Get your patient on Erythromycin And Benzoyl Peroxide - Erythromycin And Benzoyl Peroxide gel (Erythromycin And Benzoyl Peroxide)
Erythromycin And Benzoyl Peroxide - Erythromycin And Benzoyl Peroxide gel prescribing information
INDICATIONS AND USAGE
Erythromycin and Benzoyl Peroxide Topical Gel USP is indicated for the topical treatment of acne vulgaris.
DOSAGE AND ADMINISTRATION
Erythromycin and Benzoyl Peroxide Topical Gel USP should be applied twice daily, morning and evening, or as directed by a physician, to affected areas after the skin is thoroughly washed, rinsed with warm water and gently patted dry.
How Supplied and Compounding Directions
| Size (Net weight) | NDC | Benzoyl Peroxide Gel | Active Erythromycin Powder (In Plastic Vial) | 70% Ethyl Alcohol To Be Added |
| 23.3 grams (as dispensed) | 64980-328-01 | 20 grams | 0.8 grams | 3 mL |
| 46.6 grams (as dispensed) | 64980-328-02 | 40 grams | 1.6 grams | 6 mL |
Pharmacist Important - Prior to dispensing, tap vial until all powder flows freely. Add indicated amount of room temperature 70% ETHYL ALCOHOL to vial (to the mark) and immediately shake vigorously to completely dissolve erythromycin, then immediately add this solution to gel and stir until homogeneous in appearance (1 to 1-1/2 minutes). Erythromycin and Benzoyl Peroxide Topical Gel USP should then be stored under refrigeration. Do not freeze. Place a 3-month expiration date on the label.
Note: Prior to reconstitution, store at room temperature between 15˚ and 30˚C (59˚-86˚F).
After reconstitution , store under refrigeration between 2˚and 8˚C (36˚-46˚F).
Do not freeze. Keep tightly closed. Keep out of the reach of children.
Rx only
Manufactured by: Lyne Laboratories, Inc., Brockton, MA 02301 Manufactured for: Rising Pharma Holdings, Inc., East Brunswick, NJ 08816
Revised : 08/2025
PIR32846-00
CONTRAINDICATIONS
Erythromycin and Benzoyl Peroxide Topical Gel USP is contraindicated in those individuals who have shown hypersensitivity to any of its components.
ADVERSE REACTIONS
In controlled clinical trials, the incidence of adverse reactions associated with the use of Erythromycin and Benzoyl Peroxide Topical Gel USP was approximately 3%. These were dryness and urticarial reaction.
The following additional local adverse reactions have been reported occasionally: irritation of the skin including peeling, itching, burning sensation, erythema, inflammation of the face, eyes and nose, and irritation of the eyes. Skin discoloration, oiliness and tenderness of the skin have also been reported.
To report SUSPECTED ADVERSE REACTIONS, contact Rising Pharma Holdings, Inc. at 1-844-874-7464 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DESCRIPTION
Erythromycin and Benzoyl Peroxide Topical Gel USP contains erythromycin [(3R•, 4S•, 5S•, 6R•, 7R•, 9R•, 11R•, 12R•, 13S•, 14R•)-4-[(2,6-Dideoxy-3- C -methyl-3- 0 -methyl-α-L- ribo -hexopyranosyl)-oxy]-14-ethyl-7,12,13-trihydroxy-3,5,7,9,11,13-hexamethyl-6-[[3,4,6-trideoxy-3-dimethylamino)-β-D- xylo -hexopyranosyl]oxy]oxacyclotetradecane-2,10-dione]. Erythromycin is a macrolide antibiotic produced from a strain of Saccharopolyspora erythraea (formerly Streptomyces erythreus ). It is a base and readily forms salts with acids.
Chemically, erythromycin is (C 37 H 67 NO 13 ). It has the following structural formula:

Erythromycin has the molecular weight of 733.94. It is a white crystalline powder and has a solubility of approximately 1 mg/mL in water and is soluble in alcohol at 25˚C.
Erythromycin and Benzoyl Peroxide Topical Gel USP also contains benzoyl peroxide for topical use. Benzoyl peroxide is an antibacterial and keratolytic agent.
Chemically, benzoyl peroxide is (C 14 H 10 O 4 ). It has the following structural formula:
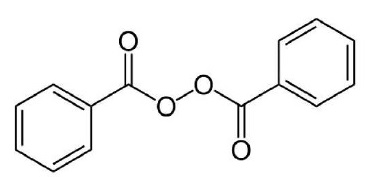
Benzoyl peroxide has the molecular weight of 242.23. It is a white granular powder and is sparingly soluble in water and alcohol and soluble in acetone, chloroform and ether.
Each gram of Erythromycin and Benzoyl Peroxide Topical Gel USP contains, as dispensed, 30 mg (3%) of erythromycin and 50 mg (5%) of benzoyl peroxide in a base of purified water USP, carbomer 940 NF, ethyl alcohol 20%, sodium hydroxide NF, docusate sodium and fragrance.
CLINICAL PHARMACOLOGY
The exact mechanism by which erythromycin reduces lesions of acne vulgaris is not fully known; however, the effect appears to be due in part to the antibacterial activity of the drug.
Benzoyl peroxide has a keratolytic and desquamative effect which may also contribute to its efficacy. Benzoyl peroxide has been shown to be absorbed by the skin where it is converted to benzoic acid.
Microbiology
Erythromycin acts by inhibition of protein synthesis in susceptible organisms by reversibly binding to 50 S ribosomal subunits, thereby inhibiting translocation of aminoacyl transfer-RNA and inhibiting polypeptide synthesis. Antagonism has been demonstrated in vitro between erythromycin, lincomycin, chloramphenicol and clindamycin.
Benzoyl peroxide is an antibacterial agent which has been shown to be effective against Propionibacterium acnes , an anaerobe found in sebaceous follicles and comedones. The antibacterial action of benzoyl peroxide is believed to be due to the release of active oxygen.
How Supplied and Compounding Directions
| Size (Net weight) | NDC | Benzoyl Peroxide Gel | Active Erythromycin Powder (In Plastic Vial) | 70% Ethyl Alcohol To Be Added |
| 23.3 grams (as dispensed) | 64980-328-01 | 20 grams | 0.8 grams | 3 mL |
| 46.6 grams (as dispensed) | 64980-328-02 | 40 grams | 1.6 grams | 6 mL |
Pharmacist Important - Prior to dispensing, tap vial until all powder flows freely. Add indicated amount of room temperature 70% ETHYL ALCOHOL to vial (to the mark) and immediately shake vigorously to completely dissolve erythromycin, then immediately add this solution to gel and stir until homogeneous in appearance (1 to 1-1/2 minutes). Erythromycin and Benzoyl Peroxide Topical Gel USP should then be stored under refrigeration. Do not freeze. Place a 3-month expiration date on the label.
Note: Prior to reconstitution, store at room temperature between 15˚ and 30˚C (59˚-86˚F).
After reconstitution , store under refrigeration between 2˚and 8˚C (36˚-46˚F).
Do not freeze. Keep tightly closed. Keep out of the reach of children.
Rx only
Manufactured by: Lyne Laboratories, Inc., Brockton, MA 02301 Manufactured for: Rising Pharma Holdings, Inc., East Brunswick, NJ 08816
Revised : 08/2025
PIR32846-00