Enbrel clinical studies.
For malignancies other than lymphoma and non-melanoma skin cancer, there was no difference in exposure-adjusted rates between the Enbrel and control arms in the controlled portions of clinical studies for all indications. Analysis of the malignancy rate in combined controlled and uncontrolled portions of studies has demonstrated that types and rates are similar to what is expected in the general U.S. population based on the SEER database and suggests no increase in rates over time. Whether treatment with Enbrel might influence the development and course of malignancies in adults is unknown.
Melanoma and Non-Melanoma Skin Cancer (NMSC)
Melanoma and non-melanoma skin cancer has been reported in patients treated with TNF antagonists including etanercept.
Among 15,401 patients treated with Enbrel in controlled and open portions of clinical trials representing approximately 23,325 patient-years of therapy, the observed rate of melanoma was 0.043 cases per 100 patient-years.
Among 3306 adult rheumatology (RA, PsA, AS) patients treated with Enbrel in controlled clinical trials representing approximately 2669 patient-years of therapy, the observed rate of NMSC was 0.41 cases per 100 patient-years versus 0.37 cases per 100 patient-years among 1521 control-treated patients representing 1077 patient-years. Among 1245 adult PsO patients treated with Enbrel in controlled clinical trials, representing approximately 283 patient-years of therapy, the observed rate of NMSC was 3.54 cases per 100 patient-years versus 1.28 cases per 100 patient-years among 720 control-treated patients representing 156 patient-years.
Postmarketing cases of Merkel cell carcinoma have been reported very infrequently in patients treated with Enbrel.
Periodic skin examinations should be considered for all patients at increased risk for skin cancer.
Pediatric Patients
Malignancies, some fatal, have been reported among children, adolescents, and young adults who received treatment with TNF-blocking agents (initiation of therapy at ≤ 18 years of age), including Enbrel. Approximately half the cases were lymphomas, including Hodgkin's and non-Hodgkin's lymphoma. The other cases represented a variety of different malignancies and included rare malignancies usually associated with immunosuppression and malignancies that are not usually observed in children and adolescents. The malignancies occurred after a median of 30 months of therapy (range 1 to 84 months). Most of the patients were receiving concomitant immunosuppressants. These cases were reported postmarketing and are derived from a variety of sources, including registries and spontaneous postmarketing reports.
In clinical trials of 1140 pediatric patients representing 1927.2 patient-years of therapy, no malignancies, including lymphoma or NMSC, have been reported.
Postmarketing Use
In global postmarketing adult and pediatric use, lymphoma and other malignancies have been reported.
New Onset or Worsening of Heart Failure
Two clinical trials evaluating the use of Enbrel in the treatment of heart failure were terminated early due to lack of efficacy. One of these studies suggested higher mortality in Enbrel-treated patients compared to placebo [see Adverse Reactions (6.2) ] . There have been postmarketing reports of worsening of congestive heart failure (CHF), with and without identifiable precipitating factors, in patients taking Enbrel. There have also been rare (< 0.1%) reports of new onset CHF, including CHF in patients without known preexisting cardiovascular disease. Some of these patients have been under 50 years of age. Physicians should exercise caution when using Enbrel in patients who also have heart failure, and monitor patients carefully.
Hematologic Reactions
Rare (< 0.1%) reports of pancytopenia, including very rare (< 0.01%) reports of aplastic anemia, some with a fatal outcome, have been reported in patients treated with Enbrel. The causal relationship to Enbrel therapy remains unclear. Although no high-risk group has been identified, caution should be exercised in patients being treated with Enbrel who have a previous history of significant hematologic abnormalities. All patients should be advised to seek immediate medical attention if they develop signs and symptoms suggestive of blood dyscrasias or infection (e.g., persistent fever, bruising, bleeding, pallor) while on Enbrel. Discontinuation of Enbrel therapy should be considered in patients with confirmed significant hematologic abnormalities.
Two percent of patients treated concurrently with Enbrel and anakinra developed neutropenia (ANC < 1 × 10 9 /L). While neutropenic, one patient developed cellulitis that resolved with antibiotic therapy.
Hepatitis B Reactivation
Reactivation of hepatitis B in patients who were previously infected with the hepatitis B virus (HBV) and had received concomitant TNF-blocking agents, including very rare cases (< 0.01%) with Enbrel, has been reported. In some instances, hepatitis B reactivation occurring in conjunction with TNF-blocker therapy has been fatal. The majority of these reports have occurred in patients concomitantly receiving other medications that suppress the immune system, which may also contribute to hepatitis B reactivation. Patients at risk for HBV infection should be evaluated for prior evidence of HBV infection before initiating TNF-blocker therapy. Prescribers should exercise caution in prescribing TNF-blockers in patients previously infected with HBV. Adequate data are not available on the safety or efficacy of treating patients who are carriers of HBV with anti-viral therapy in conjunction with TNF-blocker therapy to prevent HBV reactivation. Patients previously infected with HBV and requiring treatment with Enbrel should be closely monitored for clinical and laboratory signs of active HBV infection throughout therapy and for several months following termination of therapy. In patients who develop HBV reactivation, consideration should be given to stopping Enbrel and initiating anti-viral therapy with appropriate supportive treatment. The safety of resuming Enbrel therapy after HBV reactivation is controlled is not known. Therefore, prescribers should weigh the risks and benefits when considering resumption of therapy in this situation.
Allergic Reactions
Allergic reactions associated with administration of Enbrel during clinical trials have been reported in < 2% of patients. If an anaphylactic reaction or other serious allergic reaction occurs, discontinue administration of Enbrel and initiate appropriate therapy immediately.
Immunizations
Avoid concurrent administration of live vaccines with Enbrel. It is recommended that patients, if possible, be brought up-to-date with all immunizations in agreement with current immunization guidelines prior to initiating Enbrel therapy [see Drug Interactions (7.1) and Use in Specific Populations (8.4) ] .
Autoimmunity
Treatment with Enbrel may result in the formation of autoantibodies [see Adverse Reactions (6.1) ] and, rarely (< 0.1%), in the development of a lupus-like syndrome or autoimmune hepatitis [see Adverse Reactions (6.2) ] , which may resolve following withdrawal of Enbrel. If a patient develops symptoms and findings suggestive of a lupus-like syndrome or autoimmune hepatitis following treatment with Enbrel, discontinue treatment and evaluate the patient.
Immunosuppression
TNF mediates inflammation and modulates cellular immune responses. TNF-blocking agents, including Enbrel, affect host defenses against infections. The effect of TNF inhibition on the development and course of malignancies is not fully understood. In a study of 49 patients with RA treated with Enbrel, there was no evidence of depression of delayed-type hypersensitivity, depression of immunoglobulin levels, or change in enumeration of effector cell populations [see Warnings and Precautions (5.1 , 5.3) and Adverse Reactions (6.1) ] .
Not Recommended for Use in Patients with Granulomatosis with Polyangiitis Receiving Immunosuppressants
The use of Enbrel in patients with granulomatosis with polyangiitis receiving immunosuppressive agents is not recommended. In a study of patients with granulomatosis with polyangiitis, the addition of Enbrel to standard therapy (including cyclophosphamide) was associated with a higher incidence of non-cutaneous solid malignancies and was not associated with improved clinical outcomes when compared with standard therapy alone [see Drug Interactions (7.3) ] .
Not Recommended for Use with Anakinra or Abatacept
Use of Enbrel with anakinra or abatacept is not recommended [see Drug Interactions (7.2) ] .
Increased Mortality in Patients with Moderate to Severe Alcoholic Hepatitis
In a study of 48 hospitalized patients treated with Enbrel or placebo for moderate to severe alcoholic hepatitis, the mortality rate in patients treated with Enbrel was similar to patients treated with placebo at 1 month but significantly higher after 6 months. Physicians should use caution when using Enbrel in patients with moderate to severe alcoholic hepatitis.
ADVERSE REACTIONS
The following serious adverse reactions are discussed in greater detail in other sections of the labeling:
- Serious Infections [see Warnings and Precautions (5.1) ]
- Neurologic Reactions [see Warnings and Precautions (5.2) ]
- Malignancies [see Warnings and Precautions (5.3) ]
- Patients with Heart Failure [see Warnings and Precautions (5.4) ]
- Hematologic Reactions [see Warnings and Precautions (5.5) ]
- Hepatitis B Reactivation [see Warnings and Precautions (5.6) ]
- Allergic Reactions [see Warnings and Precautions (5.7) ]
- Autoimmunity [see Warnings and Precautions (5.9) ]
- Immunosuppression [see Warnings and Precautions (5.10) ]
Clinical Trials Experience
Across clinical studies and postmarketing experience, the most serious adverse reactions with Enbrel were infections, neurologic events, CHF, and hematologic events [see Warnings and Precautions (5) ] . The most common adverse reactions with Enbrel were infections and injection site reactions.
Because clinical trials are conducted under widely varying conditions, adverse reactions rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not predict the rates observed in clinical practice.
Adverse Reactions in Adult Patients with Rheumatoid Arthritis, Psoriatic Arthritis, Ankylosing Spondylitis, or Plaque Psoriasis
The data described below reflect exposure to Enbrel in 2219 adult patients with RA followed for up to 80 months, in 182 patients with PsA for up to 24 months, in 138 patients with AS for up to 6 months, and in 1204 adult patients with PsO for up to 18 months.
In controlled trials, the proportion of Enbrel-treated patients who discontinued treatment due to adverse events was approximately 4% in the indications studied.
Adverse Reactions in Pediatric Patients
In general, the adverse reactions in pediatric patients were similar in frequency and type as those seen in adult patients [see Warnings and Precautions (5) , Use in Specific Populations (8.4) , and Clinical Studies (14.2 , 14.6) ] .
In a 48-week clinical study in 211 children aged 4 to 17 years with pediatric PsO, the adverse reactions reported were similar to those seen in previous studies in adults with PsO. Long-term safety profile for up to 264 additional weeks was assessed in an open-label extension study and no new safety signals were identified.
In open-label clinical studies of children with JIA, adverse reactions reported in those ages 2 to 4 years were similar to adverse reactions reported in older children.
Infections
Infections, including viral, bacterial, and fungal infections, have been observed in adult and pediatric patients. Infections have been noted in all body systems and have been reported in patients receiving Enbrel alone or in combination with other immunosuppressive agents.
In controlled portions of trials, the types and severity of infection were similar between Enbrel and the respective control group (placebo or MTX for RA and PsA patients) in RA, PsA, AS and PsO patients. Rates of infections in RA and adult PsO patients are provided in Table 3 and Table 4, respectively. Infections consisted primarily of upper respiratory tract infection, sinusitis and influenza.
In controlled portions of trials in RA, PsA, AS and PsO, the rates of serious infection were similar (0.8% in placebo, 3.6% in MTX, and 1.4% in Enbrel/Enbrel + MTX-treated groups). In clinical trials in rheumatologic indications, serious infections experienced by patients have included, but are not limited to, pneumonia, cellulitis, septic arthritis, bronchitis, gastroenteritis, pyelonephritis, sepsis, abscess and osteomyelitis. In clinical trials in adult PsO patients, serious infections experienced by patients have included, but are not limited to, pneumonia, cellulitis, gastroenteritis, abscess and osteomyelitis. The rate of serious infections was not increased in open-label extension trials and was similar to that observed in Enbrel- and placebo-treated patients from controlled trials.
In 66 global clinical trials of 17,505 patients (21,015 patient-years of therapy), tuberculosis was observed in approximately 0.02% of patients. In 17,696 patients (27,169 patient-years of therapy) from 38 clinical trials and 4 cohort studies in the U.S. and Canada, tuberculosis was observed in approximately 0.006% of patients. These studies include reports of pulmonary and extrapulmonary tuberculosis [see Warnings and Precautions (5.1) ] .
The types of infections reported in pediatric patients with PsO and JIA were generally mild and consistent with those commonly seen in the general pediatric population. Two JIA patients developed varicella infection and signs and symptoms of aseptic meningitis, which resolved without sequelae.
Injection Site Reactions
In placebo-controlled trials in rheumatologic indications, approximately 37% of patients treated with Enbrel developed injection site reactions. In controlled trials in patients with PsO, 15% of adult patients and 7% of pediatric patients treated with Enbrel developed injection site reactions during the first 3 months of treatment. All injection site reactions were described as mild to moderate (erythema, itching, pain, swelling, bleeding, bruising) and generally did not necessitate drug discontinuation. Injection site reactions generally occurred in the first month and subsequently decreased in frequency. The mean duration of injection site reactions was 3 to 5 days. Seven percent of patients experienced redness at a previous injection site when subsequent injections were given.
Other Adverse Reactions
Table 3 summarizes adverse reactions reported in adult RA patients. The types of adverse reactions seen in patients with PsA or AS were similar to the types of adverse reactions seen in patients with RA.
| Placebo-Controlled Includes data from the 6-month study in which patients received concurrent MTX therapy in both arms. (Studies I, II, and a Phase 2 Study) | Active-Controlled Study duration of 2 years. (Study III) | |||
|---|---|---|---|---|
| Placebo (N = 152) | Enbrel Any dose. (N = 349) | MTX (N = 217) | Enbrel (N = 415) | |
| Adverse Reaction | Percent of Patients | Percent of Patients | ||
| Infection Includes bacterial, viral and fungal infections. (total) | 39 | 50 | 86 | 81 |
| Upper Respiratory Infections Most frequent Upper Respiratory Infections were upper respiratory tract infection, sinusitis and influenza. | 30 | 38 | 70 | 65 |
| Non-upper Respiratory Infections | 15 | 21 | 59 | 54 |
| Injection Site Reactions | 11 | 37 | 18 | 43 |
| Diarrhea | 9 | 8 | 16 | 16 |
| Rash | 2 | 3 | 19 | 13 |
| Pruritus | 1 | 2 | 5 | 5 |
| Pyrexia | - | 3 | 4 | 2 |
| Urticaria | 1 | - | 4 | 2 |
| Hypersensitivity | - | - | 1 | 1 |
In placebo-controlled adult PsO trials, the percentages of patients reporting adverse reactions in the 50 mg twice a week dose group were similar to those observed in the 25 mg twice a week dose group or placebo group.
Table 4 summarizes adverse reactions reported in adult PsO patients from Studies I and II.
| Placebo (N = 359) | Enbrel Includes 25 mg subcutaneous (SC) once weekly (QW), 25 mg SC twice weekly (BIW), 50 mg SC QW, and 50 mg SC BIW doses. (N = 876) | |
|---|---|---|
| Adverse Reaction | Percent of Patients | |
| Infection Includes bacterial, viral and fungal infections. (total) | 28 | 27 |
| Non-upper Respiratory Infections | 14 | 12 |
| Upper Respiratory Infections Most frequent Upper Respiratory Infections were upper respiratory tract infection, nasopharyngitis and sinusitis. | 17 | 17 |
| Injection Site Reactions | 6 | 15 |
| Diarrhea | 2 | 3 |
| Rash | 1 | 1 |
| Pruritus | 2 | 1 |
| Urticaria | - | 1 |
| Hypersensitivity | - | 1 |
| Pyrexia | 1 | - |
Immunogenicity
As with all therapeutic proteins, there is potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to etanercept in the studies described below with the incidence of antibodies in other studies or to other products may be misleading.
Immunogenicity
Patients with RA, PsA, AS or PsO were tested at multiple time points for antibodies to etanercept. Antibodies to the TNF receptor portion or other protein components of the Enbrel drug product were detected at least once in sera of approximately 6% of adult patients with RA, PsA, AS or PsO. These antibodies were all non-neutralizing. Results from JIA patients were similar to those seen in adult RA patients treated with Enbrel.
In adult PsO studies that evaluated the exposure of etanercept for up to 120 weeks, the percentage of patients testing positive at the assessed time points of 24, 48, 72 and 96 weeks ranged from 3.6%-8.7% and were all non-neutralizing. The percentage of patients testing positive increased with an increase in the duration of study; however, the clinical significance of this finding is unknown. No apparent correlation of antibody development to clinical response or adverse events was observed. The immunogenicity data of Enbrel beyond 120 weeks of exposure are unknown.
In pediatric PsO studies, approximately 10% of subjects developed antibodies to etanercept by Week 48 and approximately 16% of subjects developed antibodies to etanercept by Week 264. All of these antibodies were non-neutralizing. However, because of the limitations of the immunogenicity assays, the incidence of binding and neutralizing antibodies may not have been reliably determined.
The data reflect the percentage of patients whose test results were considered positive for antibodies to etanercept in an ELISA assay, and are highly dependent on the sensitivity and specificity of the assay.
Autoantibodies
Patients with RA had serum samples tested for autoantibodies at multiple time points. In RA Studies I and II, the percentage of patients evaluated for antinuclear antibodies (ANA) who developed new positive ANA (titer ≥ 1:40) was higher in patients treated with Enbrel (11%) than in placebo-treated patients (5%). The percentage of patients who developed new positive anti-double-stranded DNA antibodies was also higher by radioimmunoassay (15% of patients treated with Enbrel compared to 4% of placebo-treated patients) and by Crithidia luciliae assay (3% of patients treated with Enbrel compared to none of placebo-treated patients). The proportion of patients treated with Enbrel who developed anticardiolipin antibodies was similarly increased compared to placebo-treated patients. In RA Study III, no pattern of increased autoantibody development was seen in Enbrel patients compared to MTX patients [see Warnings and Precautions (5.9) ] .
Postmarketing Experience
Adverse reactions have been reported during post approval use of Enbrel in adults and pediatric patients. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to Enbrel exposure.
Adverse reactions are listed by body system below:
| Blood and lymphatic system disorders: | pancytopenia, anemia, leukopenia, neutropenia, thrombocytopenia, lymphadenopathy, aplastic anemia [see Warnings and Precautions (5.5) ] |
| Cardiac disorders: | congestive heart failure [see Warnings and Precautions (5.4) ] |
| Gastrointestinal disorders: | inflammatory bowel disease (IBD) |
| General disorders: | angioedema, chest pain |
| Hepatobiliary disorders: | autoimmune hepatitis, elevated transaminases, hepatitis B reactivation |
| Immune disorders: | macrophage activation syndrome, systemic vasculitis, sarcoidosis |
| Musculoskeletal and connective tissue disorders: | lupus-like syndrome |
| Neoplasms benign, malignant, and unspecified: | melanoma and non-melanoma skin cancers, Merkel cell carcinoma [see Warnings and Precautions (5.3) ] |
| Nervous system disorders: | convulsions, multiple sclerosis, demyelination, optic neuritis, transverse myelitis, paresthesias, headache [see Warnings and Precautions (5.2) ] |
| Ocular disorders: | uveitis, scleritis |
| Renal and urinary disorders: | glomerulonephritis |
| Respiratory, thoracic and mediastinal disorders: | interstitial lung disease |
| Skin and subcutaneous tissue disorders: | cutaneous lupus erythematosus, cutaneous vasculitis (including leukocytoclastic vasculitis), erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis, subcutaneous nodule, new or worsening psoriasis (all sub-types including pustular and palmoplantar) |
Opportunistic infections, including atypical mycobacterial infection, herpes zoster, aspergillosis and Pneumocystis jiroveci pneumonia, and protozoal infections have also been reported in postmarketing use.
Rare (< 0.1%) cases of IBD have been reported in JIA patients receiving Enbrel, which is not effective for the treatment of IBD.
DRUG INTERACTIONS
Specific drug interaction studies have not been conducted with Enbrel.
Vaccines
Most PsA patients receiving Enbrel were able to mount effective B-cell immune responses to pneumococcal polysaccharide vaccine, but titers in aggregate were moderately lower and fewer patients had 2-fold rises in titers compared to patients not receiving Enbrel. The clinical significance of this is unknown. Patients receiving Enbrel may receive concurrent vaccinations, except for live vaccines. No data are available on the secondary transmission of infection by live vaccines in patients receiving Enbrel.
Patients with a significant exposure to varicella virus should temporarily discontinue Enbrel therapy and be considered for prophylactic treatment with varicella zoster immune globulin [see Warnings and Precautions (5.8 , 5.10) ] .
Immune-Modulating Biologic Products
In a study in which patients with active RA were treated for up to 24 weeks with concurrent Enbrel and anakinra therapy, a 7% rate of serious infections was observed, which was higher than that observed with Enbrel alone (0%) [see Warnings and Precautions (5.12) ] and did not result in higher ACR response rates compared to Enbrel alone. The most common infections consisted of bacterial pneumonia (4 cases) and cellulitis (4 cases). One patient with pulmonary fibrosis and pneumonia died due to respiratory failure. Two percent of patients treated concurrently with Enbrel and anakinra developed neutropenia (ANC < 1 × 10 9 /L).
In clinical studies, concurrent administration of abatacept and Enbrel resulted in increased incidences of serious adverse events, including infections, and did not demonstrate increased clinical benefit [see Warnings and Precautions (5.12) ] .
Cyclophosphamide
The use of Enbrel in patients receiving concurrent cyclophosphamide therapy is not recommended [see Warnings and Precautions (5.11) ] .
Sulfasalazine
Patients in a clinical study who were on established therapy with sulfasalazine, to which Enbrel was added, were noted to develop a mild decrease in mean neutrophil counts in comparison to groups treated with either Enbrel or sulfasalazine alone. The clinical significance of this observation is unknown.
DESCRIPTION
Etanercept, a tumor necrosis factor (TNF) blocker, is a dimeric fusion protein consisting of the extracellular ligand-binding portion of the human 75 kilodalton (p75) tumor necrosis factor receptor (TNFR) linked to the Fc portion of human IgG1. The Fc component of etanercept contains the C H 2 domain, the C H 3 domain and hinge region, but not the C H 1 domain of IgG1. Etanercept is produced by recombinant DNA technology in a Chinese hamster ovary (CHO) mammalian cell expression system. It consists of 934 amino acids and has an apparent molecular weight of approximately 150 kilodaltons.
Enbrel (etanercept) Injection in the single-dose prefilled syringe, the single-dose prefilled SureClick autoinjector and the single-dose vial is clear and colorless, sterile, preservative-free solution, and is formulated at pH 6.3 ± 0.2.
Enbrel (etanercept) for Injection is supplied in a multiple-dose vial as a sterile, white, preservative-free, lyophilized powder. Reconstitution with 1 mL of the supplied Sterile Bacteriostatic Water for Injection, USP (containing 0.9% benzyl alcohol) yields a multiple-dose, clear, and colorless solution 1 mL containing 25 mg of Enbrel, with a pH of 7.4 ± 0.3.
Enbrel (etanercept) Injection in the Enbrel Mini single-dose prefilled cartridge for use with the AutoTouch reusable autoinjector is clear and colorless, sterile, preservative-free solution, and is formulated at pH 6.3 ± 0.2.
| Presentation | Active Ingredient Content | Inactive Ingredients Content |
|---|---|---|
| Enbrel 50 mg prefilled syringe and SureClick autoinjector | 50 mg etanercept in 1 mL | 25 mM L-arginine hydrochloride 120 mM sodium chloride 1% sucrose |
| Enbrel 25 mg prefilled syringe | 25 mg etanercept in 0.5 mL | 25 mM L-arginine hydrochloride 120 mM sodium chloride 1% sucrose |
| Enbrel 25 mg single-dose vial | 25 mg etanercept in 0.5 mL | 25 mM L-arginine hydrochloride 120 mM sodium chloride 1% sucrose |
| Enbrel 25 mg multiple-dose vial | After reconstitution, 25 mg etanercept in 1 mL | 40 mg mannitol 10 mg sucrose 1.2 mg tromethamine |
| Enbrel 50 mg Enbrel Mini single-dose prefilled cartridge for use with the AutoTouch reusable autoinjector only | 50 mg etanercept in 1 mL | 25 mM L-arginine hydrochloride 120 mM sodium chloride 1% sucrose |
CLINICAL PHARMACOLOGY
Mechanism of Action
TNF is a naturally occurring cytokine that is involved in normal inflammatory and immune responses. It plays an important role in the inflammatory processes of RA, polyarticular JIA, PsA, and AS and the resulting joint pathology. In addition, TNF plays a role in the inflammatory process of PsO. Elevated levels of TNF are found in involved tissues and fluids of patients with RA, JIA, PsA, AS, and PsO.
Two distinct receptors for TNF (TNFRs), a 55 kilodalton protein (p55) and a 75 kilodalton protein (p75), exist naturally as monomeric molecules on cell surfaces and in soluble forms. Biological activity of TNF is dependent upon binding to either cell surface TNFR.
Etanercept is a dimeric soluble form of the p75 TNF receptor that can bind TNF molecules. Etanercept inhibits binding of TNF-α and TNF-β (lymphotoxin alpha [LT-α]) to cell surface TNFRs, rendering TNF biologically inactive. In in vitro studies, large complexes of etanercept with TNF-α were not detected and cells expressing transmembrane TNF (that binds Enbrel) are not lysed in the presence or absence of complement.
Pharmacodynamics
Etanercept can modulate biological responses that are induced or regulated by TNF, including expression of adhesion molecules responsible for leukocyte migration (e.g. E-selectin, and to a lesser extent, intercellular adhesion molecule-1 [ICAM-1]), serum levels of cytokines (e.g. IL-6), and serum levels of matrix metalloproteinase-3 (MMP-3 or stromelysin). Etanercept has been shown to affect several animal models of inflammation, including murine collagen-induced arthritis.
Pharmacokinetics
After administration of 25 mg of Enbrel by a single SC injection to 25 patients with RA, a mean ± standard deviation half-life of 102 ± 30 hours was observed with a clearance of 160 ± 80 mL/hr. A maximum serum concentration (C max ) of 1.1 ± 0.6 mcg/mL and time to C max of 69 ± 34 hours was observed in these patients following a single 25 mg dose. After 6 months of twice weekly 25 mg doses in these same RA patients, the mean C max was 2.4 ± 1.0 mcg/mL (N = 23). Patients exhibited a 2- to 7-fold increase in peak serum concentrations and approximately 4-fold increase in AUC 0-72 hr (range 1- to 17-fold) with repeated dosing. Serum concentrations in patients with RA have not been measured for periods of dosing that exceed 6 months.
In another study, serum concentration profiles at steady-state were comparable among patients with RA treated with 50 mg Enbrel once weekly and those treated with 25 mg Enbrel twice weekly. The mean (± standard deviation) C max , C min , and partial AUC were 2.4 ± 1.5 mcg/mL, 1.2 ± 0.7 mcg/mL, and 297 ± 166 mcg∙h/mL, respectively, for patients treated with 50 mg Enbrel once weekly (N = 21); and 2.6 ± 1.2 mcg/mL, 1.4 ± 0.7 mcg/mL, and 316 ± 135 mcg∙h/mL for patients treated with 25 mg Enbrel twice weekly (N = 16).
Patients with JIA (ages 4 to 17 years) were administered 0.4 mg/kg of Enbrel twice weekly (up to a maximum dose of 50 mg per week) for up to 18 weeks. The mean serum concentration after repeated SC dosing was 2.1 mcg/mL, with a range of 0.7 to 4.3 mcg/mL. Limited data suggest that the clearance of etanercept is reduced slightly in children ages 4 to 8 years. Population pharmacokinetic analyses predict that the pharmacokinetic differences between the regimens of 0.4 mg/kg twice weekly and 0.8 mg/kg once weekly in JIA patients are of the same magnitude as the differences observed between twice weekly and weekly regimens in adult RA patients.
The mean (± SD) serum steady-state trough concentrations for 50 mg QW dosing in adult PsA subjects were 2.1 ± 1.2 mcg/mL and 2.1 ± 1.4 mcg/mL at weeks 24 and 48, respectively.
The mean (± SD) serum steady-state trough concentrations for the 50 mg QW dosing in adult PsO subjects were 1.5 ± 0.7 mcg/mL. Pediatric PsO patients (age 4 to 17 years) were administered 0.8 mg/kg of Enbrel once weekly (up to a maximum dose of 50 mg per week) for up to 48 weeks. The mean (± SD) serum steady-state trough concentrations ranged from 1.6 ± 0.8 to 2.1 ± 1.3 mcg/mL at weeks 12, 24, and 48.
Overall, the observed etanercept concentrations in patients with JIA and pediatric PsO were within the range of those observed for adult RA, PsA and PsO after administration of Enbrel.
In clinical studies with Enbrel, pharmacokinetic parameters were not different between men and women and did not vary with age in adult patients. The pharmacokinetics of etanercept were unaltered by concomitant MTX in RA patients. No formal pharmacokinetic studies have been conducted to examine the effects of renal or hepatic impairment on etanercept disposition.
NONCLINICAL TOXICOLOGY
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term animal studies have not been conducted to evaluate the carcinogenic potential of etanercept or its effect on fertility.
CLINICAL STUDIES
Adult Rheumatoid Arthritis
The safety and efficacy of Enbrel were assessed in four randomized, double-blind, controlled studies. The results of all four trials were expressed in percentage of patients with improvement in RA using ACR response criteria.
Study I evaluated 234 patients with active RA who were ≥ 18 years old, had failed therapy with at least one but no more than four disease-modifying antirheumatic drugs (DMARDs) (e.g. hydroxychloroquine, oral or injectable gold, MTX, azathioprine, D-penicillamine, sulfasalazine), and had ≥ 12 tender joints, ≥ 10 swollen joints, and either erythrocyte sedimentation rate (ESR) ≥ 28 mm/hr, C-reactive protein (CRP) > 2.0 mg/dL, or morning stiffness for ≥ 45 minutes. Doses of 10 mg or 25 mg Enbrel or placebo were administered SC twice a week for 6 consecutive months.
Study II evaluated 89 patients and had similar inclusion criteria to Study I except that patients in Study II had additionally received MTX for at least 6 months with a stable dose (12.5 to 25 mg/week) for at least 4 weeks and they had at least 6 tender or painful joints. Patients in Study II received a dose of 25 mg Enbrel or placebo SC twice a week for 6 months in addition to their stable MTX dose.
Study III compared the efficacy of Enbrel to MTX in patients with active RA. This study evaluated 632 patients who were ≥ 18 years old with early (≤ 3 years disease duration) active RA, had never received treatment with MTX, and had ≥ 12 tender joints, ≥ 10 swollen joints, and either ESR ≥ 28 mm/hr, CRP > 2.0 mg/dL, or morning stiffness for ≥ 45 minutes. Doses of 10 mg or 25 mg Enbrel were administered SC twice a week for 12 consecutive months. The study was unblinded after all patients had completed at least 12 months (and a median of 17.3 months) of therapy. The majority of patients remained in the study on the treatment to which they were randomized through 2 years, after which they entered an extension study and received open-label 25 mg Enbrel. MTX tablets (escalated from 7.5 mg/week to a maximum of 20 mg/week over the first 8 weeks of the trial) or placebo tablets were given once a week on the same day as the injection of placebo or Enbrel doses, respectively.
Study IV evaluated 682 adult patients with active RA of 6 months to 20 years duration (mean of 7 years) who had an inadequate response to at least one DMARD other than MTX. Forty-three percent of patients had previously received MTX for a mean of 2 years prior to the trial at a mean dose of 12.9 mg. Patients were excluded from this study if MTX had been discontinued for lack of efficacy or for safety considerations. The patient baseline characteristics were similar to those of patients in Study I. Patients were randomized to MTX alone (7.5 to 20 mg weekly, dose escalated as described for Study III; median dose 20 mg), Enbrel alone (25 mg twice weekly), or the combination of Enbrel and MTX initiated concurrently (at the same doses as above). The study evaluated ACR response, Sharp radiographic score, and safety.
Clinical Response
A higher percentage of patients treated with Enbrel and Enbrel in combination with MTX achieved ACR 20, ACR 50, and ACR 70 responses and Major Clinical Responses than in the comparison groups. The results of Studies I, II, and III are summarized in Table 6. The results of Study IV are summarized in Table 7.
| Placebo-Controlled | Active-Controlled | |||||
|---|---|---|---|---|---|---|
| Study I | Study II | Study III | ||||
| Placebo | Enbrel 25 mg Enbrel SC twice weekly. | MTX/Placebo | MTX/Enbrel | MTX | Enbrel | |
| Response | N = 80 | N = 78 | N = 30 | N = 59 | N = 217 | N = 207 |
| ACR 20 | ||||||
| Month 3 | 23% | 62% p < 0.01, Enbrel versus placebo. | 33% | 66% | 56% | 62% |
| Month 6 | 11% | 59% | 27% | 71% | 58% | 65% |
| Month 12 | NA | NA | NA | NA | 65% | 72% |
| ACR 50 | ||||||
| Month 3 | 8% | 41% | 0% | 42% | 24% | 29% |
| Month 6 | 5% | 40% | 3% | 39% | 32% | 40% |
| Month 12 | NA | NA | NA | NA | 43% | 49% |
| ACR 70 | ||||||
| Month 3 | 4% | 15% | 0% | 15% | 7% | 13% p < 0.05, Enbrel versus MTX. |
| Month 6 | 1% | 15% | 0% | 15% | 14% | 21% |
| Month 12 | NA | NA | NA | NA | 22% | 25% |
| Endpoint | MTX (N = 228) | Enbrel (N = 223) | Enbrel/MTX (N = 231) |
|---|---|---|---|
| ACR N Values are medians. , ACR N is the percent improvement based on the same core variables used in defining ACR 20, ACR 50, and ACR 70. | |||
| Month 12 | 40% | 47% | 63% p < 0.05 for comparisons of Enbrel/MTX versus Enbrel alone or MTX alone. |
| ACR 20 | |||
| Month 12 | 59% | 66% | 75% |
| ACR 50 | |||
| Month 12 | 36% | 43% | 63% |
| ACR 70 | |||
| Month 12 | 17% | 22% | 40% |
| Major Clinical Response Major clinical response is achieving an ACR 70 response for a continuous 6-month period. | 6% | 10% | 24% |
The time course for ACR 20 response rates for patients receiving placebo or 25 mg Enbrel in Studies I and II is summarized in Figure 1. The time course of responses to Enbrel in Study III was similar.
|
Among patients receiving Enbrel, the clinical responses generally appeared within 1 to 2 weeks after initiation of therapy and nearly always occurred by 3 months. A dose response was seen in Studies I and III: 25 mg Enbrel was more effective than 10 mg (10 mg was not evaluated in Study II). Enbrel was significantly better than placebo in all components of the ACR criteria as well as other measures of RA disease activity not included in the ACR response criteria, such as morning stiffness.
In Study III, ACR response rates and improvement in all the individual ACR response criteria were maintained through 24 months of Enbrel therapy. Over the 2-year study, 23% of Enbrel patients achieved a major clinical response, defined as maintenance of an ACR 70 response over a 6-month period.
The results of the components of the ACR response criteria for Study I are shown in Table 8. Similar results were observed for Enbrel-treated patients in Studies II and III.
| Placebo N = 80 | Enbrel 25 mg Enbrel SC twice weekly. N = 78 | |||
|---|---|---|---|---|
| Parameter (median) | Baseline | 3 Months | Baseline | 3 Months Results at 6 months showed similar improvement. |
| Number of tender joints Scale 0-71. | 34.0 | 29.5 | 31.2 | 10.0 p < 0.01, Enbrel versus placebo, based on mean percent change from baseline. |
| Number of swollen joints Scale 0-68. | 24.0 | 22.0 | 23.5 | 12.6 |
| Physician global assessment Visual analog scale: 0 = best; 10 = worst. | 7.0 | 6.5 | 7.0 | 3.0 |
| Patient global assessment | 7.0 | 7.0 | 7.0 | 3.0 |
| Pain | 6.9 | 6.6 | 6.9 | 2.4 |
| Disability index Health Assessment Questionnaire: 0 = best; 3 = worst; includes eight categories: dressing and grooming, arising, eating, walking, hygiene, reach, grip, and activities. | 1.7 | 1.8 | 1.6 | 1.0 |
| ESR (mm/hr) | 31.0 | 32.0 | 28.0 | 15.5 |
| CRP (mg/dL) | 2.8 | 3.9 | 3.5 | 0.9 |
After discontinuation of Enbrel, symptoms of arthritis generally returned within a month. Reintroduction of treatment with Enbrel after discontinuations of up to 18 months resulted in the same magnitudes of response as in patients who received Enbrel without interruption of therapy, based on results of open-label studies.
Continued durable responses were seen for over 60 months in open-label extension treatment trials when patients received Enbrel without interruption. A substantial number of patients who initially received concomitant MTX or corticosteroids were able to reduce their doses or discontinue these concomitant therapies while maintaining their clinical responses.
Physical Function Response
In Studies I, II, and III, physical function and disability were assessed using the Health Assessment Questionnaire (HAQ). Additionally, in Study III, patients were administered the SF-36 Health Survey. In Studies I and II, patients treated with 25 mg Enbrel twice weekly showed greater improvement from baseline in the HAQ score beginning in month 1 through month 6 in comparison to placebo (p < 0.001) for the HAQ disability domain (where 0 = none and 3 = severe). In Study I, the mean improvement in the HAQ score from baseline to month 6 was 0.6 (from 1.6 to 1.0) for the 25 mg Enbrel group and 0 (from 1.7 to 1.7) for the placebo group. In Study II, the mean improvement from baseline to month 6 was 0.6 (from 1.5 to 0.9) for the Enbrel/MTX group and 0.2 (from 1.3 to 1.2) for the placebo/MTX group. In Study III, the mean improvement in the HAQ score from baseline to month 6 was 0.7 (from 1.5 to 0.7) for 25 mg Enbrel twice weekly. All subdomains of the HAQ in Studies I and III were improved in patients treated with Enbrel.
In Study III, patients treated with 25 mg Enbrel twice weekly showed greater improvement from baseline in SF-36 physical component summary score compared to Enbrel 10 mg twice weekly and no worsening in the SF-36 mental component summary score. In open-label Enbrel studies, improvements in physical function and disability measures have been maintained for up to 4 years.
In Study IV, median HAQ scores improved from baseline levels of 1.8, 1.8, and 1.8 to 1.1, 1.0, and 0.6 at 12 months in the MTX, Enbrel, and Enbrel/MTX combination treatment groups, respectively (combination versus both MTX and Enbrel, p < 0.01). Twenty-nine percent of patients in the MTX alone treatment group had an improvement of HAQ of at least 1 unit versus 40% and 51% in the Enbrel alone and the Enbrel/MTX combination treatment groups, respectively.
Radiographic Response
In Study III, structural joint damage was assessed radiographically and expressed as change in Total Sharp Score (TSS) and its components, the erosion score and Joint Space Narrowing (JSN) score. Radiographs of hands/wrists and forefeet were obtained at baseline, 6 months, 12 months, and 24 months and scored by readers who were unaware of treatment group. The results are shown in Table 9. A significant difference for change in erosion score was observed at 6 months and maintained at 12 months.
| MTX | 25 mg Enbrel | MTX/Enbrel (95% Confidence Interval 95% confidence intervals for the differences in change scores between MTX and Enbrel. ) | P Value | ||
|---|---|---|---|---|---|
| 12 Months | Total Sharp Score | 1.59 | 1.00 | 0.59 (-0.12, 1.30) | 0.1 |
| Erosion Score | 1.03 | 0.47 | 0.56 (0.11, 1.00) | 0.002 | |
| JSN Score | 0.56 | 0.52 | 0.04 (-0.39, 0.46) | 0.5 | |
| 6 Months | Total Sharp Score | 1.06 | 0.57 | 0.49 (0.06, 0.91) | 0.001 |
| Erosion Score | 0.68 | 0.30 | 0.38 (0.09, 0.66) | 0.001 | |
| JSN Score | 0.38 | 0.27 | 0.11 (-0.14, 0.35) | 0.6 |
Patients continued on the therapy to which they were randomized for the second year of Study III. Seventy-two percent of patients had x-rays obtained at 24 months. Compared to the patients in the MTX group, greater inhibition of progression in TSS and erosion score was seen in the 25 mg Enbrel group, and, in addition, less progression was noted in the JSN score.
In the open-label extension of Study III, 48% of the original patients treated with 25 mg Enbrel have been evaluated radiographically at 5 years. Patients had continued inhibition of structural damage, as measured by the TSS, and 55% of them had no progression of structural damage. Patients originally treated with MTX had further reduction in radiographic progression once they began treatment with Enbrel.
In Study IV, less radiographic progression (TSS) was observed with Enbrel in combination with MTX compared with Enbrel alone or MTX alone at month 12 (Table 10). In the MTX treatment group, 55% of patients experienced no radiographic progression (TSS change ≤ 0.0) at 12 months compared to 63% and 76% in the Enbrel alone and the Enbrel/MTX combination treatment groups, respectively.
| MTX (N = 212) Analyzed radiographic ITT population. | Enbrel (N = 212) | Enbrel/MTX (N = 218) | |
|---|---|---|---|
| Total Sharp Score (TSS) | 2.80 (1.08, 4.51) | 0.52 p < 0.05 for comparison of Enbrel versus MTX. (-0.10, 1.15) | -0.54 p < 0.05 for comparison of Enbrel/MTX versus MTX. , p < 0.05 for comparison of Enbrel/MTX versus Enbrel. (-1.00, -0.07) |
| Erosion Score (ES) | 1.68 (0.61, 2.74) | 0.21 (-0.20, 0.61) | -0.30 (-0.65, 0.04) |
| Joint Space Narrowing (JSN) Score | 1.12 (0.34, 1.90) | 0.32 (0.00, 0.63) | -0.23 , (-0.45, -0.02) |
Once Weekly Dosing
The safety and efficacy of 50 mg Enbrel (two 25 mg SC injections) administered once weekly were evaluated in a double-blind, placebo-controlled study of 420 patients with active RA. Fifty-three patients received placebo, 214 patients received 50 mg Enbrel once weekly, and 153 patients received 25 mg Enbrel twice weekly. The safety and efficacy profiles of the two Enbrel treatment groups were similar.
Polyarticular Juvenile Idiopathic Arthritis (JIA)
The safety and efficacy of Enbrel were assessed in a 2-part study in 69 children with polyarticular JIA who had a variety of JIA onset types. Patients ages 2 to 17 years with moderately to severely active polyarticular JIA refractory to or intolerant of MTX were enrolled; patients remained on a stable dose of a single nonsteroidal anti-inflammatory drug and/or prednisone (≤ 0.2 mg/kg/day or 10 mg maximum). In part 1, all patients received 0.4 mg/kg (maximum 25 mg per dose) Enbrel SC twice weekly. In part 2, patients with a clinical response at day 90 were randomized to remain on Enbrel or receive placebo for 4 months and assessed for disease flare. Responses were measured using the JIA Definition of Improvement (DOI), defined as ≥ 30% improvement in at least three of six and ≥ 30% worsening in no more than one of the six JIA core set criteria, including active joint count, limitation of motion, physician and patient/parent global assessments, functional assessment, and ESR. Disease flare was defined as a ≥ 30% worsening in three of the six JIA core set criteria and ≥ 30% improvement in not more than one of the six JIA core set criteria and a minimum of two active joints.
In part 1 of the study, 51 of 69 (74%) patients demonstrated a clinical response and entered part 2. In part 2, 6 of 25 (24%) patients remaining on Enbrel experienced a disease flare compared to 20 of 26 (77%) patients receiving placebo (p = 0.007). From the start of part 2, the median time to flare was ≥ 116 days for patients who received Enbrel and 28 days for patients who received placebo. Each component of the JIA core set criteria worsened in the arm that received placebo and remained stable or improved in the arm that continued on Enbrel. The data suggested the possibility of a higher flare rate among those patients with a higher baseline ESR. Of patients who demonstrated a clinical response at 90 days and entered part 2 of the study, some of the patients remaining on Enbrel continued to improve from month 3 through month 7, while those who received placebo did not improve.
The majority of JIA patients who developed a disease flare in part 2 and reintroduced Enbrel treatment up to 4 months after discontinuation re-responded to Enbrel therapy in open-label studies. Most of the responding patients who continued Enbrel therapy without interruption have maintained responses for up to 48 months.
Studies have not been done in patients with polyarticular JIA to assess the effects of continued Enbrel therapy in patients who do not respond within 3 months of initiating Enbrel therapy, or to assess the combination of Enbrel with MTX.
Psoriatic Arthritis
The safety and efficacy of Enbrel were assessed in a randomized, double-blind, placebo-controlled study in 205 patients with PsA. Patients were between 18 and 70 years of age and had active PsA (≥ 3 swollen joints and ≥ 3 tender joints) in one or more of the following forms: (1) distal interphalangeal (DIP) involvement (N = 104); (2) polyarticular arthritis (absence of rheumatoid nodules and presence of psoriasis; N = 173); (3) arthritis mutilans (N = 3); (4) asymmetric psoriatic arthritis (N = 81); or (5) ankylosing spondylitis-like (N = 7). Patients also had plaque psoriasis with a qualifying target lesion ≥ 2 cm in diameter. Patients on MTX therapy at enrollment (stable for ≥ 2 months) could continue at a stable dose of ≤ 25 mg/week MTX. Doses of 25 mg Enbrel or placebo were administered SC twice a week during the initial 6-month double-blind period of the study. Patients continued to receive blinded therapy in an up to 6-month maintenance period until all patients had completed the controlled period. Following this, patients received open-label 25 mg Enbrel twice a week in a 12-month extension period.
Compared to placebo, treatment with Enbrel resulted in significant improvements in measures of disease activity (Table 11).
| Placebo N = 104 | Enbrel p < 0.001 for all comparisons between Enbrel and placebo at 6 months. N = 101 | |||
|---|---|---|---|---|
| Parameter (median) | Baseline | 6 Months | Baseline | 6 Months |
| Number of tender joints Scale 0-78. | 17.0 | 13.0 | 18.0 | 5.0 |
| Number of swollen joints Scale 0-76. | 12.5 | 9.5 | 13.0 | 5.0 |
| Physician global assessment Likert scale: 0 = best; 5 = worst. | 3.0 | 3.0 | 3.0 | 1.0 |
| Patient global assessment | 3.0 | 3.0 | 3.0 | 1.0 |
| Morning stiffness (minutes) | 60 | 60 | 60 | 15 |
| Pain | 3.0 | 3.0 | 3.0 | 1.0 |
| Disability index Health Assessment Questionnaire: 0 = best; 3 = worst; includes eight categories: dressing and grooming, arising, eating, walking, hygiene, reach, grip, and activities. | 1.0 | 0.9 | 1.1 | 0.3 |
| CRP (mg/dL) Normal range: 0-0.79 mg/dL. | 1.1 | 1.1 | 1.6 | 0.2 |
Among patients with PsA who received Enbrel, the clinical responses were apparent at the time of the first visit (4 weeks) and were maintained through 6 months of therapy. Responses were similar in patients who were or were not receiving concomitant MTX therapy at baseline. At 6 months, the ACR 20/50/70 responses were achieved by 50%, 37%, and 9%, respectively, of patients receiving Enbrel, compared to 13%, 4%, and 1%, respectively, of patients receiving placebo. Similar responses were seen in patients with each of the subtypes of PsA, although few patients were enrolled with the arthritis mutilans and ankylosing spondylitis-like subtypes. The results of this study were similar to those seen in an earlier single-center, randomized, placebo-controlled study of 60 patients with PsA.
The skin lesions of psoriasis were also improved with Enbrel, relative to placebo, as measured by percentages of patients achieving improvements in the Psoriasis Area and Severity Index (PASI). Responses increased over time, and at 6 months, the proportions of patients achieving a 50% or 75% improvement in the PASI were 47% and 23%, respectively, in the Enbrel group (N = 66), compared to 18% and 3%, respectively, in the placebo group (N = 62). Responses were similar in patients who were or were not receiving concomitant MTX therapy at baseline.
Radiographic Response
Radiographic changes were also assessed in the PsA study. Radiographs of hands and wrists were obtained at baseline and months 6, 12, and 24. A modified Total Sharp Score (TSS), which included distal interphalangeal joints (i.e., not identical to the modified TSS used for RA) was used by readers blinded to treatment group to assess the radiographs. Some radiographic features specific to PsA (e.g. pencil-and-cup deformity, joint space widening, gross osteolysis, and ankylosis) were included in the scoring system, but others (e.g. phalangeal tuft resorption, juxta-articular and shaft periostitis) were not.
Most patients showed little or no change in the modified TSS during this 24-month study (median change of 0 in both patients who initially received Enbrel or placebo). More placebo-treated patients experienced larger magnitudes of radiographic worsening (increased TSS) compared to Enbrel treatment during the controlled period of the study. At 12 months, in an exploratory analysis, 12% (12 of 104) of placebo patients compared to none of the 101 Enbrel-treated patients had increases of 3 points or more in TSS. Inhibition of radiographic progression was maintained in patients who continued on Enbrel during the second year. Of the patients with 1-year and 2-year x-rays, 3% (2 of 71) had increases of 3 points or more in TSS at 1 and 2 years.
Physical Function Response
In the PsA study, physical function and disability were assessed using the HAQ Disability Index (HAQ-DI) and the SF-36 Health Survey. Patients treated with 25 mg Enbrel twice weekly showed greater improvement from baseline in the HAQ-DI score (mean decreases of 54% at both months 3 and 6) in comparison to placebo (mean decreases of 6% at both months 3 and 6) (p < 0.001). At months 3 and 6, patients treated with Enbrel showed greater improvement from baseline in the SF-36 physical component summary score compared to patients treated with placebo, and no worsening in the SF-36 mental component summary score. Improvements in physical function and disability measures were maintained for up to 2 years through the open-label portion of the study.
Ankylosing Spondylitis
The safety and efficacy of Enbrel were assessed in a randomized, double-blind, placebo-controlled study in 277 patients with active AS. Patients were between 18 and 70 years of age and had AS as defined by the modified New York Criteria for Ankylosing Spondylitis. Patients were to have evidence of active disease based on values of ≥ 30 on a 0-100 unit Visual Analog Scale (VAS) for the average of morning stiffness duration and intensity, and two of the following three other parameters: a) patient global assessment, b) average of nocturnal and total back pain, and c) the average score on the Bath Ankylosing Spondylitis Functional Index (BASFI). Patients with complete ankylosis of the spine were excluded from study participation. Patients taking hydroxychloroquine, sulfasalazine, methotrexate, or prednisone (≤ 10 mg/day) could continue these drugs at stable doses for the duration of the study. Doses of 25 mg Enbrel or placebo were administered SC twice a week for 6 months.
The primary measure of efficacy was a 20% improvement in the Assessment in Ankylosing Spondylitis (ASAS) response criteria. Compared to placebo, treatment with Enbrel resulted in improvements in the ASAS and other measures of disease activity (Figure 2 and Table 12).
| Figure 2. ASAS 20 Responses in Ankylosing Spondylitis |
|---|
 |
At 12 weeks, the ASAS 20/50/70 responses were achieved by 60%, 45%, and 29%, respectively, of patients receiving Enbrel, compared to 27%, 13%, and 7%, respectively, of patients receiving placebo (p ≤ 0.0001, Enbrel versus placebo). Similar responses were seen at Week 24. Responses were similar between those patients receiving concomitant therapies at baseline and those who were not. The results of this study were similar to those seen in a single-center, randomized, placebo-controlled study of 40 patients and a multicenter, randomized, placebo-controlled study of 84 patients with AS.
| Placebo N = 139 | Enbrel p < 0.0015 for all comparisons between Enbrel and placebo at 6 months. P values for continuous endpoints were based on percent change from baseline. N = 138 | |||
|---|---|---|---|---|
| Median values at time points | Baseline | 6 Months | Baseline | 6 Months |
| ASAS response criteria | ||||
| Patient global assessment Measured on a Visual Analog Scale (VAS) with 0 = "none" and 100 = "severe". | 63 | 56 | 63 | 36 |
| Back pain Average of total nocturnal and back pain scores, measured on a VAS with 0 = "no pain" and 100 = "most severe pain". | 62 | 56 | 60 | 34 |
| BASFI Bath Ankylosing Spondylitis Functional Index (BASFI), average of 10 questions. | 56 | 55 | 52 | 36 |
| Inflammation Inflammation represented by the average of the last 2 questions on the 6-question Bath Ankylosing Spondylitis Disease Activity Index (BASDAI). | 64 | 57 | 61 | 33 |
| Acute phase reactants | ||||
| CRP (mg/dL) C-reactive protein (CRP) normal range: 0-1.0 mg/dL. | 2.0 | 1.9 | 1.9 | 0.6 |
| Spinal mobility (cm): | ||||
| Modified Schober's test | 3.0 | 2.9 | 3.1 | 3.3 |
| Chest expansion | 3.2 | 3.0 | 3.3 | 3.9 |
| Occiput-to-wall measurement | 5.3 | 6.0 | 5.6 | 4.5 |
Adult Plaque Psoriasis
The safety and efficacy of Enbrel were assessed in two randomized, double-blind, placebo-controlled studies in adults with chronic stable PsO involving ≥ 10% of the body surface area, a minimum Psoriasis Area and Severity Index (PASI) score of 10 and who had received or were candidates for systemic antipsoriatic therapy or phototherapy. Patients with guttate, erythrodermic, or pustular psoriasis and patients with severe infections within 4 weeks of screening were excluded from study. No concomitant major antipsoriatic therapies were allowed during the study.
Study I evaluated 672 subjects who received placebo or Enbrel SC at doses of 25 mg once a week, 25 mg twice a week, or 50 mg twice a week for 3 months. After 3 months, subjects continued on blinded treatments for an additional 3 months during which time subjects originally randomized to placebo began treatment with blinded Enbrel at 25 mg twice weekly (designated as placebo/Enbrel in Table 13); subjects originally randomized to Enbrel continued on the originally randomized dose (designated as Enbrel/Enbrel groups in Table 13).
Study II evaluated 611 subjects who received placebo or Enbrel SC at doses of 25 mg or 50 mg twice a week for 3 months. After 3 months of randomized, blinded treatment, subjects in all three arms began receiving open-label Enbrel at 25 mg twice weekly for 9 additional months.
Response to treatment in both studies was assessed after 3 months of therapy and was defined as the proportion of subjects who achieved a reduction in PASI score of at least 75% from baseline. The PASI is a composite score that takes into consideration both the fraction of body surface area affected and the nature and severity of psoriatic changes within the affected regions (induration, erythema and scaling).
Other evaluated outcomes included the proportion of subjects who achieved a score of "clear" or "minimal" by the Static Physician Global Assessment (sPGA) and the proportion of subjects with a reduction of PASI of at least 50% from baseline. The sPGA is a 6-category scale ranging from "5 = severe" to "0 = none" indicating the physician's overall assessment of the PsO severity focusing on induration, erythema and scaling. Treatment success of "clear" or "minimal" consisted of none or minimal elevation in plaque, up to faint red coloration in erythema and none or minimal fine scale over < 5% of the plaque.
Subjects in all treatment groups and in both studies had a median baseline PASI score ranging from 15 to 17, and the percentage of subjects with baseline sPGA classifications ranged from 54% to 66% for moderate, 17% to 26% for marked and 1% to 5% for severe. Across all treatment groups, the percentage of subjects who previously received systemic therapy for PsO ranged from 61% to 65% in Study I and 71% to 75% in Study II, and those who previously received phototherapy ranged from 44% to 50% in Study I and 72% to 73% in Study II.
More subjects randomized to Enbrel than placebo achieved at least a 75% reduction from baseline PASI score (PASI 75) with a dose response relationship across doses of 25 mg once a week, 25 mg twice a week and 50 mg twice a week (Tables 13 and 14). The individual components of the PASI (induration, erythema and scaling) contributed comparably to the overall treatment-associated improvement in PASI.
| Placebo/Enbrel | Enbrel/Enbrel | |||
|---|---|---|---|---|
| 25 mg BIW | 25 mg QW | 25 mg BIW | 50 mg BIW | |
| (N = 168) | (N = 169) | (N = 167) | (N = 168) | |
| 3 Months | ||||
| PASI 75 n (%) | 6 (4%) | 23 (14%) p = 0.001 compared with placebo. | 53 (32%) p < 0.0001 compared with placebo. | 79 (47%) |
| Difference (95% CI) | 10% (4, 16) | 28% (21, 36) | 43% (35, 52) | |
| sPGA, "clear" or "minimal" n (%) | 8 (5%) | 36 (21%) | 53 (32%) | 79 (47%) |
| Difference (95% CI) | 17% (10, 24) | 27% (19, 35) | 42% (34, 50) | |
| PASI 50 n (%) | 24 (14%) | 62 (37%) | 90 (54%) | 119 (71%) |
| Difference (95% CI) | 22% (13, 31) | 40% (30, 49) | 57% (48, 65) | |
| 6 Months | ||||
| PASI 75 n (%) | 55 (33%) | 36 (21%) | 68 (41%) | 90 (54%) |
| Placebo | Enbrel | ||
|---|---|---|---|
| 25 mg BIW | 50 mg BIW | ||
| (N = 204) | (N = 204) | (N = 203) | |
| PASI 75 n (%) | 6 (3%) | 66 (32%) p < 0.0001 compared with placebo. | 94 (46%) |
| Difference (95% CI) | 29% (23, 36) | 43% (36, 51) | |
| sPGA, "clear" or "minimal" n (%) | 7 (3%) | 75 (37%) | 109 (54%) |
| Difference (95% CI) | 34% (26, 41) | 50% (43, 58) | |
| PASI 50 n (%) | 18 (9%) | 124 (61%) | 147 (72%) |
| Difference (95% CI) | 52% (44, 60) | 64% (56, 71) | |
Among PASI 75 achievers in both studies, the median time to PASI 50 and PASI 75 was approximately 1 month and approximately 2 months, respectively, after the start of therapy with either 25 or 50 mg twice a week.
In Study I, subjects who achieved PASI 75 at month 6 were entered into a study drug withdrawal and retreatment period. Following withdrawal of study drug, these subjects had a median duration of PASI 75 of between 1 and 2 months.
In Study I, among subjects who were PASI 75 responders at 3 months, retreatment with their original blinded Enbrel dose after discontinuation of up to 5 months resulted in a similar proportion of responders as in the initial double-blind portion of the study.
In Study II, most subjects initially randomized to 50 mg twice a week continued in the study after month 3 and had their Enbrel dose decreased to 25 mg twice a week. Of the 91 subjects who were PASI 75 responders at month 3, 70 (77%) maintained their PASI 75 response at month 6.
Pediatric Plaque Psoriasis
A 48-week, randomized, double-blind, placebo-controlled study enrolled 211 pediatric subjects 4 to 17 years of age, with moderate to severe plaque psoriasis (PsO) (as defined by a sPGA score ≥ 3 [moderate, marked, or severe], involving ≥ 10% of the body surface area, and a PASI score ≥ 12) who were candidates for phototherapy or systemic therapy, or were inadequately controlled on topical therapy. Subjects in all treatment groups had a median baseline PASI score of 16.4, and the percentage of subjects with baseline sPGA classifications was 65% for moderate, 31% for marked, and 3% for severe. Across all treatment groups, the percentage of subjects who previously received systemic or phototherapy for PsO was 57%.
Subjects received Enbrel 0.8 mg/kg (up to a maximum of 50 mg per dose) or placebo once weekly for the first 12 weeks. After 12 weeks, subjects entered a 24-week open-label treatment period, in which all subjects received Enbrel at the same dose. This was followed by a 12-week withdrawal-retreatment period.
Response to treatment was assessed after 12 weeks of therapy and was defined as the proportion of subjects who achieved a reduction in PASI score of at least 75% from baseline. The PASI is a composite score that takes into consideration both the fraction of body surface area affected and the nature and severity of psoriatic changes within the affected regions (induration, erythema and scaling).
Other evaluated outcomes included the proportion of subjects who achieved a score of "clear" or "almost clear" by the sPGA and the proportion of subjects with a reduction in PASI score of at least 90% from baseline. The sPGA is a 6-category scale ranging from "5 = severe" to "0 = none" indicating the physician's overall assessment of the PsO severity focusing on induration, erythema and scaling. Treatment success of "clear" or "almost clear" consisted of none or minimal elevation in plaque, up to faint red coloration in erythema and none or minimal fine scale over < 5% of the plaque.
Efficacy results are summarized in Table 15.
| Placebo (N = 105) | Enbrel 0.8 mg/kg Once Weekly (N = 106) | |
|---|---|---|
| PASI 75, n (%) | 12 (11%) | 60 (57%) |
| PASI 90, n (%) | 7 (7%) | 29 (27%) |
| sPGA "clear" or "almost clear" n (%) | 14 (13%) | 55 (52%) |
Maintenance of Response
To evaluate maintenance of response, subjects who achieved PASI 75 response at Week 36 were re-randomized to either Enbrel or placebo during a 12-week randomized withdrawal period. The maintenance of PASI 75 response was evaluated at Week 48. The proportion of subjects who maintained PASI 75 response at Week 48 was higher for subjects treated with Enbrel (65%) compared to those treated with placebo (49%).
HOW SUPPLIED/STORAGE AND HANDLING
Enbrel (etanercept) injection is supplied as a clear and colorless sterile, preservative-free solution for subcutaneous administration in single-dose prefilled syringes, an Enbrel single-dose prefilled SureClick autoinjector with a 27-gauge, ½-inch needle, or a single-dose vial. The prefilled syringe and SureClick autoinjector are not made with natural rubber latex.
Each Enbrel ® Mini single-dose prefilled cartridge for use with the AutoTouch ® reusable autoinjector contains 1.0 mL of 50 mg/mL of etanercept. The AutoTouch reusable autoinjector and Enbrel Mini single-dose prefilled cartridge are not made with natural rubber latex.
The AutoTouch reusable autoinjector contains no drug and must use an Enbrel Mini single-dose prefilled cartridge. In addition, the AutoTouch Connect ® reusable autoinjector would allow for data connectivity via Bluetooth wireless technology.
| 50 mg/mL single-dose prefilled syringe | Carton of 4 | NDC 58406-021-04 |
| 50 mg/mL single-dose prefilled SureClick autoinjector | Carton of 4 | NDC 58406-032-04 |
| 25 mg/0.5 mL single-dose prefilled syringe | Carton of 4 | NDC 58406-010-04 |
| 50 mg/mL Enbrel Mini single-dose prefilled cartridge for use with the AutoTouch reusable autoinjector only | Cartridges: Carton of 4 | NDC 58406-044-04 NDC 58406-044-24 |
| AutoTouch Connect Reusable Autoinjector: Carton of 1 | NDC 58406-480-01 | |
| 25 mg/0.5 mL single-dose vial | Carton of 4 | NDC 58406-055-04 |
Enbrel should be refrigerated at 36°F to 46°F (2°C to 8°C) in the original carton to protect from light or physical damage. Do not store Enbrel in extreme heat or cold. DO NOT SHAKE. DO NOT FREEZE.
For convenience, storage of individual single-dose prefilled syringes, SureClick autoinjectors, single-dose vials, or Enbrel Mini cartridges at room temperature at 68°F to 77°F (20°C to 25°C) for a maximum single period of 30 days is permissible, with protection from light and sources of heat. Once a single-dose prefilled syringe, SureClick autoinjector, single-dose vial, or Enbrel Mini cartridge has been stored at room temperature, it should not be placed back into the refrigerator. If not used within 30 days at room temperature, the single-dose prefilled syringe, SureClick autoinjector, single-dose vial, or Enbrel Mini cartridge should be discarded.
Do not use Enbrel beyond the expiration date stamped on the carton or barrel/cartridge label. Keep out of the reach of children.
The AutoTouch reusable autoinjector should be stored at room temperature. Do not refrigerate the AutoTouch reusable autoinjector.
Enbrel Lyophilized Powder (Used for Weight-based Dosing)
Enbrel (etanercept) for Injection is supplied as lyophilized powder for reconstitution in a multiple-dose vial. Each vial is supplied in a carton containing four dose trays. Each dose tray contains one 25 mg vial of etanercept lyophilized powder, one diluent syringe (1 mL Sterile Bacteriostatic Water for Injection, USP, containing 0.9% benzyl alcohol), one 27-gauge ½-inch needle, one vial adapter, and one plunger. Each carton contains four "Mixing Date:" stickers.
| 25 mg multiple-dose vial | Carton of 4 | NDC 58406-425-34 |
Enbrel should be refrigerated at 36°F to 46°F (2°C to 8°C) in the original carton to protect from light or physical damage. Do not store Enbrel in extreme heat or cold. DO NOT SHAKE. DO NOT FREEZE.
For convenience, storage of an individual dose tray containing Enbrel multiple-dose vial and diluent syringe at room temperature at 68°F to 77°F (20°C to 25°C) for a maximum single period of 14 days is permissible, with protection from light, sources of heat, and humidity. Once the dose tray has been stored at room temperature, it should not be placed back into the refrigerator. If not used within 14 days at room temperature, the dose tray should be discarded. Once a vial has been reconstituted, the solution must be used immediately or may be refrigerated for up to 14 days.
Do not use Enbrel beyond the expiration date stamped on the dose tray. Keep out of the reach of children.
INSTRUCTIONS FOR USE ENBREL ® [en-brel] (etanercept) injection, for subcutaneous use 50 mg/mL single-dose prefilled SureClick ® autoinjector
This Instructions for Use contains information on how to inject ENBREL with a SureClick autoinjector.
If your healthcare provider decides that you or a caregiver may be able to give your injections of ENBREL at home, you should receive training on the right way to prepare and inject ENBREL. Do not try to inject yourself until you have been shown the right way to give the injections by your healthcare provider or nurse.
The medicine in the ENBREL autoinjector is for injection under the skin (subcutaneous injection). See the ENBREL Medication Guide for information about ENBREL.
Getting to know the prefilled autoinjector 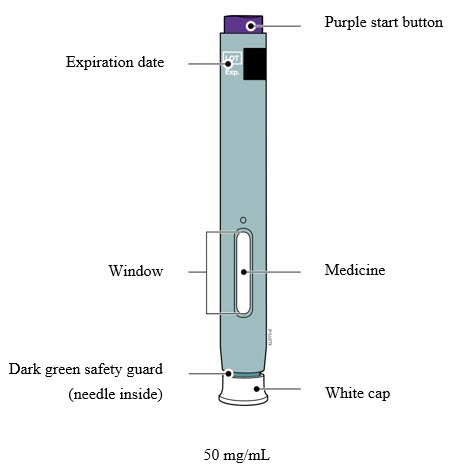 |
Important Information You Need to Know Before Injecting ENBREL
- It is important that you do not try to give the injection until you have fully read and understood this Instructions for Use.
- Check the autoinjector label and prescription to make sure you have the correct medicine and dose.
- Do not use the autoinjector if the carton is damaged or the seal is broken.
- Do not use the autoinjector after the expiration date on the label.
- Do not shake the autoinjector.
- Do not remove the white cap from the autoinjector until you are ready to inject.
- Do not use the autoinjector if it has been frozen.
- Do not use the autoinjector if it has been dropped on a hard surface. Part of the autoinjector may be broken even if you cannot see the break. Use a new autoinjector, and call 1-888-4ENBREL (1-888-436-2735).
- Children must weigh at least 138 pounds to use the ENBREL SureClick autoinjector. Children who weigh less than 138 pounds should use a different form of ENBREL.
- The autoinjector is not made with natural rubber latex.
Frequently asked questions: For additional information and answers to frequently asked questions, visit www.enbrel.com .
Where to get help: If you want more information or help using ENBREL:
- Contact your healthcare provider,
- Visit www.enbrel.com , or
- Call 1-888-4ENBREL (1-888-436-2735).
Storing and Preparing to Inject ENBREL
 |
- 1 Refrigerate the autoinjector carton until you are ready to use it.
- Keep the autoinjector in the refrigerator between 36°F to 46°F (2°C to 8°C).
- Keep the autoinjector in the original carton to protect it from light or physical damage.
- Do not freeze the autoinjector.
- Do not store the autoinjector in extreme heat or cold. For example, avoid storing in your vehicle's glove box or trunk.
| Important: Keep the autoinjector and all medicines out of the sight and reach of children. |
WAIT 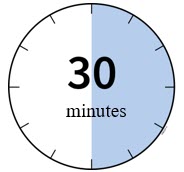 |
- 2 Wait 30 minutes for the autoinjector to reach room temperature.
- Remove the autoinjector and put any unused autoinjectors back into the refrigerator.
- Let the autoinjector warm up naturally.
- Do not heat the autoinjector with hot water, a microwave, or direct sunlight.
- Do not shake the autoinjector at any time.
- Using the autoinjector at room temperature makes sure the full dose is delivered and allows for a more comfortable injection.
 |
- 3 You may keep ENBREL at room temperature for up to 30 days, if needed.
- For example, when you are traveling, you may keep ENBREL at room temperature.
- Keep it at room temperature between 68°F to 77°F (20°C to 25°C).
- Do not put it back in the refrigerator.
- Record the date you removed it from the refrigerator and use it within 30 days.
| Important: Place the autoinjector in a sharps disposal container if it has reached room temperature and has not been used within 30 days. |
 |
- 4 Inspect the medicine. It should be clear and colorless to slightly yellow.
- It is okay to see air bubbles or small white particles in the medicine.
- Do not use the autoinjector if the medicine is cloudy, discolored, or contains large lumps, flakes or colored particles.
| Important: If the medicine is cloudy, discolored, or contains large lumps, flakes or colored particles, or if the autoinjector is damaged or expired, call 1-888-4ENBREL (1-888-436-2735). |
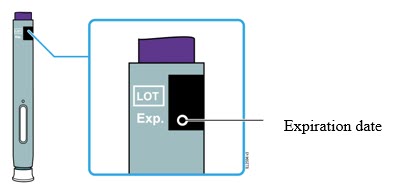 |
- 5 Check the expiration date (Exp.) and inspect the autoinjector for damage.
- Do not use the autoinjector if the expiration date has passed.
- Do not use the autoinjector if:
- the white cap is missing or loose in carton,
- it has cracks or broken parts, or
- it has been dropped on a hard surface.
Getting Ready to Inject ENBREL
 |
- 6 Gather and place the following items for your injection on a clean, flat, and well-lit surface:
- ENBREL autoinjector (room temperature),
- Sharps disposal container [see Completing the Injection and Disposal ],
- Alcohol wipe,
- Adhesive bandage, and
- Cotton balls or gauze pads.
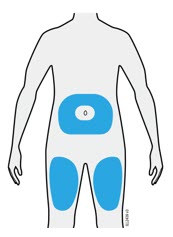 |
- 7 Select 1 of these injection sites.
- Select the thigh or stomach (except for 2 inches around the belly button).
- Someone else can inject in your thigh, stomach, or back of the upper arm.
- Change injection site each time, shifting the area of the injection to avoid skin irritation.
| Important: Avoid areas with scars or stretch marks, or where the skin is tender, bruised, red, hard, raised, thick or scaly skin patch, or lesion. |
 |
- 8 Wash your hands thoroughly with soap and water.
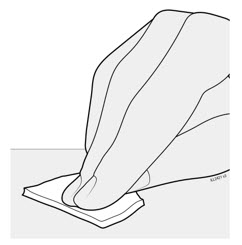 |
- 9 Clean the injection site with an alcohol wipe.
- Let the skin dry on its own.
- Do not touch this area again before injecting.
Injecting ENBREL
| Important: Only remove the white cap when you can inject right away (within 5 minutes) because the medicine can dry out. Do not recap. |
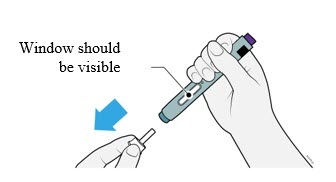 |
- 10 Grasp the autoinjector so you can see the window. Pull the white cap straight off. You may need to pull hard.
- Do not twist, bend, or wiggle the white cap to pull it off.
- Never put the white cap back on. It may damage the needle.
- Do not put your finger inside the dark green safety guard.
- It is normal to see a drop of medicine come out of the needle or dark green safety guard.
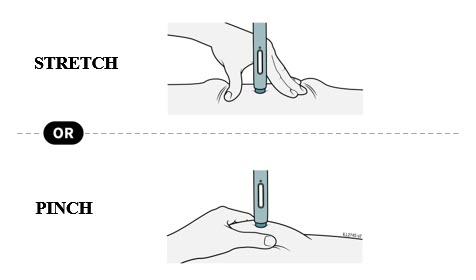 |
- 11 Stretch or pinch the skin to create a firm surface at the injection site until the injection is finished. Place the dark green safety guard straight against the skin.
- Make sure you can see the window.
- Make sure the autoinjector is positioned straight on the injection site (at a 90-degree angle).
PUSH and hold against skin 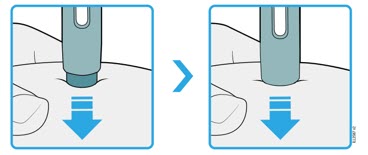 |
- 12 Firmly push the autoinjector down until the dark green safety guard stops moving. Hold the autoinjector down, do not lift.
- The dark green safety guard pushes in and unlocks the purple start button.
PRESS purple start button  |
- 13 Keep pushing the autoinjector down and press the purple start button to start the injection.
- You may hear or feel a click.
- The window starts to turn yellow.
- It is okay to let go of the purple start button.
WATCH window will turn fully yellow 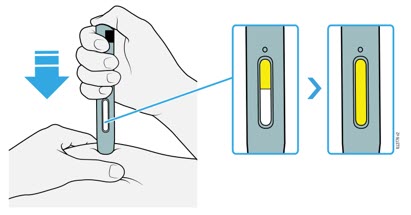 |
- 14 Keep pushing the autoinjector down. Wait for the window to turn fully yellow.
- The injection may take up to 15 seconds to complete.
- You may hear or feel a click.
- After the window turns fully yellow, lift the autoinjector away from the skin.
- The dark green safety guard locks around the needle.
Completing the Injection and Disposal
CONFIRM 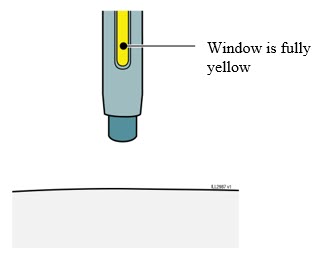 |
- 15 Confirm a full dose of medicine was injected.
- Do not touch the dark green safety guard.
- A small drop of liquid on the injection site is okay.
| Important: If the window has not turned fully yellow or if it looks like the medicine is still coming out, a full dose was not injected. Call your healthcare provider right away. |
 |
- 16 Check the injection site.
- Do not rub the injection site.
- If there is blood, press a cotton ball or gauze pad on your injection site.
- Apply an adhesive bandage if necessary.
 |
- 17 Place the used autoinjector and white cap in an FDA-cleared sharps disposal.
Important : Do not throw away the autoinjector in your household trash. - Do not reuse the autoinjector.
- Do not touch the dark green safety guard.
Additional information about your sharps disposal container
If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.
Disposing of sharps disposal containers:
When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container.
There may be state or local laws about how you should throw away used needles and syringes.
For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at:
http://www.fda.gov/safesharpsdisposal
Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this.
Do not recycle your used sharps disposal container.
Keep the autoinjector and sharps disposal container out of the sight and reach of children.
For more information or help call 1-888-4ENBREL (1-888-436-2735).
ENBREL (etanercept)
AMGEN
Manufactured by: Immunex Corporation Thousand Oaks, CA 91320-1799 U.S. License Number 1132 ©1998–2016, 2019-2025 Immunex Corporation. All rights reserved. 1XXXXXX
This Instructions for Use has been approved by the U.S. Food and Drug Administration. Revised: 6/2025 v21
Mechanism of Action
TNF is a naturally occurring cytokine that is involved in normal inflammatory and immune responses. It plays an important role in the inflammatory processes of RA, polyarticular JIA, PsA, and AS and the resulting joint pathology. In addition, TNF plays a role in the inflammatory process of PsO. Elevated levels of TNF are found in involved tissues and fluids of patients with RA, JIA, PsA, AS, and PsO.
Two distinct receptors for TNF (TNFRs), a 55 kilodalton protein (p55) and a 75 kilodalton protein (p75), exist naturally as monomeric molecules on cell surfaces and in soluble forms. Biological activity of TNF is dependent upon binding to either cell surface TNFR.
Etanercept is a dimeric soluble form of the p75 TNF receptor that can bind TNF molecules. Etanercept inhibits binding of TNF-α and TNF-β (lymphotoxin alpha [LT-α]) to cell surface TNFRs, rendering TNF biologically inactive. In in vitro studies, large complexes of etanercept with TNF-α were not detected and cells expressing transmembrane TNF (that binds Enbrel) are not lysed in the presence or absence of complement.
