Get your patient on Plegridy (Peginterferon Beta-1a)
Plegridy prior authorization resources
Most recent state uniform prior authorization forms
Brand Resources
Plegridy patient education
Patient toolkit
Dosage & administration
DOSAGE AND ADMINISTRATION
- For subcutaneous or intramuscular use only (2.1 )
- Recommended dose: 125 micrograms every 14 days (2.1 )
- PLEGRIDY dose should be titrated, starting with 63 micrograms on day 1, 94 micrograms on day 15, and 125 micrograms (full dose) on day 29 (2.1 )
- A healthcare professional should train patients in the proper technique for self-administering subcutaneous injections using the prefilled pen or syringe or intramuscular injections using the prefilled syringe (2.2 )
- Analgesics and/or antipyretics on treatment days may help ameliorate flu-like symptoms (2.3 )
Dosing Information
PLEGRIDY may only be administered subcutaneously (SC) or intramuscularly (IM).
Recommended Maintenance Dosage
After initial titration (see Table 1 and Table 2 ), the recommended dosage of PLEGRIDY is 125 micrograms injected every 14 days.
For subcutaneous injection:
Patients may rotate injection sites between the abdomen, back of the upper arm, or thigh.
For intramuscular injection:
Patients may rotate injection sites between the left and right thighs.
Treatment Initiation
Dose titration at the initiation of treatment may help to ameliorate flu-like symptoms that can occur at treatment initiation with interferons. Prophylactic and concurrent use of analgesics and/or antipyretics may prevent or ameliorate flu-like symptoms sometimes experienced during treatment with PLEGRIDY.
Switching between the subcutaneous and intramuscular routes of administration and vice versa has not been studied. It is not expected that dose titration should be repeated to ameliorate flu-like symptoms if switching between subcutaneous and intramuscular routes of administration, or vice versa based upon bioequivalence demonstrated between the two routes of administration.
Subcutaneous Administration of PLEGRIDY
Patients using PLEGRIDY for the first time should start treatment with 63 micrograms on day 1. On day 15 (14 days later), the dose is increased to 94 micrograms, reaching the full dose of 125 micrograms on day 29 (after another 14 days). Patients continue with the full dose (125 micrograms) every 14 days thereafter (see Table 1 ). A PLEGRIDY Starter Pack is available containing two prefilled pens or syringes: 63 micrograms (dose 1) and 94 micrograms (dose 2).
| Dose | Time a | Amount (micrograms) | Color of Pen or Syringe Label |
|---|---|---|---|
a Dosed every 14 days | |||
| Dose 1 | On day 1 | 63 | Orange |
| Dose 2 | On day 15 | 94 | Blue |
| Dose 3 | On day 29 and every 14 days thereafter | 125 (full dose) | Grey |
Intramuscular Administration of PLEGRIDY
For patients using PLEGRIDY injected intramuscularly for the first time, PLEGRIDY should be titrated using the PLEGRIDY Titration Kit designed for use with the prefilled syringe. The PLEGRIDY Titration Kit is supplied separately and contains two titration devices to be used only with PLEGRIDY prefilled syringes for intramuscular use.
Patients should start treatment with 63 micrograms (yellow clip) on day 1. On day 15 (14 days later), the dose is increased to 94 micrograms (purple clip), reaching the full dose of 125 micrograms on day 29 (after another 14 days). Patients continue with the full dose (125 micrograms) every 14 days thereafter (see Table 2 ).
| Dose | Time a | Amount (micrograms) | Titration Clip |
|---|---|---|---|
a Dosed every 14 days | |||
| Dose 1 | On day 1 | 63 | Yellow |
| Dose 2 | On day 15 | 94 | Purple |
| Dose 3 | On day 29 and every 14 days thereafter | 125 (full dose) | No Clips Needed |
Important Administration Instructions (All Dosage Forms)
Healthcare professionals should train patients in the proper technique for self-administering subcutaneous injections using the prefilled pen or syringe or intramuscular injections using the prefilled syringe. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration. Advise patients and caregivers to rotate injection sites with each administration to minimize the likelihood of severe injection site reactions, including necrosis or localized infection [see Warnings and Precautions (5.4 ) ].
Once removed from the refrigerator, PLEGRIDY should be allowed to warm to room temperature (about 30 minutes) prior to injection. Do not use external heat sources such as hot water to warm PLEGRIDY.
Each PLEGRIDY pen and syringe for subcutaneous injection is provided with the needle pre-attached. PLEGRIDY prefilled syringe for intramuscular injection is supplied as a prefilled syringe with a separate needle. Both intramuscular and subcutaneous prefilled syringes and subcutaneously administered prefilled pens are for one-time use in one patient only and should be discarded after use.
Premedication for Flu-like Symptoms
Prophylactic and concurrent use of analgesics and/or antipyretics may prevent or ameliorate flu-like symptoms sometimes experienced during treatment with PLEGRIDY.

By using PrescriberAI, you agree to the AI Terms of Use.
Plegridy prescribing information
| Warnings and Precautions (5.8 ) | 7/2023 |
INDICATIONS AND USAGE
PLEGRIDY is indicated for the treatment of relapsing forms of multiple sclerosis (MS), to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in adults.
DOSAGE AND ADMINISTRATION
- For subcutaneous or intramuscular use only (2.1 )
- Recommended dose: 125 micrograms every 14 days (2.1 )
- PLEGRIDY dose should be titrated, starting with 63 micrograms on day 1, 94 micrograms on day 15, and 125 micrograms (full dose) on day 29 (2.1 )
- A healthcare professional should train patients in the proper technique for self-administering subcutaneous injections using the prefilled pen or syringe or intramuscular injections using the prefilled syringe (2.2 )
- Analgesics and/or antipyretics on treatment days may help ameliorate flu-like symptoms (2.3 )
Dosing Information
PLEGRIDY may only be administered subcutaneously (SC) or intramuscularly (IM).
Recommended Maintenance Dosage
After initial titration (see Table 1 and Table 2 ), the recommended dosage of PLEGRIDY is 125 micrograms injected every 14 days.
For subcutaneous injection:
Patients may rotate injection sites between the abdomen, back of the upper arm, or thigh.
For intramuscular injection:
Patients may rotate injection sites between the left and right thighs.
Treatment Initiation
Dose titration at the initiation of treatment may help to ameliorate flu-like symptoms that can occur at treatment initiation with interferons. Prophylactic and concurrent use of analgesics and/or antipyretics may prevent or ameliorate flu-like symptoms sometimes experienced during treatment with PLEGRIDY.
Switching between the subcutaneous and intramuscular routes of administration and vice versa has not been studied. It is not expected that dose titration should be repeated to ameliorate flu-like symptoms if switching between subcutaneous and intramuscular routes of administration, or vice versa based upon bioequivalence demonstrated between the two routes of administration.
Subcutaneous Administration of PLEGRIDY
Patients using PLEGRIDY for the first time should start treatment with 63 micrograms on day 1. On day 15 (14 days later), the dose is increased to 94 micrograms, reaching the full dose of 125 micrograms on day 29 (after another 14 days). Patients continue with the full dose (125 micrograms) every 14 days thereafter (see Table 1 ). A PLEGRIDY Starter Pack is available containing two prefilled pens or syringes: 63 micrograms (dose 1) and 94 micrograms (dose 2).
| Dose | Time a | Amount (micrograms) | Color of Pen or Syringe Label |
|---|---|---|---|
a Dosed every 14 days | |||
| Dose 1 | On day 1 | 63 | Orange |
| Dose 2 | On day 15 | 94 | Blue |
| Dose 3 | On day 29 and every 14 days thereafter | 125 (full dose) | Grey |
Intramuscular Administration of PLEGRIDY
For patients using PLEGRIDY injected intramuscularly for the first time, PLEGRIDY should be titrated using the PLEGRIDY Titration Kit designed for use with the prefilled syringe. The PLEGRIDY Titration Kit is supplied separately and contains two titration devices to be used only with PLEGRIDY prefilled syringes for intramuscular use.
Patients should start treatment with 63 micrograms (yellow clip) on day 1. On day 15 (14 days later), the dose is increased to 94 micrograms (purple clip), reaching the full dose of 125 micrograms on day 29 (after another 14 days). Patients continue with the full dose (125 micrograms) every 14 days thereafter (see Table 2 ).
| Dose | Time a | Amount (micrograms) | Titration Clip |
|---|---|---|---|
a Dosed every 14 days | |||
| Dose 1 | On day 1 | 63 | Yellow |
| Dose 2 | On day 15 | 94 | Purple |
| Dose 3 | On day 29 and every 14 days thereafter | 125 (full dose) | No Clips Needed |
Important Administration Instructions (All Dosage Forms)
Healthcare professionals should train patients in the proper technique for self-administering subcutaneous injections using the prefilled pen or syringe or intramuscular injections using the prefilled syringe. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration. Advise patients and caregivers to rotate injection sites with each administration to minimize the likelihood of severe injection site reactions, including necrosis or localized infection [see Warnings and Precautions (5.4 ) ].
Once removed from the refrigerator, PLEGRIDY should be allowed to warm to room temperature (about 30 minutes) prior to injection. Do not use external heat sources such as hot water to warm PLEGRIDY.
Each PLEGRIDY pen and syringe for subcutaneous injection is provided with the needle pre-attached. PLEGRIDY prefilled syringe for intramuscular injection is supplied as a prefilled syringe with a separate needle. Both intramuscular and subcutaneous prefilled syringes and subcutaneously administered prefilled pens are for one-time use in one patient only and should be discarded after use.
Premedication for Flu-like Symptoms
Prophylactic and concurrent use of analgesics and/or antipyretics may prevent or ameliorate flu-like symptoms sometimes experienced during treatment with PLEGRIDY.
DOSAGE FORMS AND STRENGTHS
PLEGRIDY is a clear to slightly opalescent and colorless to slightly yellow solution.
Subcutaneous Administration:
- Injection: 125 mcg/0.5 mL in a single-dose prefilled pen or single-dose prefilled syringe
- Injection: 63 mcg/0.5 mL in a single-dose prefilled pen or a single-dose prefilled syringe
- Injection: 94 mcg/0.5 mL in a single-dose prefilled pen or a single-dose prefilled syringe
Intramuscular Administration:
- Injection: 125 mcg/0.5 mL in a single-dose prefilled syringe
USE IN SPECIFIC POPULATIONS
Pregnancy
Risk Summary
Data from a large population-based cohort study, as well as other published studies over several decades, have not identified a drug-associated risk of major birth defects with the use of interferon beta products during early pregnancy. Findings regarding a potential risk for low birth weight or miscarriage with the use of interferon beta products in pregnancy have been inconsistent (see Data ). In a study in pregnant monkeys, administration of interferon beta during pregnancy resulted in an increased rate of abortion ( see Data ).
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively. The background risk of major birth defects and miscarriage for the indicated population is unknown.
Data
Human Data
The majority of observational studies reporting on pregnancies exposed to interferon beta products did not identify an association between the use of interferon beta products during early pregnancy and an increased risk of major birth defects.
In a population-based cohort study conducted in Finland and Sweden, data were collected from 1996--2014 in Finland and 2005--2014 in Sweden on 2,831 pregnancy outcomes from women with MS. 797 pregnancies were in women exposed to interferon beta only. No evidence was found of an increased risk of major birth defects among women with MS exposed to interferon beta products compared to women with MS that were unexposed to any non-steroid therapy for MS (n=1,647) within the study. No increased risks were observed for miscarriages and ectopic pregnancies, though there were limitations in obtaining complete data capture for these outcomes, making the interpretation of the findings more difficult.
Two small cohort studies that examined pregnancies exposed to interferon beta products (without differentiating between subtypes of interferon beta products) suggested that a decrease in mean birth weight may be associated with interferon beta exposure during pregnancy, but this finding was not confirmed in larger observational studies. Two small studies observed an increased prevalence of miscarriage, although the finding was only statistically significant in one study. Most studies enrolled patients later in pregnancy, which made it difficult to ascertain the true percentage of miscarriages. In one small cohort study, a significantly increased risk of preterm birth following interferon beta exposure during pregnancy was observed.
Animal Data
PLEGRIDY has not been tested for developmental toxicity in pregnant animals. In monkeys given interferon beta by subcutaneous injection every other day during early pregnancy, no adverse effects on embryofetal development were observed. Abortifacient activity was evident following 3 to 5 doses.
Lactation
Risk Summary
Limited published literature has described the presence of interferon beta-1a products in human milk at low levels. There are no data on the effects of interferon beta-1a on milk production. Therefore, the developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for PLEGRIDY and any potential adverse effects on the breastfed infant from PLEGRIDY or from the underlying maternal condition.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
Geriatric Use
Clinical studies of PLEGRIDY did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients.
Renal Impairment
Monitor for adverse reactions due to increased drug exposure in patients with severe renal impairment [ see Clinical Pharmacology (12.3 ) ].
CONTRAINDICATIONS
PLEGRIDY is contraindicated in patients with a history of hypersensitivity to natural or recombinant interferon beta or peginterferon, or any other component of PLEGRIDY [see Warnings and Precautions (5.3 )] .
WARNINGS AND PRECAUTIONS
- Hepatic injury: monitor liver function tests; monitor patients for signs and symptoms of hepatic injury; consider discontinuation of PLEGRIDY if hepatic injury occurs (5.1 )
- Depression and suicide: advise patients to report immediately any symptom of depression or suicidal ideation to their healthcare provider; consider discontinuation of PLEGRIDY if depression occurs (5.2 )
- Anaphylaxis and other allergic reactions: Discontinue PLEGRIDY if a serious allergic reaction occurs (5.3 )
- Injection site reactions: Do not administer PLEGRIDY into affected area until fully healed; if multiple lesions occur, change injection site or discontinue PLEGRIDY until healing of skin lesions (5.4 )
- Congestive heart failure: monitor patients with pre-existing significant cardiac disease for worsening of cardiac symptoms (5.5 )
- Decreased peripheral blood counts: monitor complete blood counts (5.6 )
- Thrombotic Microangiopathy: Cases of thrombotic microangiopathy have been reported with interferon beta products. Discontinue PLEGRIDY if clinical symptoms and laboratory findings consistent with TMA occur (5.7 )
- Pulmonary Arterial Hypertension: Cases of pulmonary arterial hypertension (PAH) have been reported in patients treated with interferon beta products, including PLEGRIDY. Discontinue PLEGRIDY if PAH is diagnosed (5.8 )
- Autoimmune disorders: consider discontinuation of PLEGRIDY if a new autoimmune disorder occurs (5.9 )
Hepatic Injury
Severe hepatic injury, including hepatitis, autoimmune hepatitis, and rare cases of severe hepatic failure, have been reported with interferon beta. Asymptomatic elevation of hepatic transaminases has also been reported, and in some patients has recurred upon rechallenge with interferon beta.
Elevations in hepatic enzymes and hepatic injury have been observed with the use of PLEGRIDY in clinical studies. The incidence of increases in hepatic transaminases was greater in patients taking PLEGRIDY than in those taking placebo. The incidence of elevations of alanine aminotransferase above 5 times the upper limit of normal was 1% in placebo-treated patients and 2% in PLEGRIDY-treated patients. The incidence of elevations of aspartate aminotransferase above 5 times the upper limit of normal was less than 1% in placebo-treated patients and less than 1% in PLEGRIDY-treated patients. Elevations of serum hepatic transaminases combined with elevated bilirubin occurred in 2 patients. Both cases resolved following discontinuation of PLEGRIDY.
Cases of noninfectious hepatitis have been reported in the postmarketing setting with use of PLEGRIDY.
Monitor patients for signs and symptoms of hepatic injury.
Depression and Suicide
Depression, suicidal ideation, and suicide occur more frequently in patients receiving interferon beta than in patients receiving placebo.
In clinical studies, the overall incidence of adverse events related to depression and suicidal ideation in multiple sclerosis patients was 8% in both the PLEGRIDY and placebo groups. The incidence of serious events related to depression and suicidal ideation was similar and less than 1% in both groups.
Advise patients to report immediately any symptom of depression or suicidal ideation to their healthcare provider. If a patient develops depression or other severe psychiatric symptoms, consider stopping treatment with PLEGRIDY.
Anaphylaxis and Other Allergic Reactions
Serious allergic reactions are rare complications of treatment with interferon beta; anaphylaxis has been reported with use of PLEGRIDY in the postmarketing setting.
Less than 1% of PLEGRIDY-treated patients experienced a serious allergic reaction such as angioedema or urticaria. Those who did have serious allergic reactions recovered promptly after treatment with antihistamines or corticosteroids. Discontinue PLEGRIDY if a serious allergic reaction occurs.
The protective rubber cover of the PLEGRIDY prefilled syringe for intramuscular administration contains natural rubber latex which may cause allergic reactions and should not be handled by latex-sensitive individuals. The safe use of PLEGRIDY prefilled syringe in latex-sensitive individuals has not been studied.
Injection Site Reactions Including Necrosis
Injection site reactions, including injection site necrosis, can occur with the use of interferon beta, including PLEGRIDY.
In clinical studies of subcutaneous PLEGRIDY, the incidence of injection site reactions (e.g., injection site erythema, pain, pruritus, or edema) was 66% in the PLEGRIDY group and 11% in the placebo group; the incidence of severe injection site reactions was 3% in the PLEGRIDY group and 0% in the placebo group. One patient out of 1468 patients who received PLEGRIDY in clinical studies experienced injection site necrosis. The injury resolved with standard medical treatment.
In Study 3, which compared single doses of intramuscular and subcutaneous PLEGRIDY [see Adverse Reactions (6.1 )] , the incidence of injection site reactions (e.g., injection site erythema, pain, pruritus, or edema) was 14% in the intramuscular PLEGRIDY group and 32% in the subcutaneous PLEGRIDY group.
Injection site abscesses and cellulitis have been reported in the postmarketing setting with use of interferon beta. Some cases required treatment with hospitalization for surgical drainage and intravenous antibiotics.
Periodically evaluate patient understanding and use of aseptic self-injection techniques and procedures, particularly if injection site necrosis has occurred.
Decisions to discontinue therapy following necrosis at a single injection site should be based on the extent of the necrosis. For patients who continue therapy with PLEGRIDY after injection site necrosis has occurred, avoid administration of PLEGRIDY near the affected area until it is fully healed. If multiple lesions occur, change injection site or discontinue PLEGRIDY until healing occurs.
Congestive Heart Failure
Congestive heart failure, cardiomyopathy, and cardiomyopathy with congestive heart failure occur in patients receiving interferon beta.
In clinical studies, the incidence of cardiovascular events was 7% in both PLEGRIDY and placebo treatment groups. No serious cardiovascular events were reported in the PLEGRIDY group.
Monitor patients with significant cardiac disease for worsening of their cardiac condition during initiation and continuation of treatment with PLEGRIDY.
Decreased Peripheral Blood Counts
Interferon beta can cause decreased peripheral blood counts in all cell lines, including rare instances of pancytopenia and severe thrombocytopenia.
In clinical studies, decreases in white blood cell counts below 3.0 x 10 9 /L occurred in 7% of patients receiving PLEGRIDY and in 1% receiving placebo. There is no apparent association between decreases in white blood cell counts and an increased risk of infections or serious infections. The incidence of clinically significant decreases in lymphocyte counts (below 0.5 x 10 9 /L), neutrophil counts (below 1.0 x 10 9 /L), and platelet counts (below 100 x 10 9 /L) were all less than 1% and similar in both placebo and PLEGRIDY groups. Two serious cases were reported in patients treated with PLEGRIDY: one patient (less than 1%) experienced severe thrombocytopenia (defined as a platelet count less than or equal to 10 x 10 9 /L), and another patient (less than 1%) experienced severe neutropenia (defined as a neutrophil count less than or equal to 0.5 x 10 9 /L). In both patients, cell counts recovered after discontinuation of PLEGRIDY. Compared to placebo, there were no significant differences in red blood cell counts in patients treated with PLEGRIDY.
Monitor patients for infections, bleeding, and symptoms of anemia. Monitor complete blood cell counts, differential white blood cell counts, and platelet counts during treatment with PLEGRIDY. Patients with myelosuppression may require more intensive monitoring of blood cell counts.
Thrombotic Microangiopathy
Cases of thrombotic microangiopathy (TMA), including thrombotic thrombocytopenic purpura and hemolytic uremic syndrome, some fatal, have been reported with interferon beta products. Cases have been reported several weeks to years after starting interferon beta products. Discontinue PLEGRIDY if clinical symptoms and laboratory findings consistent with TMA occur, and manage as clinically indicated.
Pulmonary Arterial Hypertension
Cases of pulmonary arterial hypertension (PAH) have been reported in patients treated with interferon beta products, including PLEGRIDY. PAH has occurred in patients treated with interferon beta products in the absence of other contributory factors. Many of the reported cases required hospitalization, including one case with interferon beta in which the patient underwent a lung transplant. PAH has developed at various time points after initiating therapy with interferon beta products and may occur several years after starting treatment.
Patients who develop unexplained symptoms (e.g., dyspnea, new or increasing fatigue) should be assessed for PAH. If alternative etiologies have been ruled out and a diagnosis of PAH is confirmed, discontinue treatment and manage as clinically indicated.
Autoimmune Disorders
Autoimmune disorders of multiple target organs including idiopathic thrombocytopenia, hyper- and hypothyroidism, and autoimmune hepatitis have been reported with interferon beta.
In clinical studies, the incidence of autoimmune disorders was less than 1% in both PLEGRIDY and placebo treatment groups.
If patients develop a new autoimmune disorder, consider stopping PLEGRIDY.
Seizures
Seizures are associated with the use of interferon beta.
The incidence of seizures in multiple sclerosis clinical studies was less than 1% in patients receiving PLEGRIDY and placebo.
Exercise caution when administering PLEGRIDY to patients with a seizure disorder.
ADVERSE REACTIONS
The following serious adverse reactions are discussed in more detail in other sections of labeling:
- Hepatic Injury [see Warnings and Precautions (5.1 )]
- Depression and Suicide [see Warnings and Precautions (5.2 )]
- Anaphylaxis and Other Allergic Reactions [see Warnings and Precautions (5.3 )]
- Injection Site Reactions Including Necrosis [see Warnings and Precautions (5.4 )]
- Congestive Heart Failure [see Warnings and Precautions (Section 5.5 )]
- Decreased Peripheral Blood Counts [see Warnings and Precautions (5.6 )]
- Thrombotic Microangiopathy [see Warnings and Precautions (5.7 )]
- Pulmonary Arterial Hypertension [see Warnings and Precautions (5.8 )]
- Autoimmune Disorders [see Warnings and Precautions (5.9 )]
- Seizures [see Warnings and Precautions (5.10 )]
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of PLEGRIDY cannot be directly compared to rates in clinical trials of other drugs and may not reflect the rates observed in practice.
PLEGRIDY Via Subcutaneous Administration
In clinical studies (Study 1 and Study 2), a total of 1468 patients with relapsing multiple sclerosis received PLEGRIDY by subcutaneous injection for up to 177 weeks (41 months), with an overall exposure equivalent to 1932 person-years. A total of 1093 patients received at least 1 year, and 415 patients at least 2 years of treatment with PLEGRIDY. A total of 512 and 500 patients, respectively, received PLEGRIDY 125 micrograms every 14 days or every 28 days during the placebo-controlled phase of Study 1 (year 1). The experience in year 2 of Study 1 and in the 2-year safety extension study (Study 2) was consistent with the experience in the 1-year placebo-controlled phase of Study 1.
In the placebo-controlled phase of Study 1, the most common adverse drug reactions for PLEGRIDY 125 micrograms subcutaneously every 14 days were injection site erythema, influenza-like illness, pyrexia, headache, myalgia, chills, injection site pain, asthenia, injection site pruritus, and arthralgia (all had incidence more than 10% and at least 2% more than placebo). The most commonly reported adverse event leading to discontinuation in patients treated with PLEGRIDY 125 micrograms subcutaneously every 14 days was influenza-like illness (in less than 1% of patients).
Table 3 summarizes adverse reactions reported over 48 weeks from patients treated in the placebo-controlled phase of Study 1 who received subcutaneous PLEGRIDY 125 micrograms (n=512), or placebo (n=500), every 14 days.
| PLEGRIDY (N=512) % | Placebo (N=500) % | |
|---|---|---|
| Nervous System Disorders | ||
| Headache | 44 | 33 |
| Gastrointestinal Disorders | ||
| Nausea | 9 | 6 |
| Vomiting | 5 | 2 |
| Musculoskeletal and Connective Tissue Disorders | ||
| Myalgia | 19 | 6 |
| Arthralgia | 11 | 7 |
| General Disorders and Administration Site Conditions | ||
| Injection site erythema | 62 | 7 |
| Influenza like illness | 47 | 13 |
| Pyrexia | 45 | 15 |
| Chills | 17 | 5 |
| Injection site pain | 15 | 3 |
| Asthenia | 13 | 8 |
| Injection site pruritus | 13 | 1 |
| Hyperthermia | 4 | 1 |
| Pain | 5 | 3 |
| Injection site edema | 3 | 0 |
| Injection site warmth | 3 | 0 |
| Injection site hematoma | 3 | 1 |
| Injection site rash | 2 | 0 |
| Investigations | ||
| Body temperature increased | 6 | 3 |
| Alanine aminotransferase increased | 6 | 3 |
| Aspartate aminotransferase increased | 4 | 2 |
| Gamma-glutamyl-transferase increased | 3 | 1 |
| Skin and Subcutaneous Tissue Disorder | ||
| Pruritus | 4 | 1 |
Flu-Like Symptoms
Influenza-like illness was experienced by 47% of patients receiving PLEGRIDY 125 micrograms every 14 days and 13% of patients receiving placebo. Fewer than 1% of PLEGRIDY-treated patients in Study 1 discontinued treatment due to flu-like symptoms.
Comparison Between Subcutaneous and Intramuscular Administration
An open-label, crossover study analyzed findings from 130 healthy volunteers to assess the bioequivalence of single doses of 125 micrograms of PLEGRIDY administered as a subcutaneous and intramuscular injection (Study 3).
The most commonly reported adverse reactions (with >10% incidence in either arm) across both treatment periods were chills (36% in IM vs 27% in SC), pain (22% in IM vs 14% in SC), headache (36% in IM vs 41% in SC), injection site pain (11% in IM vs 15% in SC), and injection site erythema (2% in IM vs 25% in SC). Overall, injection site reactions were reported in 14% via IM route as compared to 32% via SC route.
Immunogenicity
As with all therapeutic proteins, there is a potential for immunogenicity.
The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies in the studies described below with the incidence of antibodies in other studies or to other interferon beta-1a products may be misleading.
In Study 1, fewer than 1% of patients treated with PLEGRIDY SC every 14 days for 1 year developed neutralizing antibodies. Approximately 7% of PLEGRIDY SC-treated patients developed antibodies to the polyethylene glycol moiety.
No formal studies have been conducted with regards to immunogenicity of the intramuscular route of administration of PLEGRIDY.
Postmarketing Experience
The following adverse reactions have been identified during post-approval use of PLEGRIDY. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Anaphylactic reactions
In post marketing experience, serious hypersensitivity reactions, including cases of anaphylaxis, have been reported following PLEGRIDY administration [see Warnings and Precautions (5.3 )].
Hepatic injury
In post marketing experience, noninfectious hepatitis (including serious hepatitis) cases have been reported following PLEGRIDY administration [see Warnings and Precautions (5.1 )] .
Pulmonary Arterial Hypertension
In postmarketing experience, pulmonary arterial hypertension has been reported following PLEGRIDY administration [see Warnings and Precautions (5.8 )] .
DESCRIPTION
Peginterferon beta-1a is a covalent conjugate of recombinant interferon beta-1a (approximate molecular weight [MW] 20,000 daltons) with a single, linear methoxy poly(ethyleneglycol)-O-2-methylpropionaldehyde molecule (approximate MW 20,000 daltons). Interferon beta-1a is produced as a glycosylated protein using genetically-engineered Chinese hamster ovary cells into which the human interferon beta gene has been introduced. The amino acid sequence of recombinant interferon beta-1a is identical to that of the human interferon beta counterpart.
The molecular weight of peginterferon beta-1a is approximately 44,000 daltons, consistent with the mass of the protein, the carbohydrate moieties (approximately 2,500 daltons), and the attached poly(ethylene glycol).
Peginterferon beta-1a 125 mcg contains 125 mcg of interferon beta-1a plus 125 mcg of poly(ethylene glycol). Using the World Health Organization International Standard for interferon beta, peginterferon beta-1a has a specific antiviral activity of approximately 100 million International Units (MIU) per mg of protein as determined using an in vitro cytopathic effect assay. Peginterferon beta-1a 125 mcg contains approximately 12 MIU of antiviral activity.
Subcutaneous Administration
PLEGRIDY (peginterferon beta-1a) injection is a sterile, preservative-free solution in a single-dose prefilled pen or single-dose prefilled syringe with a 29-gauge, 0.5-inch needle for subcutaneous use. Each prefilled pen or prefilled syringe delivers 0.5 mL. Each 0.5 mL contains 63 mcg, 94 mcg, or 125 mcg of peginterferon beta-1a, and L-arginine HCl (15.8 mg), glacial acetic acid (0.25 mg), polysorbate 20 (0.025 mg), and sodium acetate trihydrate (0.79 mg) in Water for Injection, USP. The pH is approximately 4.8.
Intramuscular Administration
PLEGRIDY (peginterferon beta-1a) injection is a sterile, preservative-free solution in a single-dose prefilled syringe with a 23-gauge, 1.25-inch needle for intramuscular use. Each prefilled syringe delivers 0.5 mL. Each 0.5 mL contains 125 mcg of peginterferon beta-1a, and L-arginine HCl (15.8 mg), glacial acetic acid (0.25 mg), polysorbate 20 (0.025 mg), and sodium acetate trihydrate (0.79 mg) in Water for Injection, USP. The pH is approximately 4.8.
CLINICAL PHARMACOLOGY
Mechanism of Action
The mechanism by which PLEGRIDY exerts its effects in patients with multiple sclerosis is unknown.
Pharmacodynamics
There is no biochemical or physiologic effect known to relate directly to the clinical effect of PLEGRIDY.
Pharmacokinetics
After single-dose or multiple-dose subcutaneous administration of PLEGRIDY to healthy subjects, serum PLEGRIDY peak concentration (C max ) and total exposure over time (area under the curve, or AUC) increased in proportion to doses from 63 to 188 micrograms. PLEGRIDY did not accumulate in the serum after multiple doses of 125 micrograms every 14 days. Pharmacokinetic parameters for PLEGRIDY, including C max and AUC, did not differ significantly between healthy volunteers and multiple sclerosis patients or between single-dose and multiple-dose administrations. However, the coefficient of variation between individual patients for AUC, C max , and half-life was high (41% to 68%, 74% to 89%, and 45% to 93%, respectively).
Absorption
After 125 microgram subcutaneous doses of PLEGRIDY in multiple sclerosis patients, the maximum concentration occurred between 1 and 1.5 days, the mean C max was 280 pg/mL, and the AUC over the 14 day dosing interval was 34.8 ng.hr/mL.
Distribution
In multiple sclerosis patients taking 125 microgram subcutaneous doses of PLEGRIDY every 14 days, the estimated volume of distribution was 481 liters.
Metabolism and Elimination
Clearance mechanisms for PLEGRIDY include catabolism and excretion. The major pathway of elimination is renal. The half-life is approximately 78 hours in multiple sclerosis patients. The mean steady state clearance of PLEGRIDY is approximately 4.1 L/hr. PLEGRIDY is not extensively metabolized in the liver.
The pharmacokinetics of 125 μg single dose of PLEGRIDY administered subcutaneously and intramuscularly were similar.
Specific Populations
Body weight, gender, and age do not require dosage adjustment.
Renal impairment can increase the C max and AUC for PLEGRIDY. Results of a pharmacokinetic study in patients with mild, moderate, and severe renal impairment (creatinine clearance 50 to 80, 30 to 50, and less than 30 mL/minute, respectively) showed increases above normal for C max of 27%, 26%, and 42%, and for AUC, increases of 30%, 40%, and 53%. The half-life was 53, 49, and 82 hours in patients with mild, moderate, and severe renal impairment, respectively, compared to 54 hours in normal subjects.
In the same study, subjects with end stage renal disease requiring hemodialysis two or three times weekly had AUC and C max of PLEGRIDY values that were similar to those of normal controls. Each hemodialysis session removed approximately 24% of circulating PLEGRIDY from the systemic circulation [see Use in Specific Populations (8.6 )] .
NONCLINICAL TOXICOLOGY
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
The carcinogenic potential of PLEGRIDY has not been tested in animals.
Mutagenesis
PLEGRIDY was not mutagenic when tested in an in vitro bacterial reverse mutation (Ames) test and was not clastogenic in an in vitro assay in human lymphocytes.
Impairment of Fertility
In monkeys administered interferon beta by subcutaneous injection over the course of one menstrual cycle, menstrual irregularities, anovulation, and decreased serum progesterone levels were observed. These effects were reversible after discontinuation of drug.
CLINICAL STUDIES
The efficacy of PLEGRIDY was demonstrated in the randomized, double-blind, and placebo-controlled phase (year 1) of Study 1. The trial compared clinical and MRI outcomes at 48 weeks in patients who received PLEGRIDY 125 micrograms (n=512) or placebo (n=500) by the subcutaneous route, once every 14 days.
Study 1 enrolled patients who had a baseline Expanded Disability Status Scale (EDSS) score from 0 to 5, who had experienced at least 2 relapses within the previous three years, and had experienced at least 1 relapse in the previous year. The trial excluded patients with progressive forms of multiple sclerosis. The mean age of the study population was 37 years, the mean disease duration was 3.6 years, and the mean EDSS score at baseline was 2.46. The majority of the patients were women (71%).
The trial scheduled neurological evaluations at baseline, every 12 weeks, and at the time of a suspected relapse. Brain MRI evaluations were scheduled at baseline, week 24, and week 48.
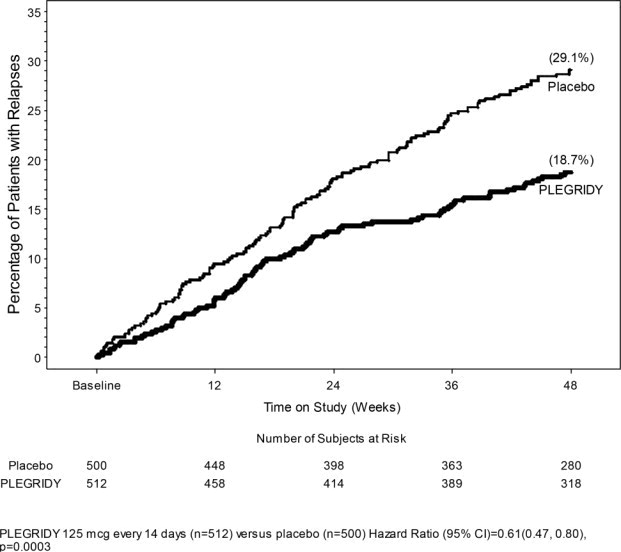
The primary outcome was the annualized relapse rate over 1 year. Secondary outcomes included the proportion of patients relapsing, number of new or newly enlarging T2 hyperintense lesions, and time to confirmed disability progression. Confirmed disability progression was defined as follows: if the baseline EDSS score was 0, a sustained 12-week increase in EDSS score of 1.5 points was required; if the baseline EDSS score was greater than 0, a sustained 12-week increase in EDSS score of 1 point was required. Table 4 and Figure 1 show the results of Study 1.
| Endpoint | PLEGRIDY 125 micrograms every 14 days | Placebo | p-value |
|---|---|---|---|
| Clinical outcomes at 48 weeks | N=512 | N=500 | |
| Annualized relapse rate | 0.26 | 0.40 | 0.0007 |
| Relative reduction | 36% | ||
| Proportion of patients with relapses | 0.19 | 0.29 | 0.0003 |
| Relative risk reduction | 39% | ||
| Proportion of patients with disability progression | 0.07 | 0.11 | 0.0383 |
| Relative risk reduction | 38% | ||
| MRI outcomes at 48 weeks | N=457 | N=476 | |
| Mean number of new or newly enlarging T2 hyperintense lesions | 3.6 | 10.9 | <0.0001 |
| Relative reduction | 67% | ||
| Mean number of Gd enhancing lesions | 0.2 | 1.4 | <0.0001 |
| Relative reduction | 86% |
Figure 1: Time to first relapse
HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
PLEGRIDY (peginterferon beta-1a) injection is a sterile, preservative-free, clear to slightly opalescent and colorless to slightly yellow solution supplied as a 0.5 mL single-dose prefilled pen or a 0.5 mL single-dose prefilled syringe.
Subcutaneous Administration
PLEGRIDY (peginterferon beta-1a) injection for subcutaneous use is supplied as a single-dose prefilled pen or single-dose prefilled syringe with a rubber stopper and a 29-gauge, 0.5-inch staked needle with a rigid needle shield in the following packaging configurations:
- Carton containing two-125 mcg/0.5 mL single-dose prefilled pens of PLEGRIDY (NDC 64406-011-01).
- Starter Pack carton containing two single-dose prefilled pens; dose 1 provides 63 mcg/0.5 mL of PLEGRIDY and dose 2 provides 94 mcg/0.5 mL of PLEGRIDY (NDC 64406-012-01).
- Carton containing two-125 mcg/0.5 mL single-dose prefilled syringes of PLEGRIDY (NDC 64406-015-01).
- Starter Pack carton containing two single-dose prefilled syringes; dose 1 provides 63 mcg/0.5 mL of PLEGRIDY, and dose 2 provides 94 mcg/0.5 mL of PLEGRIDY (NDC 64406-016-01).
Intramuscular Administration
PLEGRIDY (peginterferon beta-1a) injection for intramuscular use is supplied as a single-dose prefilled syringe with a rubber stopper and a 23-gauge, 1.25-inch staked needle provided separately with the syringe in the following packaging configurations:
- Carton containing two-125 mcg/0.5 mL single-dose prefilled syringes of PLEGRIDY (NDC 64406-017-01).
- The PLEGRIDY Titration Kit must be prescribed and dispensed separately for treatment initiation. The Titration Kit contains two titration clips: The yellow clip (for dose 1) allows a delivered dose of 63 mcg of PLEGRIDY, and the purple clip (for dose 2) allows a delivered dose of 94 mcg of PLEGRIDY.
Storage and Handling
Store PLEGRIDY prefilled pens and prefilled syringes in a refrigerator between 2°C to 8°C (36°F to 46°F) in the closed original carton to protect from light until ready for injection. Do not freeze. Discard if frozen.
If refrigeration is unavailable, PLEGRIDY may be stored at room temperature up to 25°C (77°F) for a period up to 30 days, protected from light. PLEGRIDY can be removed from, and returned to, a refrigerator if necessary. The total combined time out of refrigeration should not exceed 30 days.
PLEGRIDY prefilled syringe for intramuscular administration contains natural rubber latex which may cause allergic reactions.
Dispose in a sharps-bin container or other hard plastic or metal sealable container. Always follow local regulations for disposal.
Instructions for Use
PLEGRIDY ® (PLEGG-rih-dee)
(peginterferon beta-1a) injection, for Subcutaneous Use
Single-Dose Prefilled Pen
Starter Pack
Important: Do not remove the Plegridy Pen cap until you are ready to inject.
How to Inject your Plegridy Pen
Read this Instructions for Use before you start using Plegridy and each time you get a refill of your prescription. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
Note:
- Before you use your Plegridy Pen for the first time, your healthcare provider should show you or your caregiver how to prepare and inject your Plegridy Pen the right way.
- If you experience difficulty or have questions, call 1-800-456-2255.
- Plegridy Pen is for use under the skin only (subcutaneous).
- Each Plegridy Pen can be used 1 time only. Do not share your Plegridy Pen with anyone else. By sharing the Pen, you may give an infection to them or get an infection from them.
- Take your Plegridy Pen out of the refrigerator and let it come to room temperature for at least 30 minutes before your injection.
- Do not use more than 1 Plegridy Pen every 14 days.
- Do not use your Plegridy Pen if it has been dropped or is visibly damaged.
How should I store Plegridy?
- Store Plegridy in the refrigerator between 36°F to 46°F (2°C to 8°C).
- If a refrigerator is not available, PLEGRIDY may be stored at room temperature up to 77°F (25°C) for up to 30 days in total.
- Keep Plegridy in the original carton to protect it from light.
- Do not freeze Plegridy.
- Keep Plegridy Pens, needles, and all medicines out of the reach of children.
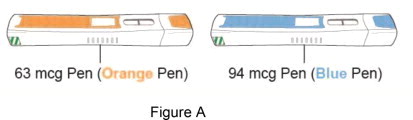
Supplies needed for your Starter Pack for Plegridy Pen injection (See Figure A ):
- 1 Starter Pack for Plegridy Pen which contains:
- 1 Plegridy 63 mcg Pen (orange pen)
- 1 Plegridy 94 mcg Pen (blue pen)
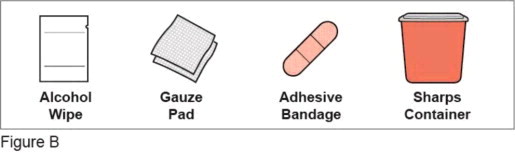
Additional supplies which are not included in the pack (See Figure B ):
- alcohol wipe
- gauze pad
- adhesive bandage
- 1 sharps container for throwing away used needles and Plegridy Pens. See “ Disposing of your used Plegridy Pens ” at the end of these instructions.
- a well-lit area and a clean, flat surface to work on, like a table
Dose Schedule
- Choose the right Plegridy Pen for your dose. The Starter Pack for Plegridy Pen contains your first 2 injections to slowly adjust your dose.
| When | Which Dose | Choose |
| Day 1 (63 mcg) | First Dose: 63 mcg | Orange Pen (See Figure C )  |
| When | Which Dose | Choose |
| Day 15 (94 mcg) | Second Dose: 94 mcg | Blue Pen (See Figure D )  |
- Make sure you use the 63 mcg orange pen for your first dose (on day 1).
- Make sure you use the 94 mcg blue pen for your second dose (on day 15).
Ask your healthcare provider which Plegridy Pen you should use for your correct dose if you are not sure.
Before Use – Parts of your Plegridy Pen before use (See Figure E ):
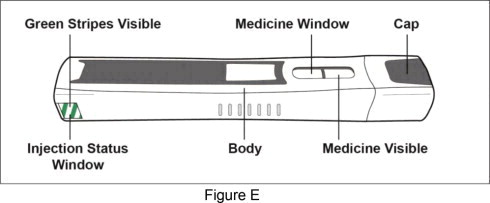
Important: Do not remove the Plegridy Pen cap until you are ready to inject. If you remove the cap before you are ready to inject, do not re-cap the pen. Re-capping could cause the pen to lock.
Preparing for your injection:
Step 1: Take your Plegridy Pen out of the refrigerator and let it come to room temperature for at least 30 minutes.
- Do not use external heat sources such as hot water to warm your Plegridy Pen.
Step 2: Collect your supplies and wash your hands
- Find a well-lit area and a clean, flat surface like a table and collect all the supplies you will need to give yourself or to receive an injection.
- Wash your hands with soap and water.
Step 3: Check your Plegridy Pen (see Figure F )
| 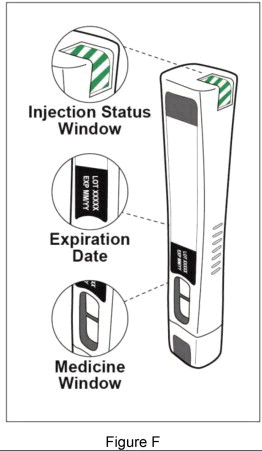 |
| |
|
| Step 4: Choose and clean your injection site | |||
| 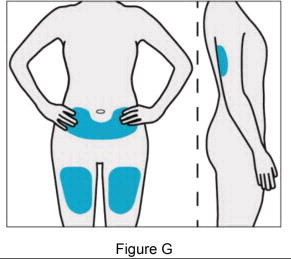 | ||
| Giving your injection: Step 5: Remove the Plegridy Pen cap | |||
| Figure H | ||
| Step 6: Give your injection | |||
| 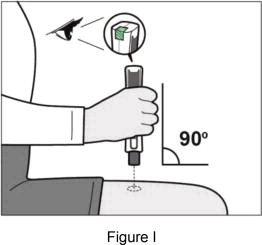 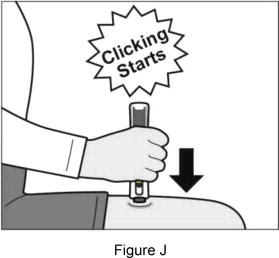 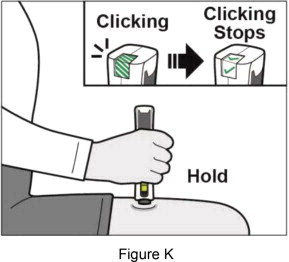 | ||
| If you do not hear clicking sounds, or you do not see green checkmarks in the injection status window after trying to give your injection, your Plegridy Pen may have locked and you should call 1-800-456-2255 for help. Step 7: Remove your Plegridy Pen from your injection site | |||
| 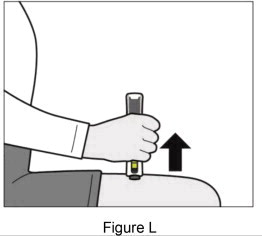 | ||
| Step 8: Check to make sure you have received your full dose of Plegridy (see Figure M ) | |||
| 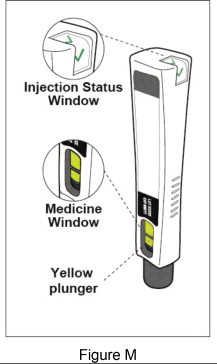 | ||
After your injection:
After Use – Parts of your Plegridy Pen (Figure N )
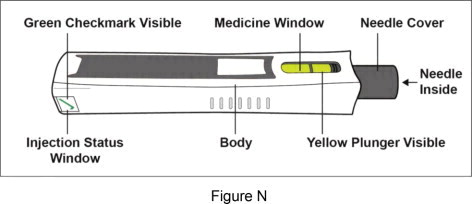
Important: Do not touch the needle cover in any way. You could get a needle stick injury.
Note: After the pen has been removed from the injection site, the needle cover will lock to protect against needle stick injury. Do not recap your Plegridy Pen.
Step 9: Disposing of your used Plegridy Pens
- Put your used Plegridy Pens in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) Plegridy Pens in your household trash.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak resistant, and
- properly labeled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and pens. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal.
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
- Do not recap your Plegridy Pen.
Step 10: Check your injection site
- Plegridy may commonly cause redness, pain or swelling of your skin at the injection site.
- After 2 hours check your injection site for redness, swelling or tenderness.
- Call your healthcare provider right away if your injection site becomes swollen and painful or the area looks infected and does not heal within a few days.
Questions?
For product or service-related questions, call 1-800-456-2255 or go to www.plegridy.com .
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured by: Biogen Inc., Cambridge, MA 02142, U.S. License # 1697
©2013-2022 Biogen. All rights reserved. 1-800-456-2255
Issued: 03/2020
43839-03
Mechanism of Action
The mechanism by which PLEGRIDY exerts its effects in patients with multiple sclerosis is unknown.