Mounjaro prior authorization resources
Most recent state uniform prior authorization forms
Brand Resources
Mounjaro patient education
Patient toolkit
Dosage & administration
DOSAGE AND ADMINISTRATION
- The recommended starting dosage is 2.5 mg injected subcutaneously once weekly. (2.1 )
- After 4 weeks, increase to 5 mg injected subcutaneously once weekly. (2.1 )
- If additional glycemic control is needed, increase the dosage in 2.5 mg increments after at least 4 weeks on the current dose. (2.1 )
- Maximum dosage (2.1 ):
- Adults: 15 mg subcutaneously once weekly.
- Pediatric patients 10 years of age and older: 10 mg subcutaneously once weekly.
- Administer once weekly at any time of day, with or without meals. (2.2 )
- Inject subcutaneously in the abdomen, thigh, or another person should inject in the back of the upper arm. Rotate injection sites with each dose. (2.2 )
- Refer to the Full Prescribing Information for additional important administration instructions about MOUNJARO presentations. (2.2 )
Recommended Dosage
- The recommended starting dosage of MOUNJARO is 2.5 mg injected subcutaneously once weekly [see Dosage and Administration (2.2 )] . Follow the dosage escalation below to reduce the risk of gastrointestinal adverse reactions [see Warnings and Precautions (5.6 ) and Adverse Reactions (6.1 )] . The 2.5 mg dosage is for treatment initiation and is not intended for glycemic control.
- After 4 weeks, increase the dosage to 5 mg injected subcutaneously once weekly.
- If additional glycemic control is needed, increase the dosage in 2.5 mg increments after at least 4 weeks on the current dose. The maximum dosage of MOUNJARO is:
- 15 mg injected subcutaneously once weekly in adults.
- 10 mg injected subcutaneously once weekly in pediatric patients.
- If a dose is missed, instruct patients to administer MOUNJARO as soon as possible within 4 days (96 hours) after the missed dose. If more than 4 days have passed, skip the missed dose and administer the next dose on the regularly scheduled day. In each case, patients can then resume their regular once weekly dosing schedule.
- The day of weekly administration can be changed, if necessary, as long as the time between the two doses is at least 3 days (72 hours).
Important Administration Instructions
- Inform patients and their caregiver(s) which MOUNJARO presentation (e.g., vial, pre-filled single-dose pen, single-patient-use KwikPen) they will receive and ensure they receive training appropriate for that specific presentation. If the prescribed MOUNJARO presentation changes, ensure patients and caregivers receive appropriate training and instruct them to consult the Instructions for Use for the newly prescribed presentation.
- Prior to initiation, train patients and their caregiver(s) on proper injection technique for the prescribed MOUNJARO presentation [see Instructions for Use] . After training, a patient may self-inject MOUNJARO if the healthcare provider determines that it can be properly administered, except for the following:
- MOUNJARO KwikPen is not recommended for self-administration by pediatric patients.
- MOUNJARO KwikPen is not recommended for self-administration by those who are visually impaired.
- Instruct patients using MOUNJARO vials to use a syringe appropriate for dose administration (e.g., a 1 mL syringe capable of measuring a 0.5 mL or 0.6 mL dose) and always use a new syringe and needle for each injection.
- Administer MOUNJARO once weekly, any time of day, with or without meals.
- Inject MOUNJARO subcutaneously in the abdomen, thigh, or another person should inject in the back of the upper arm.
- Rotate injection sites with each dose.
- Inspect MOUNJARO visually before use. It should appear clear and colorless to slightly yellow. Do not use MOUNJARO if particulate matter or discoloration is seen.
- When using MOUNJARO with insulin, administer as separate injections and never mix. It is acceptable to inject MOUNJARO and insulin in the same body region, but the injections should not be adjacent to each other.

By using PrescriberAI, you agree to the AI Terms of Use.
Mounjaro prescribing information
WARNING: RISK OF THYROID C-CELL TUMORS
- In both male and female rats, tirzepatide causes dose-dependent and treatment-duration-dependent thyroid C-cell tumors at clinically relevant exposures. It is unknown whether MOUNJARO causes thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans as human relevance of tirzepatide-induced rodent thyroid C-cell tumors has not been determined [see Warnings and Precautions (5.1 ) and Nonclinical Toxicology (13.1 )].
- MOUNJARO is contraindicated in patients with a personal or family history of MTC or in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2) [see Contraindications (4 )] . Counsel patients regarding the potential risk for MTC with the use of MOUNJARO and inform them of symptoms of thyroid tumors (e.g., a mass in the neck, dysphagia, dyspnea, persistent hoarseness). Routine monitoring of serum calcitonin or using thyroid ultrasound is of uncertain value for early detection of MTC in patients treated with MOUNJARO [see Contraindications (4 ) and Warnings and Precautions (5.1 )].
INDICATIONS AND USAGE
MOUNJARO ® is indicated as an adjunct to diet and exercise to improve glycemic control in adults and pediatric patients 10 years of age and older with type 2 diabetes mellitus.
DOSAGE AND ADMINISTRATION
- The recommended starting dosage is 2.5 mg injected subcutaneously once weekly. (2.1 )
- After 4 weeks, increase to 5 mg injected subcutaneously once weekly. (2.1 )
- If additional glycemic control is needed, increase the dosage in 2.5 mg increments after at least 4 weeks on the current dose. (2.1 )
- Maximum dosage (2.1 ):
- Adults: 15 mg subcutaneously once weekly.
- Pediatric patients 10 years of age and older: 10 mg subcutaneously once weekly.
- Administer once weekly at any time of day, with or without meals. (2.2 )
- Inject subcutaneously in the abdomen, thigh, or another person should inject in the back of the upper arm. Rotate injection sites with each dose. (2.2 )
- Refer to the Full Prescribing Information for additional important administration instructions about MOUNJARO presentations. (2.2 )
Recommended Dosage
- The recommended starting dosage of MOUNJARO is 2.5 mg injected subcutaneously once weekly [see Dosage and Administration (2.2 )] . Follow the dosage escalation below to reduce the risk of gastrointestinal adverse reactions [see Warnings and Precautions (5.6 ) and Adverse Reactions (6.1 )] . The 2.5 mg dosage is for treatment initiation and is not intended for glycemic control.
- After 4 weeks, increase the dosage to 5 mg injected subcutaneously once weekly.
- If additional glycemic control is needed, increase the dosage in 2.5 mg increments after at least 4 weeks on the current dose. The maximum dosage of MOUNJARO is:
- 15 mg injected subcutaneously once weekly in adults.
- 10 mg injected subcutaneously once weekly in pediatric patients.
- If a dose is missed, instruct patients to administer MOUNJARO as soon as possible within 4 days (96 hours) after the missed dose. If more than 4 days have passed, skip the missed dose and administer the next dose on the regularly scheduled day. In each case, patients can then resume their regular once weekly dosing schedule.
- The day of weekly administration can be changed, if necessary, as long as the time between the two doses is at least 3 days (72 hours).
Important Administration Instructions
- Inform patients and their caregiver(s) which MOUNJARO presentation (e.g., vial, pre-filled single-dose pen, single-patient-use KwikPen) they will receive and ensure they receive training appropriate for that specific presentation. If the prescribed MOUNJARO presentation changes, ensure patients and caregivers receive appropriate training and instruct them to consult the Instructions for Use for the newly prescribed presentation.
- Prior to initiation, train patients and their caregiver(s) on proper injection technique for the prescribed MOUNJARO presentation [see Instructions for Use] . After training, a patient may self-inject MOUNJARO if the healthcare provider determines that it can be properly administered, except for the following:
- MOUNJARO KwikPen is not recommended for self-administration by pediatric patients.
- MOUNJARO KwikPen is not recommended for self-administration by those who are visually impaired.
- Instruct patients using MOUNJARO vials to use a syringe appropriate for dose administration (e.g., a 1 mL syringe capable of measuring a 0.5 mL or 0.6 mL dose) and always use a new syringe and needle for each injection.
- Administer MOUNJARO once weekly, any time of day, with or without meals.
- Inject MOUNJARO subcutaneously in the abdomen, thigh, or another person should inject in the back of the upper arm.
- Rotate injection sites with each dose.
- Inspect MOUNJARO visually before use. It should appear clear and colorless to slightly yellow. Do not use MOUNJARO if particulate matter or discoloration is seen.
- When using MOUNJARO with insulin, administer as separate injections and never mix. It is acceptable to inject MOUNJARO and insulin in the same body region, but the injections should not be adjacent to each other.
DOSAGE FORMS AND STRENGTHS
Injection: Clear, colorless to slightly yellow solution in pre-filled single-dose pens, single-dose vials, multi-dose vials, or single-patient-use KwikPens, each available in the following strengths. The multi-dose vials and single-patient-use KwikPen each contain 4 doses:
| Single-dose Pen or Vial |
| 2.5 mg/0.5 mL |
| 5 mg/0.5 mL |
| 7.5 mg/0.5 mL |
| 10 mg/0.5 mL |
| 12.5 mg/0.5 mL |
| 15 mg/0.5 mL |
| Multi-dose Vial (4 doses per vial) | ||
| Dose per Injection | Total Strength per Total Volume | Strength per mL |
| 2.5 mg/0.6 mL | 10 mg/2.4 mL | 4.17 mg/mL |
| 5 mg/0.6 mL | 20 mg/2.4 mL | 8.33 mg/mL |
| 7.5 mg/0.6 mL | 30 mg/2.4 mL | 12.5 mg/mL |
| 10 mg/0.6 mL | 40 mg/2.4 mL | 16.7 mg/mL |
| 12.5 mg/0.6 mL | 50 mg/2.4 mL | 20.8 mg/mL |
| 15 mg/0.6 mL | 60 mg/2.4 mL | 25 mg/mL |
| Single-Patient-Use KwikPen (4 doses per KwikPen) | ||
| Dose per Injection | Total Strength per Total Volume | Strength per mL |
| 2.5 mg | 10 mg/2.4 mL | 4.17 mg/mL |
| 5 mg | 20 mg/2.4 mL | 8.33 mg/mL |
| 7.5 mg | 30 mg/2.4 mL | 12.5 mg/mL |
| 10 mg | 40 mg/2.4 mL | 16.7 mg/mL |
| 12.5 mg | 50 mg/2.4 mL | 20.8 mg/mL |
| 15 mg | 60 mg/2.4 mL | 25 mg/mL |
USE IN SPECIFIC POPULATIONS
- Pregnancy: Based on animal studies, may cause fetal harm. (8.1 )
- Females of Reproductive Potential: Advise females using oral contraceptives to switch to a non-oral contraceptive method or add a barrier method of contraception for 4 weeks after initiation and for 4 weeks after each dose escalation. (8.3 )
Pregnancy
Risk Summary
Available data with MOUNJARO use in pregnant women are insufficient to evaluate for a drug-related risk of major birth defects, miscarriage, or other adverse maternal or fetal outcomes. There are risks to the mother and fetus associated with poorly controlled diabetes in pregnancy (see Clinical Considerations) . Based on animal reproduction studies, there may be risks to the fetus from exposure to tirzepatide during pregnancy. MOUNJARO should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
In pregnant rats administered tirzepatide during organogenesis, fetal growth reductions and fetal abnormalities occurred at clinical exposure in maternal rats based on AUC. In rabbits administered tirzepatide during organogenesis, fetal growth reductions were observed at clinically relevant exposures based on AUC. These adverse embryo/fetal effects in animals coincided with pharmacological effects on maternal weight and food consumption (see Data) .
The estimated background risk of major birth defects is 6–10% in women with pre-gestational diabetes with an HbA1c >7% and has been reported to be as high as 20–25% in women with an HbA1c >10%. The estimated background risk of miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2–4% and 15–20%, respectively.
Clinical Considerations
Disease-Associated Maternal and/or Embryo/Fetal Risk
Poorly controlled diabetes in pregnancy increases the maternal risk for diabetic ketoacidosis, pre-eclampsia, spontaneous abortions, preterm delivery, and delivery complications. Poorly controlled diabetes increases the fetal risk for major birth defects, stillbirth, and macrosomia-related morbidity.
Data
Animal Data
In pregnant rats given twice weekly subcutaneous doses of 0.02, 0.1, and 0.5 mg/kg tirzepatide (0.03-, 0.07-, and 0.5-fold the MRHD of 15 mg once weekly based on AUC) during organogenesis, increased incidences of external, visceral, and skeletal malformations, increased incidences of visceral and skeletal developmental variations, and decreased fetal weights coincided with pharmacologically-mediated reductions in maternal body weights and food consumption at 0.5 mg/kg. In pregnant rabbits given once weekly subcutaneous doses of 0.01, 0.03, or 0.1 mg/kg tirzepatide (0.01-, 0.06-, and 0.2-fold the MRHD) during organogenesis, pharmacologically-mediated effects on the gastrointestinal system resulting in maternal mortality or abortion in a few rabbits occurred at all dose levels. Reduced fetal weights associated with decreased maternal food consumption and body weights were observed at 0.1 mg/kg. In a pre- and post-natal study in rats administered subcutaneous doses of 0.02, 0.10, or 0.25 mg/kg tirzepatide twice weekly from implantation through lactation, F 1 pups from F 0 maternal rats given 0.25 mg/kg tirzepatide had statistically significant lower mean body weight when compared to controls from post-natal day 7 through post-natal day 126 for males and post-natal day 56 for females.
Lactation
Risk Summary
In a single-dose clinical lactation study, the concentration of tirzepatide in breast milk was found to be either undetectable or low compared to the maternal administered dose ( see Data ). There are no available data on the effects of tirzepatide on the breastfed infant or on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for MOUNJARO and any potential adverse effects on the breastfed infant from MOUNJARO or from the underlying maternal condition.
Data
Following subcutaneous administration of a single 5 mg dose to 11 healthy lactating adult females, the concentration of tirzepatide in breast milk was found to be undetectable (limit of detection in breast milk 4 ng/mL) in 164/171 samples assayed. The cumulative amount of tirzepatide detected in the remaining 7 breast milk samples over the 28-day sampling window was equivalent to less than 0.02% of the maternal administered dose, with the last measurable concentrations occurring 5 days post-dose. The AUC of tirzepatide in breast milk could not be calculated, due to insufficient quantifiable concentrations.
Females and Males of Reproductive Potential
Contraception
Use of MOUNJARO may reduce the efficacy of oral hormonal contraceptives due to delayed gastric emptying. This delay is largest after the first dose and diminishes over time. Advise patients using oral hormonal contraceptives to switch to a non-oral contraceptive method or add a barrier method of contraception for 4 weeks after initiation and for 4 weeks after each dose escalation with MOUNJARO [see Drug Interactions (7.2 ) and Clinical Pharmacology (12.2 , 12.3 )].
Pediatric Use
The safety and effectiveness of MOUNJARO as an adjunct to diet and exercise to improve glycemic control in pediatric patients 10 years of age and older with type 2 diabetes mellitus have been established. Use of MOUNJARO for this indication is supported by a 30-week, randomized, double-blind, placebo-controlled trial with a 22-week open label extension in 99 pediatric patients [see Clinical Studies (14.5 )] .
Adverse reactions reported in pediatric patients 10 years of age and older treated with MOUNJARO were similar to those reported in adults with the exception of a higher incidence of vomiting, abdominal pain, and hypoglycemia [see Adverse Reactions (6.1 )] .
The safety and effectiveness of MOUNJARO have not been established in pediatric patients less than 10 years of age.
Geriatric Use
In the pool of seven clinical trials, 1539 (30.1%) MOUNJARO-treated patients were 65 years of age or older, and 212 (4.1%) MOUNJARO-treated patients were 75 years of age or older at baseline.
No overall differences in safety or efficacy were detected between these patients and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
Renal Impairment
No dosage adjustment of MOUNJARO is recommended for patients with renal impairment. In subjects with renal impairment including end-stage renal disease (ESRD), no change in tirzepatide pharmacokinetics (PK) was observed [see Clinical Pharmacology (12.3 )] . Monitor renal function when initiating or escalating doses of MOUNJARO in patients with renal impairment reporting severe adverse gastrointestinal reactions [see Warnings and Precautions (5.5 )].
Hepatic Impairment
No dosage adjustment of MOUNJARO is recommended for patients with hepatic impairment. In a clinical pharmacology study in subjects with varying degrees of hepatic impairment, no change in tirzepatide PK was observed [see Clinical Pharmacology (12.3 )] .
CONTRAINDICATIONS
MOUNJARO is contraindicated in patients with:
- A personal or family history of medullary thyroid carcinoma (MTC) or in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2) [see Warnings and Precautions (5.1 )] .
- Known serious hypersensitivity to tirzepatide or any of the excipients in MOUNJARO. Serious hypersensitivity reactions, including anaphylaxis and angioedema, have been reported with MOUNJARO [see Warnings and Precautions (5.4 )] .
WARNINGS AND PRECAUTIONS
- Acute Pancreatitis: Has been observed in patients treated with GLP-1 receptor agonists, or MOUNJARO. Discontinue if pancreatitis is suspected. (5.2 )
- Hypoglycemia with Concomitant Use of Insulin Secretagogues or Insulin: Concomitant use with an insulin secretagogue or insulin may increase the risk of hypoglycemia, including severe hypoglycemia. Reducing dose of insulin secretagogue or insulin may be necessary. (5.3 )
- Hypersensitivity Reactions: Serious hypersensitivity reactions (e.g., anaphylaxis and angioedema) have been reported. Discontinue MOUNJARO if suspected and promptly seek medical advice. (5.4 )
- Acute Kidney Injury Due to Volume Depletion: Monitor renal function in patients reporting adverse reactions that could lead to volume depletion. (5.5 )
- Severe Gastrointestinal Adverse Reactions: Use has been associated with gastrointestinal adverse reactions, sometimes severe. MOUNJARO is not recommended in patients with severe gastroparesis. (5.6 )
- Diabetic Retinopathy Complications in Patients with a History of Diabetic Retinopathy: Has not been studied in patients with non-proliferative diabetic retinopathy requiring acute therapy, proliferative diabetic retinopathy, or diabetic macular edema. Monitor patients with a history of diabetic retinopathy for progression. (5.7 )
- Acute Gallbladder Disease: Has occurred in clinical trials. If cholelithiasis is suspected, gallbladder studies and clinical follow-up are indicated. (5.8 )
- Pulmonary Aspiration During General Anesthesia or Deep Sedation: Has been reported in patients receiving GLP-1 receptor agonists undergoing elective surgeries or procedures. Instruct patients to inform healthcare providers of any planned surgeries or procedures. (5.9 )
- Never share a MOUNJARO KwikPen between patients, even if the pen needle is changed. (5.10 )
Risk of Thyroid C-Cell Tumors
In both sexes of rats, tirzepatide caused a dose-dependent and treatment-duration-dependent increase in the incidence of thyroid C-cell tumors (adenomas and carcinomas) in a 2-year study at clinically relevant plasma exposures [see Nonclinical Toxicology (13.1 )] . It is unknown whether MOUNJARO causes thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans as human relevance of tirzepatide-induced rodent thyroid C-cell tumors has not been determined.
MOUNJARO is contraindicated in patients with a personal or family history of MTC or in patients with MEN 2. Counsel patients regarding the potential risk for MTC with the use of MOUNJARO and inform them of symptoms of thyroid tumors (e.g., a mass in the neck, dysphagia, dyspnea, persistent hoarseness).
Routine monitoring of serum calcitonin or using thyroid ultrasound is of uncertain value for early detection of MTC in patients treated with MOUNJARO. Such monitoring may increase the risk of unnecessary procedures, due to the low test specificity for serum calcitonin and a high background incidence of thyroid disease. Significantly elevated serum calcitonin values may indicate MTC and patients with MTC usually have calcitonin values >50 ng/L. If serum calcitonin is measured and found to be elevated, the patient should be further evaluated. Patients with thyroid nodules noted on physical examination or neck imaging should also be further evaluated.
Acute Pancreatitis
Acute pancreatitis, including fatal and non-fatal hemorrhagic or necrotizing pancreatitis, has been observed in patients treated with GLP-1 receptor agonists, or MOUNJARO [see Adverse Reactions (6 )] .
After initiation of MOUNJARO, observe patients carefully for signs and symptoms of acute pancreatitis, which may include persistent or severe abdominal pain (sometimes radiating to the back) and which may or may not be accompanied by nausea or vomiting. If pancreatitis is suspected, discontinue MOUNJARO and initiate appropriate management.
Hypoglycemia with Concomitant Use of Insulin Secretagogues or Insulin
Patients receiving MOUNJARO in combination with an insulin secretagogue (e.g., sulfonylurea) or insulin may have an increased risk of hypoglycemia, including severe hypoglycemia [see Adverse Reactions (6.1 ), Drug Interactions (7.1 )] .
The risk of hypoglycemia may be lowered by a reduction in the dose of sulfonylurea (or other concomitantly administered insulin secretagogue) or insulin. Inform patients using these concomitant medications of the risk of hypoglycemia and educate them on the signs and symptoms of hypoglycemia.
Hypersensitivity Reactions
Serious hypersensitivity reactions (e.g., anaphylaxis, angioedema) have been reported in patients treated with MOUNJARO. If hypersensitivity reactions occur, discontinue use of MOUNJARO; treat promptly per standard of care, and monitor until signs and symptoms resolve. Do not use in patients with a previous serious hypersensitivity reaction to tirzepatide or any of the excipients in MOUNJARO [see Contraindications (4 ), Adverse Reactions (6.2 )] .
Anaphylaxis and angioedema have been reported with GLP-1 receptor agonists. Use caution in patients with a history of angioedema or anaphylaxis with a GLP-1 receptor agonist because it is unknown whether such patients will be predisposed to these reactions with MOUNJARO.
Acute Kidney Injury Due to Volume Depletion
There have been postmarketing reports of acute kidney injury, in some cases requiring hemodialysis, in patients treated with GLP-1 receptor agonists, or MOUNJARO. The majority of the reported events occurred in patients who experienced gastrointestinal adverse reactions leading to dehydration such as nausea, vomiting, or diarrhea [see Adverse Reactions (6 )] .
Monitor renal function in patients reporting adverse reactions to MOUNJARO that could lead to volume depletion, especially during dosage initiation and escalation of MOUNJARO.
Severe Gastrointestinal Adverse Reactions
Use of MOUNJARO has been associated with gastrointestinal adverse reactions, sometimes severe [see Adverse Reactions (6 )] . In the pool of placebo-controlled trials in adults, severe gastrointestinal adverse reactions occurred more frequently among patients receiving MOUNJARO (5 mg 1.3%, 10 mg 0.4%, 15 mg 1.2%) than placebo (0.9%). Severe gastrointestinal adverse reactions have also been reported postmarketing with GLP-1 receptor agonists.
MOUNJARO is not recommended in patients with severe gastroparesis.
Diabetic Retinopathy Complications in Patients with a History of Diabetic Retinopathy
Rapid improvement in glucose control has been associated with a temporary worsening of diabetic retinopathy. MOUNJARO has not been studied in patients with non-proliferative diabetic retinopathy requiring acute therapy, proliferative diabetic retinopathy, or diabetic macular edema. Patients with a history of diabetic retinopathy should be monitored for progression of diabetic retinopathy.
Acute Gallbladder Disease
Acute events of gallbladder disease such as cholelithiasis or cholecystitis have been reported in GLP-1 receptor agonist trials and postmarketing.
In MOUNJARO placebo-controlled clinical trials in adults, acute gallbladder disease (cholelithiasis, biliary colic, and cholecystectomy) was reported by 0.6% of MOUNJARO-treated patients and 0% of placebo-treated patients. If cholelithiasis is suspected, gallbladder diagnostic studies and appropriate clinical follow-up are indicated.
Pulmonary Aspiration During General Anesthesia or Deep Sedation
MOUNJARO delays gastric emptying [see Clinical Pharmacology (12.2 )] . There have been rare postmarketing reports of pulmonary aspiration in patients receiving GLP-1 receptor agonists undergoing elective surgeries or procedures requiring general anesthesia or deep sedation who had residual gastric contents despite reported adherence to preoperative fasting recommendations.
Available data are insufficient to inform recommendations to mitigate the risk of pulmonary aspiration during general anesthesia or deep sedation in patients taking MOUNJARO, including whether modifying preoperative fasting recommendations or temporarily discontinuing MOUNJARO could reduce the incidence of retained gastric contents. Instruct patients to inform healthcare providers prior to any planned surgeries or procedures if they are taking MOUNJARO.
Never Share a MOUNJARO KwikPen Between Patients
Never share MOUNJARO KwikPen between patients, even if the pen needle is changed. Sharing poses a risk for transmission of blood-borne pathogens.
ADVERSE REACTIONS
The following serious adverse reactions are described below or elsewhere in the prescribing information:
- Risk of Thyroid C-cell Tumors [see Warnings and Precautions (5.1 )]
- Acute Pancreatitis [see Warnings and Precautions (5.2 )]
- Hypoglycemia with Concomitant Use of Insulin Secretagogues or Insulin [see Warnings and Precautions (5.3 )]
- Hypersensitivity Reactions [see Warnings and Precautions (5.4 )]
- Acute Kidney Injury Due to Volume Depletion [see Warnings and Precautions (5.5 )]
- Severe Gastrointestinal Adverse Reactions [see Warnings and Precautions (5.6 )]
- Diabetic Retinopathy Complications in Patients with a History of Diabetic Retinopathy [see Warnings and Precautions (5.7 )]
- Acute Gallbladder Disease [see Warnings and Precautions (5.8 )]
- Pulmonary Aspiration During General Anesthesia or Deep Sedation [see Warnings and Precautions (5.9 )]
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adverse Reactions in the Clinical Trials of Adults with Type 2 Diabetes Mellitus
Pool of Two Placebo-Controlled Clinical Trials in Adults
The data in Table 1 are derived from 2 placebo-controlled trials [1 monotherapy trial (SURPASS-1) and 1 trial in combination with basal insulin with or without metformin (SURPASS-5)] in adult patients with type 2 diabetes mellitus [see Clinical Studies (14.2 , 14.4 )] . These data reflect exposure of 718 patients to MOUNJARO and a mean duration of exposure to MOUNJARO of 36.6 weeks. The mean age of patients was 58 years, 4% were 75 years or older and 54% were male. The population was 57% White, 27% Asian, 13% American Indian or Alaska Native, and 3% Black or African American; 25% identified as Hispanic or Latino ethnicity. At baseline, patients had type 2 diabetes mellitus for an average of 9.1 years with a mean HbA1c of 8.1%. As assessed by baseline fundoscopic examination, 13% of the population had retinopathy. At baseline, eGFR was ≥90 mL/min/1.73 m 2 in 53%, 60 to 90 mL/min/1.73 m 2 in 39%, 45 to 60 mL/min/1.73 m 2 in 7%, and 30 to 45 mL/min/1.73 m 2 in 1% of patients.
Pool of Seven Controlled Clinical Trials
Adverse reactions were also evaluated in a larger pool of adult patients with type 2 diabetes mellitus participating in seven controlled clinical trials which included two placebo-controlled trials (SURPASS-1 and -5), three trials of MOUNJARO in combination with metformin, sulfonylureas, and/or SGLT2 Inhibitors (SURPASS-2, -3, -4) [see Clinical Studies (14.3 )] and two additional trials conducted in Japan. In this pool, a total of 5119 adult patients with type 2 diabetes mellitus were treated with MOUNJARO for a mean duration of 48.1 weeks. The mean age of patients was 58 years, 4% were 75 years or older and 58% were male. The population was 65% White, 24% Asian, 7% American Indian or Alaska Native, and 3% Black or African American; 38% identified as Hispanic or Latino ethnicity. At baseline, patients had type 2 diabetes mellitus for an average of 9.1 years with a mean HbA1c of 8.3%. As assessed by baseline fundoscopic examination, 15% of the population had retinopathy. At baseline, eGFR was ≥90 mL/min/1.73 m 2 in 52%, 60 to 90 mL/min/1.73 m 2 in 40%, 45 to 60 mL/min/1.73 m 2 in 6%, and 30 to 45 mL/min/1.73 m 2 in 1% of patients.
Common Adverse Reactions
Table 1 shows common adverse reactions, not including hypoglycemia, associated with the use of MOUNJARO in the pool of placebo-controlled trials in adults. These adverse reactions occurred more commonly on MOUNJARO than on placebo and occurred in at least 5% of patients treated with MOUNJARO.
Note: Percentages reflect the number of patients who reported at least 1 occurrence of the adverse reaction. | ||||
| Adverse Reaction | Placebo (N=235) % | MOUNJARO 5 mg (N=237) % | MOUNJARO 10 mg (N=240) % | MOUNJARO 15 mg (N=241) % |
| Nausea | 4 | 12 | 15 | 18 |
| Diarrhea | 9 | 12 | 13 | 17 |
| Decreased Appetite | 1 | 5 | 10 | 11 |
| Vomiting | 2 | 5 | 5 | 9 |
| Constipation | 1 | 6 | 6 | 7 |
| Dyspepsia | 3 | 8 | 8 | 5 |
| Abdominal Pain | 4 | 6 | 5 | 5 |
In the pool of seven clinical trials in adults, the types and frequency of common adverse reactions, not including hypoglycemia, were similar to those listed in Table 1 .
Gastrointestinal Adverse Reactions
In the pool of placebo-controlled trials in adults, gastrointestinal adverse reactions occurred more frequently among patients receiving MOUNJARO than placebo (placebo 20.4%, MOUNJARO 5 mg 37.1%, MOUNJARO 10 mg 39.6%, MOUNJARO 15 mg 43.6%). More patients receiving MOUNJARO 5 mg (3.0%), MOUNJARO 10 mg (5.4%), and MOUNJARO 15 mg (6.6%) discontinued treatment due to gastrointestinal adverse reactions than patients receiving placebo (0.4%). The majority of reports of nausea, vomiting, and/or diarrhea occurred during dose escalation and decreased over time.
The following gastrointestinal adverse reactions were reported more frequently in MOUNJARO-treated adult patients than placebo-treated patients (frequencies listed, respectively, as: placebo; 5 mg; 10 mg; 15 mg): eructation (0.4%, 3.0%, 2.5%, 3.3%), flatulence (0%, 1.3%, 2.5%, 2.9%), gastroesophageal reflux disease (0.4%, 1.7%, 2.5%, 1.7%), abdominal distension (0.4%, 0.4%, 2.9%, 0.8%).
Other Adverse Reactions in Adults
Hypoglycemia
Table 2 summarizes the incidence of hypoglycemic events in the placebo-controlled trials in adults.
Note: Percentages reflect the number of patients who reported at least 1 episode of hypoglycemia in respective categories. | ||||
• Reflects the study treatment period. Data include events occurring during 4 weeks of treatment-free safety follow up. Events after introduction of a new glucose-lowering treatment are excluded. | ||||
•• Episodes requiring the assistance of another person to actively administer carbohydrate, glucagon, or other resuscitative actions. | ||||
| Placebo % | MOUNJARO 5 mg % | MOUNJARO 10 mg % | MOUNJARO 15 mg % | |
| Monotherapy | ||||
| (40 weeks)• | N=115 | N=121 | N=119 | N=120 |
| Blood glucose <54 mg/dL | 1 | 0 | 0 | 0 |
| Severe hypoglycemia•• | 0 | 0 | 0 | 0 |
| Add-on to Basal Insulin with or without Metformin | ||||
| (40 weeks)• | N=120 | N=116 | N=119 | N=120 |
| Blood glucose <54 mg/dL | 13 | 16 | 19 | 14 |
| Severe hypoglycemia•• | 0 | 0 | 2 | 1 |
Hypoglycemia was more frequent when MOUNJARO was used in combination with a sulfonylurea [see Clinical Studies (14 )] . In an adult clinical trial up to 104 weeks of treatment, when administered with a sulfonylurea, hypoglycemia (glucose level <54 mg/dL) occurred in 13.8%, 9.9%, and 12.8%, and severe hypoglycemia occurred in 0.5%, 0%, and 0.6% of patients treated with MOUNJARO 5 mg, 10 mg, and 15 mg, respectively.
Acute Pancreatitis
In clinical studies, 14 events of acute pancreatitis were confirmed by adjudication in 13 MOUNJARO-treated adult patients (0.23 patients per 100 years of exposure) versus 3 events in 3 comparator-treated patients (0.11 patients per 100 years of exposure).
Heart Rate Increase
In the pool of placebo-controlled trials, treatment of adults with MOUNJARO resulted in a mean increase in heart rate of 2 to 4 beats per minute compared to a mean increase of 1 beat per minute in placebo-treated patients. Episodes of sinus tachycardia, associated with a concomitant increase from baseline in heart rate of ≥15 beats per minute, also were reported in 4.3%, 4.6%, 5.9% and 10% of subjects treated with placebo, MOUNJARO 5 mg, 10 mg, and 15 mg, respectively. For patients enrolled in Japan, these episodes were reported in 7% (3/43), 7.1% (3/42), 9.3% (4/43), and 23% (10/43) of patients treated with placebo, MOUNJARO 5 mg, 10 mg, and 15 mg, respectively. The clinical relevance of heart rate increases is uncertain.
Hypersensitivity Reactions
Hypersensitivity reactions have been reported with MOUNJARO in the pool of placebo-controlled trials in adults, sometimes severe (e.g., urticaria and eczema); hypersensitivity reactions were reported in 3.2% of MOUNJARO-treated patients compared to 1.7% of placebo-treated patients.
In the pool of seven clinical trials in adults , hypersensitivity reactions occurred in 106/2,570 (4.1%) of MOUNJARO-treated adult patients with anti-tirzepatide antibodies and in 73/2,455 (3.0%) of MOUNJARO-treated patients who did not develop anti-tirzepatide antibodies. In the clinical trial in pediatric patients 10 years of age and older, hypersensitivity reactions occurred in 2/50 (4%) of MOUNJARO-treated pediatric patients with anti-tirzepatide antibodies and in 0/43 (0%) of MOUNJARO-treated pediatric patients who did not develop anti-tirzepatide antibodies [see Clinical Pharmacology (12.6 )].
Injection Site Reactions
In the pool of placebo-controlled trials in adults, injection site reactions were reported in 3.2% of MOUNJARO-treated patients compared to 0.4% of placebo-treated patients.
In the pool of seven clinical trials, injection site reactions occurred in 119/2,570 (4.6%) of MOUNJARO-treated adult patients with anti-tirzepatide antibodies and in 18/2,455 (0.7%) of MOUNJARO-treated adult patients who did not develop anti-tirzepatide antibodies. In the clinical trial in pediatric patients 10 years of age and older, injection site reactions occurred in 3/50 (6%) of MOUNJARO-treated pediatric patients with anti-tirzepatide antibodies and in 0/43 (0%) of MOUNJARO-treated pediatric patients who did not develop anti-tirzepatide antibodies [see Clinical Pharmacology (12.6 )].
Acute Gallbladder Disease
In the pool of placebo-controlled clinical trials in adults, acute gallbladder disease (cholelithiasis, biliary colic and cholecystectomy) was reported by 0.6% of MOUNJARO-treated patients and 0% of placebo-treated patients.
Dysesthesia
In the pool of placebo-controlled clinical trials in adults, dysesthesia was reported by 0.4%, 0.4%, and 0.4% of patients treated with MOUNJARO 5 mg, 10 mg, and 15 mg, respectively. No events were reported by patients receiving placebo.
Dysgeusia
In the pool of placebo-controlled clinical trials in adults, dysgeusia was reported by 0.1% of MOUNJARO-treated patients and 0% of placebo-treated patients.
Laboratory Abnormalities
Amylase and Lipase Increase
In the pool of placebo-controlled adult clinical trials, treatment with MOUNJARO resulted in mean increases from baseline in serum pancreatic amylase concentrations of 33% to 38% and serum lipase concentrations of 31% to 42%. Placebo-treated patients had a mean increase from baseline in pancreatic amylase of 4% and no changes were observed in lipase. The clinical significance of elevations in lipase or amylase with MOUNJARO is unknown in the absence of other signs and symptoms of pancreatitis.
Adverse Reactions in the Clinical Trial of Pediatric Patients 10 Years of Age and Older with Type 2 Diabetes Mellitus
MOUNJARO was administered to 97 pediatric patients 10 years of age and older with type 2 diabetes mellitus for a mean duration of 39.9 weeks [see Clinical Studies (14.5 )] . The mean age was 15 years and 61% of patients were female. The population was 58% White, 11% Black or African American, 6% Asian, 20% American Indian or Alaska Native, and 5% were other races; 66% identified as Hispanic or Latino ethnicity. At baseline, pediatric patients had type 2 diabetes mellitus for an average of 2.4 years with a mean HbA1c of 8.0%.
The incidences of adverse reactions reported in pediatric patients treated with MOUNJARO 5 mg and 10 mg subcutaneously once-weekly were consistent with those described above for adult patients with type 2 diabetes mellitus with the exception of a higher incidence of vomiting, abdominal pain, and hypoglycemia. During the 30-week placebo-controlled period of the study, vomiting occurred in 3%, 16%, and 12% of patients and abdominal pain occurred in 9%, 22%, and 15% of patients treated with placebo, MOUNJARO 5 mg, and 10 mg, respectively.
No severe hypoglycemia episodes were reported during the trial. Table 3 summarizes the incidence of hypoglycemic events with blood glucose <54 mg/dL in the trial.
Note: Percentages reflect the number of patients who reported at least 1 episode of blood glucose <54 mg/dL. | |||
a Events after the introduction of a new glucose-lowering treatment are excluded. | |||
| Placebo % | MOUNJARO 5 mg % | MOUNJARO 10 mg % | |
| Add on to basal insulin with or without metformin a | N=10 | N=10 | N=11 |
| Blood glucose <54 mg/dL | 10 | 30 | 27 |
| Add on to metformin alone a | N=24 | N=22 | N=22 |
| Blood glucose <54 mg/dL | 4 | 9 | 9 |
Postmarketing Experience
The following adverse reactions have been reported during post-approval use of MOUNJARO. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Hypersensitivity : anaphylaxis, angioedema
Gastrointestinal : acute pancreatitis, hemorrhagic and necrotizing pancreatitis sometimes resulting in death, ileus, intestinal obstruction, severe constipation including fecal impaction
Pulmonary : Pulmonary aspiration has occurred in patients receiving GLP-1 receptor agonists undergoing elective surgeries or procedures requiring general anesthesia or deep sedation
Renal : acute renal failure or worsening of chronic renal failure, sometimes requiring hemodialysis
Skin and Subcutaneous Tissue : alopecia
DRUG INTERACTIONS
MOUNJARO delays gastric emptying and has the potential to impact the absorption of concomitantly administered oral medications. (7.2 )
Concomitant Use with an Insulin Secretagogue (e.g., Sulfonylurea) or with Insulin
When initiating MOUNJARO, consider reducing the dose of concomitantly administered insulin secretagogues (e.g., sulfonylureas) or insulin to reduce the risk of hypoglycemia [see Warnings and Precautions (5.3 )].
Oral Medications
MOUNJARO delays gastric emptying and thereby has the potential to impact the absorption of concomitantly administered oral medications. Caution should be exercised when oral medications are concomitantly administered with MOUNJARO.
Monitor patients on oral medications dependent on threshold concentrations for efficacy and those with a narrow therapeutic index (e.g., warfarin) when concomitantly administered with MOUNJARO.
Advise patients using oral hormonal contraceptives to switch to a non-oral contraceptive method or add a barrier method of contraception for 4 weeks after initiation and for 4 weeks after each dose escalation with MOUNJARO. Hormonal contraceptives that are not administered orally should not be affected [see Use in Specific Populations (8.3 ) and Clinical Pharmacology (12.2 , 12.3 )].
DESCRIPTION
MOUNJARO (tirzepatide) injection, for subcutaneous use, contains tirzepatide, a once weekly GIP receptor and GLP-1 receptor agonist. Tirzepatide is based on the GIP sequence and contains aminoisobutyric acid (Aib) in positions 2 and 13, a C-terminal amide, and Lys residue at position 20 that is attached to 1,20-eicosanedioic acid via a linker. The molecular weight is 4813.53 Da and the empirical formula is C 225 H 348 N 48 O 68 .
Structural formula:

MOUNJARO is a clear, colorless to slightly yellow, sterile solution for subcutaneous use. Each single-dose pen or single-dose vial contains a 0.5 mL solution of 2.5 mg, 5 mg, 7.5 mg, 10 mg, 12.5 mg, or 15 mg of tirzepatide and the following excipients: sodium chloride (4.1 mg), sodium phosphate dibasic heptahydrate (0.7 mg), and water for injection. Each multi-dose vial or single-patient-use KwikPen contains 2.4 mL of solution, which provides 4 doses of 2.5 mg, 5 mg, 7.5 mg, 10 mg, 12.5 mg, or 15 mg of tirzepatide per 0.6 mL. Each dose contains the following excipients: benzyl alcohol (5.4 mg), glycerin (4.8 mg), phenol (1.08 mg), sodium chloride (1.05 mg), sodium phosphate dibasic heptahydrate (0.8 mg), and water for injection. Hydrochloric acid solution and/or sodium hydroxide solution may have been added to adjust the pH. MOUNJARO has a pH of 6.5 to 7.5.
Each single-patient-use KwikPen contains additional volume to allow for device priming.
CLINICAL PHARMACOLOGY
Mechanism of Action
Tirzepatide is a GIP receptor and GLP-1 receptor agonist. It contains a C20 fatty diacid that enables albumin binding and prolongs the half-life. Tirzepatide selectively binds to and activates both the GIP and GLP-1 receptors, the targets for native GIP and GLP-1.
Tirzepatide enhances first- and second-phase insulin secretion, and reduces glucagon levels, both in a glucose-dependent manner.
Pharmacodynamics
Tirzepatide lowers fasting and postprandial glucose concentration, decreases food intake, and reduces body weight in patients with type 2 diabetes mellitus.
First and Second-Phase Insulin Secretion
Tirzepatide enhances the first- and second-phase insulin secretion. (Figure 1 )
Figure 1: Mean insulin concentration at 0-120 minutes during hyperglycemic clamp at baseline and Week 28

Insulin Sensitivity
Tirzepatide increases insulin sensitivity, as demonstrated in a hyperinsulinemic euglycemic clamp study after 28 weeks of treatment.
Glucagon Secretion
Tirzepatide reduces fasting and postprandial glucagon concentrations. Tirzepatide 15 mg reduced fasting glucagon concentration by 28% and glucagon AUC after a mixed meal by 43%, compared with no change for placebo after 28 weeks of treatment.
Gastric Emptying
Tirzepatide delays gastric emptying. The delay is largest after the first dose and this effect diminishes over time. Tirzepatide slows post-meal glucose absorption, reducing postprandial glucose.
Pharmacokinetics
The pharmacokinetics of tirzepatide is similar between healthy subjects and patients with type 2 diabetes mellitus. Steady-state plasma tirzepatide concentrations were achieved following 4 weeks of once weekly administration. Tirzepatide exposure increases in a dose-proportional manner.
Absorption
Following subcutaneous administration, the time to maximum plasma concentration of tirzepatide ranges from 8 to 72 hours. The mean absolute bioavailability of tirzepatide following subcutaneous administration is 80%. Similar exposure was achieved with subcutaneous administration of tirzepatide in the abdomen, thigh, or upper arm.
Distribution
The mean apparent steady-state volume of distribution of tirzepatide following subcutaneous administration in patients with type 2 diabetes mellitus is approximately 10.3 L. Tirzepatide is highly bound to plasma albumin (99%).
Elimination
The apparent population mean clearance of tirzepatide is 0.061 L/h with an elimination half-life of approximately 5 days, enabling once-weekly dosing.
Metabolism
Tirzepatide is metabolized by proteolytic cleavage of the peptide backbone, beta-oxidation of the C20 fatty diacid and amide hydrolysis.
Excretion
The primary excretion routes of tirzepatide metabolites are via urine and feces. Intact tirzepatide is not observed in urine or feces.
Specific Populations
The intrinsic factors of age, gender, race, ethnicity, or body weight do not have a clinically relevant effect on the PK of tirzepatide.
Pediatric Patients
A population pharmacokinetic analysis was conducted for tirzepatide 5 mg and 10 mg using data from 93 pediatric patients 10 years of age and older with type 2 diabetes mellitus. The tirzepatide exposure in pediatric patients was within the range observed in adult patients.
Patients with Renal Impairment
Renal impairment does not impact the pharmacokinetics of tirzepatide. The pharmacokinetics of tirzepatide after a single 5 mg dose was evaluated in patients with different degrees of renal impairment (mild, moderate, severe, ESRD) compared with subjects with normal renal function. This was also shown for patients with both type 2 diabetes mellitus and renal impairment based on data from clinical studies [see Use in Specific Populations (8.6 )] .
Patients with Hepatic Impairment
Hepatic impairment does not impact the pharmacokinetics of tirzepatide. The pharmacokinetics of tirzepatide after a single 5 mg dose was evaluated in patients with different degrees of hepatic impairment (mild, moderate, severe) compared with subjects with normal hepatic function [see Use in Specific Populations (8.7 )] .
Drug Interactions Studies
Potential for Tirzepatide to Influence the Pharmacokinetics of Other Drugs
In vitro studies have shown low potential for tirzepatide to inhibit or induce CYP enzymes, and to inhibit drug transporters.
MOUNJARO delays gastric emptying and thereby has the potential to impact the absorption of concomitantly administered oral medications [see Drug Interactions (7.2 )] .
The impact of tirzepatide on gastric emptying was greatest after a single dose of 5 mg and diminished after subsequent doses.
Following a first dose of tirzepatide 5 mg, acetaminophen maximum concentration (C max ) was reduced by 50%, and the median peak plasma concentration (t max ) occurred 1 hour later. After coadministration at week 4, there was no meaningful impact on acetaminophen C max and t max . Overall acetaminophen exposure (AUC 0-24hr ) was not influenced.
Following administration of a combined oral contraceptive (0.035 mg ethinyl estradiol and 0.25 mg norgestimate) in the presence of a single dose of tirzepatide 5 mg, mean C max of ethinyl estradiol, norgestimate, and norelgestromin was reduced by 59%,66%, and 55%, while mean AUC was reduced by 20%, 21%, and 23%, respectively. A delay in t max of 2.5 to 4.5 hours was observed.
Immunogenicity
The observed incidence of anti-drug antibodies is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of anti-drug antibodies in the trials described below with the incidence of anti-drug antibodies in other trials.
During the 40- to 104-week treatment periods with ADA sampling conducted up to 44 to 108 weeks in seven clinical trials in adults with type 2 diabetes mellitus [see Clinical Studies (14 )], 51% (2,570/5,025) of MOUNJARO-treated patients developed anti-tirzepatide antibodies. In these trials, anti-tirzepatide antibody formation in 34% and 14% of MOUNJARO-treated adult patients showed cross-reactivity to native GIP or native GLP-1, respectively.
Of the 2,570 MOUNJARO-treated patients who developed anti-tirzepatide antibodies during the treatment periods in these seven trials, 2% and 2% developed neutralizing antibodies against tirzepatide activity on the GIP or GLP-1 receptors, respectively, and 0.9% and 0.4% developed neutralizing antibodies against native GIP or GLP-1, respectively.
During the 30-week double-blind placebo-controlled period of the glycemic control trial in pediatric patients 10 years of age or older with type 2 diabetes mellitus [see Clinical Studies (14.5 )] , 30/61 (49%) of MOUNJARO-treated pediatric patients developed anti-tirzepatide antibodies. Anti-tirzepatide antibodies showed cross reactivity to native GIP or native GLP-1 in 26% and 8% of MOUNJARO-treated pediatric patients, respectively. There were no neutralizing antibodies against tirzepatide activity on the GIP or GLP-1 receptors. No pediatric patients developed neutralizing antibodies against native GIP or GLP-1.
There was no identified clinically significant effect of anti-tirzepatide antibodies on pharmacokinetics or effectiveness of MOUNJARO. More MOUNJARO-treated adult and pediatric patients who developed anti-tirzepatide antibodies experienced hypersensitivity reactions or injection site reactions than those who did not develop these antibodies [see Adverse Reactions (6.1 )].
NONCLINICAL TOXICOLOGY
Carcinogenesis, Mutagenesis, Impairment of Fertility
A 2-year carcinogenicity study was conducted with tirzepatide in male and female rats at doses of 0.15, 0.50, and 1.5 mg/kg (0.1-, 0.4-, and 1-fold the MRHD of 15 mg once weekly based on AUC) administered by subcutaneous injection twice weekly. A statistically significant increase in thyroid C-cell adenomas was observed in males (≥0.5 mg/kg) and females (≥0.15 mg/kg), and a statistically significant increase in thyroid C-cell adenomas and carcinomas combined was observed in males and females at all doses examined. In a 6-month carcinogenicity study in rasH2 transgenic mice, tirzepatide at doses of 1, 3, and 10 mg/kg administered by subcutaneous injection twice weekly was not tumorigenic.
Tirzepatide was not genotoxic in a rat bone marrow micronucleus assay.
In fertility and early embryonic development studies, male and female rats were administered twice weekly subcutaneous doses of 0.5, 1.5, or 3 mg/kg (0.3-, 1-, and 2-fold and 0.3-, 0.9-, and 2-fold, respectively, the MRHD of 15 mg once weekly based on AUC). No effects of tirzepatide were observed on sperm morphology, mating, fertility, and conception. In female rats, an increase in the number of females with prolonged diestrus and a decrease in the mean number of corpora lutea resulting in a decrease in the mean number of implantation sites and viable embryos was observed at all dose levels. These effects were considered secondary to the pharmacological effects of tirzepatide on food consumption and body weight.
CLINICAL STUDIES
Overview of Clinical Studies
The effectiveness of MOUNJARO as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus was established in five trials. In these trials, MOUNJARO was studied as monotherapy (SURPASS-1); as an add-on to metformin, sulfonylureas, and/or sodium-glucose co-transporter 2 inhibitors (SGLT2 inhibitors) (SURPASS-2, -3, and -4); and in combination with basal insulin with or without metformin (SURPASS-5). In these trials, MOUNJARO (5 mg, 10 mg, and 15 mg given subcutaneously once weekly) was compared with placebo, semaglutide 1 mg, insulin degludec, and/or insulin glargine [see Clinical Studies (14.2 , 14.3 , 14.4 )] .
In adult patients with type 2 diabetes mellitus, treatment with MOUNJARO produced a statistically significant reduction from baseline in HbA1c compared to placebo. The effectiveness of MOUNJARO was not impacted by age, gender, race, ethnicity, region, or by baseline BMI, HbA1c, diabetes duration, or renal function.
MOUNJARO 5 mg and 10 mg was studied in pediatric patients 10 years of age and older with type 2 diabetes in combination with metformin and/or basal insulin [see Clinical Studies (14.5 )] .
Monotherapy Use of MOUNJARO in Adult Patients with Type 2 Diabetes Mellitus
SURPASS-1 (NCT03954834) was a 40-week double-blind trial that randomized 478 adult patients with type 2 diabetes mellitus with inadequate glycemic control with diet and exercise to subcutaneous MOUNJARO 5 mg, MOUNJARO 10 mg, MOUNJARO 15 mg, or placebo once weekly.
Patients had a mean age of 54 years, and 52% were men. The mean duration of type 2 diabetes mellitus was 4.7 years, and the mean BMI was 32 kg/m 2 . Overall, 36% were White, 35% were Asian, 25% were American Indians/Alaska Natives, and 5% were Black or African American; 43% identified as Hispanic or Latino ethnicity.
Monotherapy with MOUNJARO 5 mg, 10 mg and 15 mg once weekly for 40 weeks resulted in a statistically significant reduction in HbA1c compared with placebo (see Table 4 ).
a The modified intent-to-treat population consists of all randomly assigned participants who were exposed to at least 1 dose of study drug. Patients who discontinued study treatment because they did not meet study enrollment criteria were excluded. During the trial, rescue medication (additional antihyperglycemic medication) was initiated by 25%, 2%, 3%, and 2% of patients randomized to placebo, MOUNJARO 5 mg, 10 mg, and 15 mg, respectively. At Week 40 the HbA1c data were missing for 12%, 6%, 7%, and 14% of patients randomized to placebo, MOUNJARO 5 mg, 10 mg, and 15 mg, respectively. Missing Week 40 data were imputed using placebo-based multiple imputation. | ||||
b Least-squares mean from ANCOVA adjusted for baseline value and other stratification factors. | ||||
c p<0.001 (two-sided) for superiority versus placebo, adjusted for multiplicity. | ||||
d Analyzed using logistic regression adjusted for baseline value and other stratification factors. | ||||
| Placebo | MOUNJARO 5 mg | MOUNJARO 10 mg | MOUNJARO 15 mg | |
| Modified Intent-to-Treat (mITT) Population (N) a | 113 | 121 | 121 | 120 |
| HbA1c (%) | ||||
| Baseline (mean) | 8.1 | 8.0 | 7.9 | 7.9 |
| Change at Week 40 b | -0.1 | -1.8 | -1.7 | -1.7 |
| Difference from placebo b (95% CI) | -- | -1.7 c (-2.0, -1.4) | -1.6 c (-1.9, -1.3) | -1.6 c (-1.9, -1.3) |
| Patients (%) achieving HbA1c <7% d | 23 | 82 c | 85 c | 78 c |
| Fasting Serum Glucose (mg/dL) | ||||
| Baseline (mean) | 155 | 154 | 153 | 154 |
| Change at Week 40 b | 4 | -40 | -40 | -39 |
| Difference from placebo b (95% CI) | -- | -43 c (-55, -32) | -43 c (-55, -32) | -42 c (-54, -30) |
| Body Weight (kg) | ||||
| Baseline (mean) | 84.5 | 87.0 | 86.2 | 85.5 |
| Change at Week 40 b | -1.0 | -6.3 | -7.0 | -7.8 |
| Difference from placebo b (95% CI) | -- | -5.3 c (-6.8, -3.9) | -6.0 c (-7.4, -4.6) | -6.8 c (-8.3, -5.4) |
MOUNJARO Use in Combination with Metformin, Sulfonylureas, and/or SGLT2 Inhibitors in Adult Patients with Type 2 Diabetes Mellitus
Add-on to metformin
SURPASS-2 (NCT03987919) was a 40-week open-label trial (double-blind with respect to MOUNJARO dose assignment) that randomized 1879 adult patients with type 2 diabetes mellitus with inadequate glycemic control on stable doses of metformin alone to the addition of subcutaneous MOUNJARO 5 mg, MOUNJARO 10 mg, or MOUNJARO 15 mg once weekly or subcutaneous semaglutide 1 mg once weekly.
Patients had a mean age of 57 years and 47% were men. The mean duration of type 2 diabetes mellitus was 8.6 years, and the mean BMI was 34 kg/m 2 . Overall, 83% were White, 4% were Black or African American, and 1% were Asian; 70% identified as Hispanic or Latino ethnicity.
Treatment with MOUNJARO 10 mg and 15 mg once weekly for 40 weeks resulted in a statistically significant reduction in HbA1c compared with semaglutide 1 mg once weekly (see Table 5 and Figure 2 ).
a The modified intent-to-treat population consists of all randomly assigned participants who were exposed to at least 1 dose of study drug. Patients who discontinued study treatment because they did not meet study enrollment criteria were excluded. During the trial, rescue medication (additional antihyperglycemic medication) was initiated by 3%, 2%, 1%, and 1% of patients randomized to semaglutide 1 mg, MOUNJARO 5 mg, 10 mg, and 15 mg, respectively. At Week 40 the HbA1c endpoint was missing for 5%, 4%, 5%, and 5% of patients randomized to semaglutide 1 mg, MOUNJARO 5 mg, 10 mg, and 15 mg, respectively. Missing Week 40 data were imputed using multiple imputation with retrieved dropout. | ||||
b Least-squares mean from ANCOVA adjusted for baseline value and other stratification factors. | ||||
c p<0.05 (two-sided) for superiority versus semaglutide, adjusted for multiplicity. | ||||
d p<0.001 (two-sided) for superiority versus semaglutide, adjusted for multiplicity. | ||||
e Analyzed using logistic regression adjusted for baseline value and other stratification factors. | ||||
f p<0.01 (two-sided) for superiority versus semaglutide, adjusted for multiplicity. | ||||
| Semaglutide 1 mg | MOUNJARO 5 mg | MOUNJARO 10 mg | MOUNJARO 15 mg | |
| Modified Intent-to-Treat (mITT) Population (N) a | 468 | 470 | 469 | 469 |
| HbA1c (%) | ||||
| Baseline (mean) | 8.3 | 8.3 | 8.3 | 8.3 |
| Change at Week 40 b | -1.9 | -2.0 | -2.2 | -2.3 |
| Difference from semaglutide b (95% CI) | -- | -0.2 c (-0.3, -0.0) | -0.4 d (-0.5, -0.3) | -0.5 d (-0.6, -0.3) |
| Patients (%) achieving HbA1c <7% e | 79 | 82 | 86 f | 86 f |
| Fasting Serum Glucose (mg/dL) | ||||
| Baseline (mean) | 171 | 174 | 174 | 172 |
| Change at Week 40 b | -49 | -55 | -59 | -60 |
| Body Weight (kg) | ||||
| Baseline (mean) | 93.7 | 92.5 | 94.8 | 93.8 |
| Change at Week 40 b | -5.7 | -7.6 | -9.3 | -11.2 |
| Difference from semaglutide b (95% CI) | -- | -1.9 c (-2.8, -1.0) | -3.6 d (-4.5, -2.7) | -5.5 d (-6.4, -4.6) |
Figure 2: Mean HbA1c (%) Over Time - Baseline to Week 40

| Number of patients | |||
| MOUNJARO 5mg | 470 | 451 | 470 |
| MOUNJARO 10mg | 469 | 445 | 469 |
| MOUNJARO 15mg | 469 | 447 | 469 |
| Semaglutide 1mg | 468 | 443 | 468 |
Note: Displayed results are from modified Intent-to-Treat Full Analysis Set. (1) Observed mean value from Week 0 to Week 40, and (2) least-squares mean ± standard error at Week 40 multiple imputation (MI).
Add-on to metformin with or without SGLT2 inhibitor
SURPASS-3 (NCT03882970) was a 52-week open-label trial that randomized 1444 adult patients with type 2 diabetes mellitus with inadequate glycemic control on stable doses of metformin with or without SGLT2 inhibitor to the addition of subcutaneous MOUNJARO 5 mg, MOUNJARO 10 mg, MOUNJARO 15 mg once weekly, or insulin degludec 100 units/mL once daily. In this trial, 32% of patients were on SGLT2 inhibitor. Insulin degludec was initiated at 10 units once daily and adjusted weekly throughout the trial using a treat-to-target algorithm based on self-measured fasting blood glucose values. At Week 52, 26% of patients randomized to insulin degludec achieved the fasting serum glucose target of <90 mg/dL, and the mean daily insulin degludec dose was 49 U (0.5 U per kilogram).
Patients had a mean age of 57 years, and 56% were men. The mean duration of type 2 diabetes mellitus was 8.4 years, and the mean baseline BMI was 34 kg/m 2 . Overall, 91% were White, 3% were Black or African American, and 5% were Asian; 29% identified as Hispanic or Latino ethnicity.
Treatment with MOUNJARO 10 mg and 15 mg once weekly for 52 weeks resulted in a statistically significant reduction in HbA1c compared with daily insulin degludec (see Table 6 ).
a The modified intent-to-treat population consists of all randomly assigned participants who were exposed to at least 1 dose of study drug. Patients who discontinued study treatment because they did not meet study enrollment criteria were excluded. During the trial, rescue medication (additional antihyperglycemic medication) was initiated by 1%,1%, 1%, and 2% of patients randomized to insulin degludec, MOUNJARO 5 mg, 10 mg, and 15 mg, respectively. At Week 52 the HbA1c endpoint was missing for 9%, 6%, 10%, and 5% of patients randomized to insulin degludec, MOUNJARO 5 mg, 10 mg, and 15 mg, respectively. Missing Week 52 data were imputed using multiple imputation with retrieved dropout. | ||||
b Least-squares mean from ANCOVA adjusted for baseline value and other stratification factors. | ||||
c p<0.001 (two-sided) for superiority versus insulin degludec, adjusted for multiplicity. | ||||
d Analyzed using logistic regression adjusted for baseline value and other stratification factors. | ||||
| Insulin Degludec | MOUNJARO 5 mg | MOUNJARO 10 mg | MOUNJARO 15 mg | |
| Modified Intent-to-Treat (mITT) a Population (N) | 359 | 358 | 360 | 358 |
| HbA1c (%) | ||||
| Baseline (mean) | 8.1 | 8.2 | 8.2 | 8.2 |
| Change at Week 52 b | -1.3 | -1.9 | -2.0 | -2.1 |
| Difference from insulin degludec b (95% CI) | -- | -0.6 c (-0.7, -0.5) | -0.8 c (-0.9, -0.6) | -0.9 c (-1.0, -0.7) |
| Patients (%) achieving HbA1c <7% d | 58 | 79 c | 82 c | 83 c |
| Fasting Serum Glucose (mg/dL) | ||||
| Baseline (mean) | 167 | 172 | 170 | 168 |
| Change at Week 52 b | -51 | -47 | -50 | -54 |
| Body Weight (kg) | ||||
| Baseline (mean) | 94.0 | 94.4 | 93.8 | 94.9 |
| Change at Week 52 b | 1.9 | -7.0 | -9.6 | -11.3 |
| Difference from insulin degludec b (95% CI) | -- | -8.9 c (-10.0, -7.8) | -11.5 c (-12.6, -10.4) | -13.2 c (-14.3, -12.1) |
Add-on to 1-3 oral anti-hyperglycemic agents (metformin, sulfonylurea, or SGLT-2 inhibitor)
SURPASS-4 (NCT03730662) was a 104-week open-label trial (52-week primary endpoint) that randomized 2002 adult patients with type 2 diabetes mellitus with increased cardiovascular risk to subcutaneous MOUNJARO 5 mg, MOUNJARO 10 mg, MOUNJARO 15 mg once weekly, or insulin glargine 100 units/mL once daily (1:1:1:3 ratio) on a background of metformin (95%) and/or sulfonylureas (54%) and/or SGLT2 inhibitors (25%).
Patients had a mean age of 64 years, and 63% were men. The mean duration of type 2 diabetes mellitus was 11.8 years, and the mean baseline BMI was 33 kg/m 2 . Overall, 82% were White, 4% were Black or African American, and 4% were Asian; 48% identified as Hispanic or Latino ethnicity. Across all treatment groups, 87% had a history of cardiovascular disease. At baseline, eGFR was ≥90 mL/min/1.73 m 2 in 43%, 60 to 90 mL/min/1.73 m 2 in 40%, 45 to 60 mL/min/1.73 m 2 in 10%, and 30 to 45 mL/min/1.73 m 2 in 6% of patients.
Insulin glargine was initiated at 10 U once daily and adjusted weekly throughout the trial using a treat-to-target algorithm based on self-measured fasting blood glucose values. At Week 52, 30% of patients randomized to insulin glargine achieved the fasting serum glucose target of <100 mg/dL, and the mean daily insulin glargine dose was 44 U (0.5 U per kilogram).
Treatment with MOUNJARO 10 mg and 15 mg once weekly for 52 weeks resulted in a statistically significant reduction in HbA1c compared with insulin glargine once daily (see Table 7 ).
a The modified intent-to-treat population consists of all randomly assigned participants who were exposed to at least 1 dose of study drug. Patients who discontinued study treatment because they did not meet study enrollment criteria were excluded. During the trial, rescue medication (additional antihyperglycemic medication) was initiated by 1%, 0%, 0%, and 1% of patients randomized to insulin glargine, MOUNJARO 5 mg, 10 mg, and 15 mg, respectively. At Week 52 the HbA1c endpoint was missing for 9%, 9%, 6%, and 4% of patients randomized to insulin glargine, MOUNJARO 5 mg, 10 mg, and 15 mg, respectively. Missing Week 52 data were imputed using multiple imputation with retrieved dropout. | ||||
b Least-squares mean from ANCOVA adjusted for baseline value and other stratification factors. | ||||
c p<0.001 (two-sided) for superiority versus insulin glargine, adjusted for multiplicity. | ||||
d Analyzed using logistic regression adjusted for baseline value and other stratification factors. | ||||
| Insulin Glargine | MOUNJARO 5 mg | MOUNJARO 10 mg | MOUNJARO 15 mg | |
| Modified Intent-to-Treat (mITT) Population (N) a | 998 | 328 | 326 | 337 |
| HbA1c (%) | ||||
| Baseline (mean) | 8.5 | 8.5 | 8.6 | 8.5 |
| Change at Week 52 b | -1.4 | -2.1 | -2.3 | -2.4 |
| Difference from insulin glargine b (95% CI) | -- | -0.7 c (-0.9, -0.6) | -0.9 c (-1.1, -0.8) | -1.0 c (-1.2, -0.9) |
| Patients (%) achieving HbA1c <7% d | 49 | 75 c | 83 c | 85 c |
| Fasting Serum Glucose (mg/dL) | ||||
| Baseline (mean) | 168 | 172 | 176 | 174 |
| Change at Week 52 b | -49 | -44 | -50 | -55 |
| Body Weight (kg) | ||||
| Baseline (mean) | 90.2 | 90.3 | 90.6 | 90.0 |
| Change at Week 52 b | 1.7 | -6.4 | -8.9 | -10.6 |
| Difference from insulin glargine b (95% CI) | -- | -8.1 c (-8.9, -7.3) | -10.6 c (-11.4, -9.8) | -12.2 c (-13.0, -11.5) |
MOUNJARO Use in Combination with Basal Insulin with or without Metformin in Adult Patients with Type 2 Diabetes Mellitus
SURPASS-5 (NCT04039503) was a 40-week double-blind trial that randomized 475 adult patients with type 2 diabetes mellitus with inadequate glycemic control on insulin glargine 100 units/mL, with or without metformin, to subcutaneous MOUNJARO 5 mg, MOUNJARO 10 mg, MOUNJARO 15 mg once weekly, or placebo. The dose of background insulin glargine was adjusted using a treat-to-target algorithm based on self-measured fasting blood glucose values, targeting <100 mg/dL.
Patients had a mean age of 61 years, and 56% were men. The mean duration of type 2 diabetes mellitus was 13.3 years, and the mean baseline BMI was 33 kg/m 2 . Overall, 80% were White, 1% were Black or African American, and 18% were Asian; 5% identified as Hispanic or Latino ethnicity.
The mean dose of insulin glargine at baseline was 34, 32, 35, and 33 units/day for patients receiving MOUNJARO 5 mg, 10 mg, 15 mg, and placebo, respectively. At randomization, the initial insulin glargine dose in patients with HbA1c ≤8.0% was reduced by 20%. At week 40, mean dose of insulin glargine was 38, 36, 29, and 59 units/day for patients receiving MOUNJARO 5 mg, 10 mg, 15 mg, and placebo, respectively.
Treatment with MOUNJARO 5 mg once weekly, 10 mg once weekly and 15 mg once weekly for 40 weeks resulted in a statistically significant reduction in HbA1c compared with placebo (see Table 8 ).
a The modified intent-to-treat population consists of all randomly assigned participants who were exposed to at least 1 dose of study drug. Patients who discontinued study treatment because they did not meet study enrollment criteria were excluded. During the trial, rescue medication (additional antihyperglycemic medication) was initiated by 4%, 1%, 0%, and 1% of patients randomized to placebo, MOUNJARO 5 mg, 10 mg, and 15 mg, respectively. At Week 40 the HbA1c endpoint was missing for 2%, 6%, 3%, and 7% of patients randomized to placebo, MOUNJARO 5 mg, 10 mg, and 15 mg, respectively. Missing Week 40 data were imputed using placebo-based multiple imputation. | ||||
b Least-squares mean from ANCOVA adjusted for baseline value and other stratification factors. | ||||
c p<0.001 (two-sided) for superiority versus placebo, adjusted for multiplicity. | ||||
d Analyzed using logistic regression adjusted for baseline value and other stratification factors. | ||||
| Placebo | MOUNJARO 5 mg | MOUNJARO 10 mg | MOUNJARO 15 mg | |
| Modified Intent-to-Treat (mITT) Population (N) a | 119 | 116 | 118 | 118 |
| HbA1c (%) | ||||
| Baseline (mean) | 8.4 | 8.3 | 8.4 | 8.2 |
| Change at Week 40 b | -0.9 | -2.1 | -2.4 | -2.3 |
| Difference from placebo b (95% CI) | -- | -1.2 c (-1.5, -1.0) | -1.5 c (-1.8, -1.3) | -1.5 c (-1.7, -1.2) |
| Patients (%) achieving HbA1c <7% d | 35 | 87 c | 90 c | 85 c |
| Fasting Serum Glucose (mg/dL) | ||||
| Baseline (mean) | 164 | 163 | 163 | 160 |
| Change at Week 40 b | -39 | -58 | -64 | -63 |
| Difference from placebo b (95% CI) | -- | -19 c (-27, -11) | -25 c (-32, -17) | -23 c (-31, -16) |
| Body Weight (kg) | ||||
| Baseline (mean) | 94.2 | 95.8 | 94.6 | 96.0 |
| Change at Week 40 b | 1.6 | -5.4 | -7.5 | -8.8 |
| Difference from placebo b (95% CI) | -- | -7.1 c (-8.7, -5.4) | -9.1 c (-10.7, -7.5) | -10.5 c (-12.1, -8.8) |
MOUNJARO Use in Combination with Metformin or Basal Insulin, or Both in Pediatric Patients 10 Years of Age and Older with Type 2 Diabetes Mellitus
SURPASS-PEDS (NCT05260021) was a 30-week double-blind, placebo-controlled trial with a 22-week open-label extension that randomized 99 pediatric patients 10 years of age and older with type 2 diabetes mellitus with inadequate glycemic control on metformin (69%), or basal insulin (8%), or both (23%) to receive subcutaneous MOUNJARO 5 mg, MOUNJARO 10 mg, or placebo once weekly as add-on therapy.
Patients had a mean age of 15 years, and 61% were female. The mean duration of type 2 diabetes mellitus was 2.4 years, mean HbA1c was 8.0%, mean weight was 97 kg, and the mean baseline BMI was 35 kg/m 2 . Overall, 58% were White, 11% were Black or African American, 6% were Asian, 20% were American Indian or Alaska Native, and 5% were other races; 66% identified as Hispanic or Latino ethnicity.
Treatment with MOUNJARO 5 mg once weekly and 10 mg once weekly for 30 weeks, both pooled and individually, resulted in a statistically significant reduction in HbA1c compared with placebo (see Table 9 ).
a The modified intent-to-treat population consists of all randomly assigned participants who were exposed to at least 1 dose of study drug. During the trial, rescue medication (additional antihyperglycemic medication) was initiated by 6%, 0%, and 0% of patients randomized to placebo, MOUNJARO 5 mg, and 10 mg, respectively. At Week 30 the HbA1c endpoint was missing for 6%, 9%, and 18% of patients randomized to placebo, MOUNJARO 5 mg, and 10 mg, respectively. Missing Week 30 data were imputed using multiple imputation with placebo wash-out or with assumption of missing at random. | ||||
b Least-squares mean from ANCOVA adjusted for baseline value and other stratification factors. | ||||
c p<0.001 (two-sided) for superiority versus placebo, adjusted for multiplicity. | ||||
d Response in (%) is calculated by combining proportion of participants achieving target in imputed datasets using Rubin's rule. | ||||
e p<0.01 (two-sided) for superiority versus placebo, adjusted for multiplicity. | ||||
f Any missing values at baseline were imputed as missing at random. | ||||
| Placebo | MOUNJARO 5 mg | MOUNJARO 10 mg | MOUNJARO 5 mg/10 mg pooled | |
| Modified Intent-to-Treat (mITT) Population (N) a | 34 | 32 | 33 | 65 |
| HbA1c (%) | ||||
| Baseline (mean) | 8.0 | 8.2 | 7.9 | 8.1 |
| Change at Week 30 b | -0.2 | -1.9 | -2.2 | -2.0 |
| Difference from placebo b (95% CI) | -- | -1.7 c (-2.4, -1.0) | -2.0 c (-2.7, -1.3) | -1.8 c (-2.4, -1.2) |
| Patients (%) with HbA1c ≤6.5% at Week 30 d | 28 | 68 c | 81 c | 75 c |
| Fasting Serum Glucose (mg/dL) | ||||
| Baseline (mean) | 156 f | 148 f | 152 f | 150 f |
| Change at Week 30 b | -5 | -35 | -51 | -43 |
| Difference from placebo b (95% CI) | -- | -30 e (-53, -8) | -46 c (-68, -24) | -38 c (-57, -19) |
| BMI (kg/m 2 ) | ||||
| Baseline (mean) | 34.7 | 33.9 | 37.7 | 35.8 |
| Percent Change at Week 30 b | -0.5 | -6.9 | -10.8 | -8.8 |
| Difference from placebo (%) b (95% CI) | -- | -6.4 c (-9.5, -3.2) | -10.3 c (-13.5, -7.1) | -8.3 c (-11.0, -5.6) |
HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
MOUNJARO is a clear, colorless to slightly yellow solution available in cartons containing 4 pre-filled single-dose pens, 1 single-dose vial, 1 multi-dose vial, or 1 Single-Patient-Use KwikPen as follows:
| Single-Dose Vial and Prefilled Pen | ||
| Strength | 4 pack Single-dose Pen NDC | 1 pack Single-dose Vial NDC |
| 2.5 mg/0.5 mL | 0002-1506-80 | 0002-1152-01 |
| 5 mg/0.5 mL | 0002-1495-80 | 0002-1243-01 |
| 7.5 mg/0.5 mL | 0002-1484-80 | 0002-2214-01 |
| 10 mg/0.5 mL | 0002-1471-80 | 0002-2340-01 |
| 12.5 mg/0.5 mL | 0002-1460-80 | 0002-2423-01 |
| 15 mg/0.5 mL | 0002-1457-80 | 0002-3002-01 |
| Multi-Dose Vial | ||
| Doses per Vial | Strength | 1-pack Multi-dose Vial NDC |
| 4 doses of 2.5 mg/0.6 mL | 10 mg/2.4 mL (4.17 mg/mL) | 0002-4052-11 |
| 4 doses of 5 mg/0.6 mL | 20 mg/2.4 mL (8.33 mg/mL) | 0002-4103-11 |
| 4 doses of 7.5 mg/0.6 mL | 30 mg/2.4 mL (12.5 mg/mL) | 0002-4210-11 |
| 4 doses of 10 mg/0.6 mL | 40 mg/2.4 mL (16.7 mg/mL) | 0002-4304-11 |
| 4 doses of 12.5 mg/0.6 mL | 50 mg/2.4 mL (20.8 mg/mL) | 0002-4523-11 |
| 4 doses of 15 mg/0.6 mL | 60 mg/2.4 mL (25 mg/mL) | 0002-4612-11 |
| Single-Patient-Use KwikPen (with four weekly doses) | ||
| Doses per KwikPen | Strength | 1-pack Single-Patient-Use KwikPen NDC |
| 4 doses of 2.5 mg | 10 mg/2.4 mL (4.17 mg/mL) | 0002-3466-11 |
| 4 doses of 5 mg | 20 mg/2.4 mL (8.33 mg/mL) | 0002-3455-11 |
| 4 doses of 7.5 mg | 30 mg/2.4 mL (12.5 mg/mL) | 0002-3444-11 |
| 4 doses of 10 mg | 40 mg/2.4 mL (16.7 mg/mL) | 0002-3433-11 |
| 4 doses of 12.5 mg | 50 mg/2.4 mL (20.8 mg/mL) | 0002-3422-11 |
| 4 doses of 15 mg | 60 mg/2.4 mL (25 mg/mL) | 0002-3411-11 |
Storage and Handling
- Do not freeze MOUNJARO. Do not use MOUNJARO if frozen.
- Protect MOUNJARO from heat and light.
- Store MOUNJARO in the original carton to protect from light.
MOUNJARO Single-dose Pen and Single-dose Vial
- Store MOUNJARO single-dose pen and single-dose vial in a refrigerator at 2°C to 8°C (36°F to 46°F).
- If needed, each single-dose pen or single-dose vial can be stored unrefrigerated at temperatures not to exceed 30°C (86°F) for up to 21 days.
MOUNJARO Multi-dose Vial or Single-Patient-Use KwikPen
Unopened vial or single-patient-use KwikPen:
- Store unopened multi-dose vial or single-patient-use KwikPen in the refrigerator at 2°C to 8°C (36°F to 46°F). The unopened multi-dose vial or single-patient-use KwikPen can be used until the expiration date on the label if kept in the refrigerator.
- If stored at room temperature [up to 30°C (86°F)], throw away unopened multi-dose vial or single-patient-use KwikPen after 30 days.
After vial or single-patient-use KwikPen has been opened:
- Store opened (in-use) multi-dose vial or single-patient-use KwikPen in the original carton in the refrigerator at 2°C to 8°C (36°F to 46°F) or at room temperature [up to 30°C (86°F)].
- Throw away opened multi-dose vial or single-patient-use KwikPen after a total of 30 days at room temperature, 30 days after first use, or after taking 4 weekly doses, even if there is medicine left in it.
Mounjaro Single-Dose Pen Instructions for Use
INSTRUCTIONS FOR USE MOUNJARO ® (mown-JAHR-OH) (tirzepatide) injection, for subcutaneous use  2.5 mg/0.5 mL single-dose pen 5 mg/0.5 mL single-dose pen 7.5 mg/0.5 mL single-dose pen 10 mg/0.5 mL single-dose pen 12.5 mg/0.5 mL single-dose pen 15 mg/0.5 mL single-dose pen use 1 time each week 2.5 mg/0.5 mL single-dose pen 5 mg/0.5 mL single-dose pen 7.5 mg/0.5 mL single-dose pen 10 mg/0.5 mL single-dose pen 12.5 mg/0.5 mL single-dose pen 15 mg/0.5 mL single-dose pen use 1 time each week |
Important information you need to know before injecting MOUNJARO
Read this Instructions for Use and the Medication Guide before using your MOUNJARO Pen and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or treatment.
Talk to your healthcare provider about how to inject MOUNJARO the right way.
- MOUNJARO is a single-dose prefilled pen.
- MOUNJARO is used 1 time each week.
- Inject under the skin (subcutaneously) only.
- You or another person can inject into your stomach (abdomen) or thigh.
- Another person can inject into the back of your upper arm.
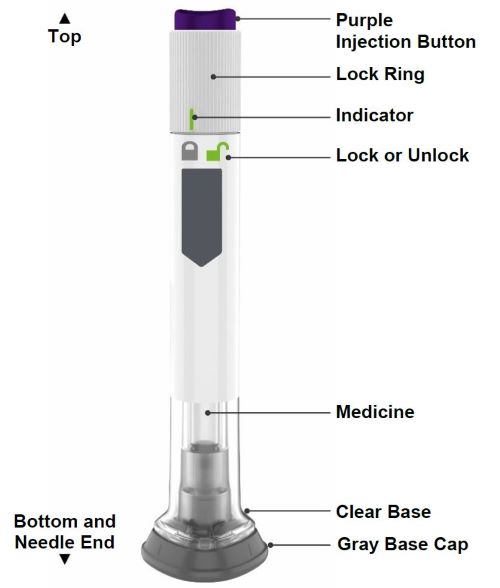
Guide to parts
Preparing to inject MOUNJARO
| Remove the Pen from the refrigerator. Leave the gray base cap on until you are ready to inject. | ||
| Check the Pen label to make sure you have the right medicine and dose, and that it has not expired. Inspect the Pen to make sure that it is not damaged. | 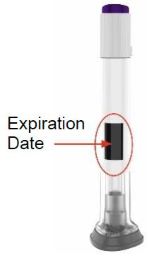 | |
| Make sure the medicine: | ||
|
| |
| Wash your hands. | ||
| Step 1 | Choose your injection site |
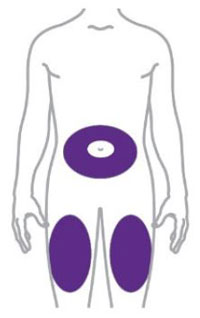 | Your healthcare provider can help you choose the injection site that is best for you. You or another person can inject the medicine in your stomach (abdomen) or thigh. |
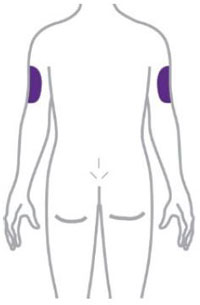 | Another person should give you the injection in the back of your upper arm. Change (rotate) your injection site each week. You may use the same area of your body but be sure to choose a different injection site in that area. |
| Step 2 | Pull off the gray base cap | |
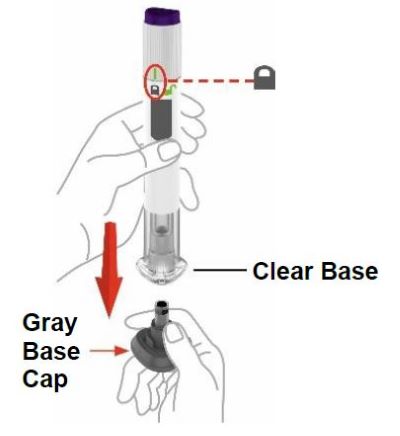 | Make sure the Pen is locked . Do not unlock the Pen until you place the clear base on your skin and are ready to inject. | |
| Pull the gray base cap straight off and throw it away in your household trash. Do not put the gray base cap back on – this could damage the needle. Do not touch the needle. | ||
| Step 3 | Place clear base on skin, then unlock | |
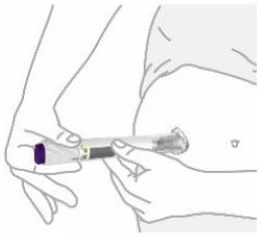 | Place the clear base flat against your skin at the injection site. | |
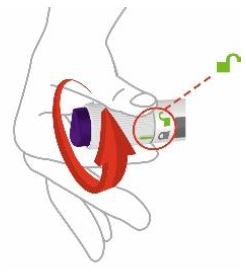 | Unlock by turning the lock ring. | |
Step 4 | Press and hold up to 10 seconds | |
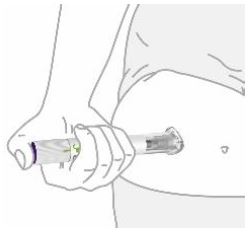 | Press and hold the purple injection button for up to 10 seconds. Listen for: • First click = injection started • Second click = injection completed | |
 | You will know your injection is complete when the gray plunger is visible. | |
| After your injection, place the used Pen in a sharps container. |
| See Disposing of your used Pen. |
Disposing of your used Pen
|
Storage and handling
- Store your Pen in the refrigerator between 36°F to 46°F (2°C to 8°C).
- You may store your Pen at room temperature up to 86°F (30°C) for up to 21 days.
- Do not freeze your Pen. If the Pen has been frozen, throw the Pen away and use a new Pen.
- Store your Pen in the original carton to protect your Pen from light.
- The Pen has glass parts. Handle it carefully. If you drop the Pen on a hard surface, do not use it. Use a new Pen for your injection.
- Keep your MOUNJARO Pen and all medicines out of the reach of children.
Commonly asked questions
What if I see air bubbles in my Pen?
Air bubbles are normal.
What if my Pen is not at room temperature?
It is not necessary to warm the Pen to room temperature.
What if I unlock the Pen and press the purple injection button before pulling off the gray base cap?
Do not remove the gray base cap. Throw away the Pen and get a new Pen.
What if there is a drop of liquid on the tip of the needle when I remove the gray base cap?
A drop of liquid on the tip of the needle is normal. Do not touch the needle.
Do I need to hold the injection button down until the injection is complete?
This is not necessary, but it may help you keep the Pen steady against your skin.
I heard more than 2 clicks during my injection—2 loud clicks and 1 soft one. Did I get my complete injection?
Some people may hear a soft click right before the second loud click. That is the normal operation of the Pen. Do not remove the Pen from your skin until you hear the second loud click.
I am not sure if my Pen worked the right way.
 | Check to see if you have received your dose. Your dose was delivered the right way if the gray plunger is visible. Also, see Step 4 of the instructions. If you do not see the gray plunger, contact Lilly at 1-800-Lilly-Rx (1-800-545-5979) for further instructions. Until then, store your Pen safely to avoid an accidental needle stick. |
What if there is a drop of liquid or blood on my skin after my injection?
This is normal. Press a cotton ball or gauze over the injection site. Do not rub the injection site.
Other information
• If you have vision problems, do not use your Pen without help from a person trained to use the MOUNJARO Pen.
Where to learn more
• If you have questions or problems with your MOUNJARO Pen, contact Lilly at 1-800-Lilly-Rx (1-800-545-5979) or call your healthcare provider.
• For more information about the MOUNJARO Pen, visit our website at www.mounjaro.com.
 | Scan this code to launch www.mounjaro.com |
Marketed by:
Lilly USA, LLC
Indianapolis, IN 46285, USA
MOUNJARO is a registered trademark of Eli Lilly and Company.
Copyright © 2022, 2024, Eli Lilly and Company. All rights reserved.
This Instructions for Use has been approved by the U.S. Food and Drug Administration. Revised: May 2024
MOU-0003-IFU-20240516

Mechanism of Action
Tirzepatide is a GIP receptor and GLP-1 receptor agonist. It contains a C20 fatty diacid that enables albumin binding and prolongs the half-life. Tirzepatide selectively binds to and activates both the GIP and GLP-1 receptors, the targets for native GIP and GLP-1.
Tirzepatide enhances first- and second-phase insulin secretion, and reduces glucagon levels, both in a glucose-dependent manner.