Get your patient on Ponvory (Ponesimod)
Ponvory prior authorization resources
Most recent state uniform prior authorization forms
Ponvory patient education
Patient toolkit
Dosage & administration
DOSAGE AND ADMINISTRATION
- Assessments are required prior to initiating PONVORY (2.1 )
- Titration is required for treatment initiation (2.2 )
- The recommended maintenance dosage is 20 mg taken orally once daily (2.2 )
- First-dose monitoring is recommended for patients with sinus bradycardia, first- or second-degree [Mobitz type I] atrioventricular (AV) block, or a history of myocardial infarction or heart failure (2.3 )
Assessments Prior to First Dose of PONVORY
Before initiation of treatment with PONVORY, assess the following:
Complete Blood Count
Obtain a recent (i.e., within the last 6 months or after discontinuation of prior MS therapy) complete blood count (CBC), including lymphocyte count [see Warnings and Precautions (5.1) ] .
Cardiac Evaluation
Obtain an electrocardiogram (ECG) to determine whether preexisting conduction abnormalities are present. In patients with certain preexisting conditions, advice from a cardiologist should be sought and first-dose monitoring is recommended [see Dosage and Administration (2.3) and Warnings and Precautions (5.2) ] .
Determine whether patients are taking drugs that could slow heart rate or atrioventricular (AV) conduction [see Warnings and Precautions (5.2) and Drug Interactions (7.2 , 7.3) ] .
Liver Function Tests
Obtain recent (i.e., within the last 6 months) transaminase and bilirubin levels [see Warnings and Precautions (5.4) ] .
Ophthalmic Evaluation
Obtain an evaluation of the fundus, including the macula [see Warnings and Precautions (5.8) ] .
Current or Prior Medications with Immune System Effects
If patients are taking anti-neoplastic, immunosuppressive, or immune-modulating therapies, or if there is a history of prior use of these drugs, consider possible unintended additive immunosuppressive effects before initiating treatment with PONVORY [see Warnings and Precautions (5.1 , 5.10) and Drug Interactions (7.1) ] .
Vaccinations
Test patients for antibodies to varicella zoster virus (VZV) before initiating PONVORY; VZV vaccination of antibody-negative patients is recommended prior to commencing treatment with PONVORY [see Warnings and Precautions (5.1) ] . If live attenuated vaccine immunizations are required, administer at least 1 month prior to initiation of PONVORY.
Recommended Dosage
Maintenance Dosage
After dose titration is complete (see Treatment Initiation ) , the recommended maintenance dosage of PONVORY is 20 mg taken orally once daily starting on Day 15.
Administer PONVORY orally once daily. Swallow the tablet whole. PONVORY can be taken with or without food.
Treatment Initiation
A starter pack must be used for patients initiating treatment with PONVORY [see How Supplied/Storage and Handling (16.1) ] . Initiate PONVORY treatment with a 14-day titration; start with one 2 mg tablet orally once daily and progress with the titration schedule as shown in Table 1 [see Warnings and Precautions (5.2) ] .
| Titration Day | Daily Dose |
|---|---|
| Days 1 and 2 | 2 mg |
| Days 3 and 4 | 3 mg |
| Days 5 and 6 | 4 mg |
| Day 7 | 5 mg |
| Day 8 | 6 mg |
| Day 9 | 7 mg |
| Day 10 | 8 mg |
| Day 11 | 9 mg |
| Days 12, 13, and 14 | 10 mg |
| Maintenance | Daily Dose |
|---|---|
| Day 15 and thereafter | 20 mg |
If dose titration is interrupted, missed dose instructions must be followed [see Dosage and Administration (2.4) ] .
First Dose Monitoring in Patients with Certain Preexisting Cardiac Conditions
Because initiation of PONVORY treatment results in a decrease in heart rate (HR), first-dose 4-hour monitoring is recommended for patients with sinus bradycardia [HR less than 55 beats per minute (bpm)], first- or second-degree [Mobitz type I] AV block, or a history of myocardial infarction or heart failure occurring more than 6 months prior to treatment initiation and in stable condition [see Warnings and Precautions (5.3) and Clinical Pharmacology (12.2) ] .
First Dose 4-Hour Monitoring
Administer the first dose of PONVORY in a setting where resources to appropriately manage symptomatic bradycardia are available. Monitor patients for 4 hours after the first dose for signs and symptoms of bradycardia with a minimum of hourly pulse and blood pressure measurements. Obtain an ECG in these patients prior to dosing and at the end of the 4-hour observation period.
Additional Monitoring After 4-Hour Monitoring
If any of the following abnormalities are present after 4 hours (even in the absence of symptoms), continue monitoring until the abnormality resolves:
- The heart rate 4 hours post-dose is less than 45 bpm
- The heart rate 4 hours post-dose is at the lowest value post-dose, suggesting that the maximum pharmacodynamic effect on the heart may not have occurred
- The ECG 4 hours post-dose shows new onset second-degree or higher AV block
If post-dose symptomatic bradycardia, bradyarrhythmia, or conduction related symptoms occur, or if ECG 4 hours post-dose shows new onset second degree or higher AV block or QTc greater than or equal to 500 msec, initiate appropriate management, begin continuous ECG monitoring, and continue monitoring until the symptoms have resolved if no pharmacological treatment is required. If pharmacological treatment is required, continue monitoring overnight and repeat 4-hour monitoring after the second dose.
Advice from a cardiologist should be sought to determine the most appropriate monitoring strategy (which may include overnight monitoring) during treatment initiation, if treatment with PONVORY is considered in patients:
- With some preexisting heart and cerebrovascular conditions [see Warnings and Precautions (5.2) ]
- With a prolonged QTc interval before dosing or during the 4-hour observation, or at additional risk for QT prolongation, or on concurrent therapy with QT prolonging drugs with a known risk of torsades de pointes [see Warnings and Precautions (5.2) and Drug Interactions (7.2) ]
- Receiving concurrent therapy with drugs that slow heart rate or AV conduction [see Drug Interactions (7.2 , 7.3) ]
Reinitiation of PONVORY After Treatment Interruption
Interruption during treatment, especially during titration, is not recommended; however:
- If fewer than 4 consecutive doses are missed:
- during titration: resume treatment with the first missed titration dose and resume the titration schedule at that dose and titration day.
- during maintenance: resume treatment with the maintenance dosage.
- If 4 or more consecutive doses are missed during titration or maintenance:
- treatment should be reinitiated with Day 1 of the titration regimen (new starter pack).
If treatment needs to be reinitiated with Day 1 of the titration regimen (new starter pack), complete first-dose monitoring in patients for whom it is recommended [see Dosage and Administration (2.3) ] .

By using PrescriberAI, you agree to the AI Terms of Use.
Ponvory prescribing information
INDICATIONS AND USAGE
PONVORY is indicated for the treatment of relapsing forms of multiple sclerosis (MS), to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in adults.
DOSAGE AND ADMINISTRATION
- Assessments are required prior to initiating PONVORY (2.1 )
- Titration is required for treatment initiation (2.2 )
- The recommended maintenance dosage is 20 mg taken orally once daily (2.2 )
- First-dose monitoring is recommended for patients with sinus bradycardia, first- or second-degree [Mobitz type I] atrioventricular (AV) block, or a history of myocardial infarction or heart failure (2.3 )
Assessments Prior to First Dose of PONVORY
Before initiation of treatment with PONVORY, assess the following:
Complete Blood Count
Obtain a recent (i.e., within the last 6 months or after discontinuation of prior MS therapy) complete blood count (CBC), including lymphocyte count [see Warnings and Precautions (5.1) ] .
Cardiac Evaluation
Obtain an electrocardiogram (ECG) to determine whether preexisting conduction abnormalities are present. In patients with certain preexisting conditions, advice from a cardiologist should be sought and first-dose monitoring is recommended [see Dosage and Administration (2.3) and Warnings and Precautions (5.2) ] .
Determine whether patients are taking drugs that could slow heart rate or atrioventricular (AV) conduction [see Warnings and Precautions (5.2) and Drug Interactions (7.2 , 7.3) ] .
Liver Function Tests
Obtain recent (i.e., within the last 6 months) transaminase and bilirubin levels [see Warnings and Precautions (5.4) ] .
Ophthalmic Evaluation
Obtain an evaluation of the fundus, including the macula [see Warnings and Precautions (5.8) ] .
Current or Prior Medications with Immune System Effects
If patients are taking anti-neoplastic, immunosuppressive, or immune-modulating therapies, or if there is a history of prior use of these drugs, consider possible unintended additive immunosuppressive effects before initiating treatment with PONVORY [see Warnings and Precautions (5.1 , 5.10) and Drug Interactions (7.1) ] .
Vaccinations
Test patients for antibodies to varicella zoster virus (VZV) before initiating PONVORY; VZV vaccination of antibody-negative patients is recommended prior to commencing treatment with PONVORY [see Warnings and Precautions (5.1) ] . If live attenuated vaccine immunizations are required, administer at least 1 month prior to initiation of PONVORY.
Recommended Dosage
Maintenance Dosage
After dose titration is complete (see Treatment Initiation ) , the recommended maintenance dosage of PONVORY is 20 mg taken orally once daily starting on Day 15.
Administer PONVORY orally once daily. Swallow the tablet whole. PONVORY can be taken with or without food.
Treatment Initiation
A starter pack must be used for patients initiating treatment with PONVORY [see How Supplied/Storage and Handling (16.1) ] . Initiate PONVORY treatment with a 14-day titration; start with one 2 mg tablet orally once daily and progress with the titration schedule as shown in Table 1 [see Warnings and Precautions (5.2) ] .
| Titration Day | Daily Dose |
|---|---|
| Days 1 and 2 | 2 mg |
| Days 3 and 4 | 3 mg |
| Days 5 and 6 | 4 mg |
| Day 7 | 5 mg |
| Day 8 | 6 mg |
| Day 9 | 7 mg |
| Day 10 | 8 mg |
| Day 11 | 9 mg |
| Days 12, 13, and 14 | 10 mg |
| Maintenance | Daily Dose |
|---|---|
| Day 15 and thereafter | 20 mg |
If dose titration is interrupted, missed dose instructions must be followed [see Dosage and Administration (2.4) ] .
First Dose Monitoring in Patients with Certain Preexisting Cardiac Conditions
Because initiation of PONVORY treatment results in a decrease in heart rate (HR), first-dose 4-hour monitoring is recommended for patients with sinus bradycardia [HR less than 55 beats per minute (bpm)], first- or second-degree [Mobitz type I] AV block, or a history of myocardial infarction or heart failure occurring more than 6 months prior to treatment initiation and in stable condition [see Warnings and Precautions (5.3) and Clinical Pharmacology (12.2) ] .
First Dose 4-Hour Monitoring
Administer the first dose of PONVORY in a setting where resources to appropriately manage symptomatic bradycardia are available. Monitor patients for 4 hours after the first dose for signs and symptoms of bradycardia with a minimum of hourly pulse and blood pressure measurements. Obtain an ECG in these patients prior to dosing and at the end of the 4-hour observation period.
Additional Monitoring After 4-Hour Monitoring
If any of the following abnormalities are present after 4 hours (even in the absence of symptoms), continue monitoring until the abnormality resolves:
- The heart rate 4 hours post-dose is less than 45 bpm
- The heart rate 4 hours post-dose is at the lowest value post-dose, suggesting that the maximum pharmacodynamic effect on the heart may not have occurred
- The ECG 4 hours post-dose shows new onset second-degree or higher AV block
If post-dose symptomatic bradycardia, bradyarrhythmia, or conduction related symptoms occur, or if ECG 4 hours post-dose shows new onset second degree or higher AV block or QTc greater than or equal to 500 msec, initiate appropriate management, begin continuous ECG monitoring, and continue monitoring until the symptoms have resolved if no pharmacological treatment is required. If pharmacological treatment is required, continue monitoring overnight and repeat 4-hour monitoring after the second dose.
Advice from a cardiologist should be sought to determine the most appropriate monitoring strategy (which may include overnight monitoring) during treatment initiation, if treatment with PONVORY is considered in patients:
- With some preexisting heart and cerebrovascular conditions [see Warnings and Precautions (5.2) ]
- With a prolonged QTc interval before dosing or during the 4-hour observation, or at additional risk for QT prolongation, or on concurrent therapy with QT prolonging drugs with a known risk of torsades de pointes [see Warnings and Precautions (5.2) and Drug Interactions (7.2) ]
- Receiving concurrent therapy with drugs that slow heart rate or AV conduction [see Drug Interactions (7.2 , 7.3) ]
Reinitiation of PONVORY After Treatment Interruption
Interruption during treatment, especially during titration, is not recommended; however:
- If fewer than 4 consecutive doses are missed:
- during titration: resume treatment with the first missed titration dose and resume the titration schedule at that dose and titration day.
- during maintenance: resume treatment with the maintenance dosage.
- If 4 or more consecutive doses are missed during titration or maintenance:
- treatment should be reinitiated with Day 1 of the titration regimen (new starter pack).
If treatment needs to be reinitiated with Day 1 of the titration regimen (new starter pack), complete first-dose monitoring in patients for whom it is recommended [see Dosage and Administration (2.3) ] .
DOSAGE FORMS AND STRENGTHS
PONVORY is available as round, biconvex, film-coated tablets for oral use. PONVORY contains ponesimod in the following dosage strengths (see Table 2 ):
| Tablet Strength | Tablet Color | Tablet Size | Tablet Debossing |
|---|---|---|---|
| 2 mg | White | 5.0 mm | "2" on one side and an arch on the other side. |
| 3 mg | Red | 5.0 mm | "3" on one side and an arch on the other side. |
| 4 mg | Purple | 5.0 mm | "4" on one side and an arch on the other side. |
| 5 mg | Green | 8.6 mm | "5" on one side and an arch and an "A" on the other side. |
| 6 mg | White | 8.6 mm | " 6 " on one side and an arch and an "A" on the other side. |
| 7 mg | Red | 8.6 mm | "7" on one side and an arch and an "A" on the other side. |
| 8 mg | Purple | 8.6 mm | "8" on one side and an arch and an "A" on the other side. |
| 9 mg | Brown | 8.6 mm | " 9 " on one side and an arch and an "A" on the other side. |
| 10 mg | Orange | 8.6 mm | "10" on one side and an arch and an "A" on the other side. |
| 20 mg | Yellow | 8.6 mm | "20" on one side and an arch and an "A" on the other side. |
USE IN SPECIFIC POPULATIONS
Hepatic Impairment : PONVORY is not recommended in patients with moderate or severe hepatic impairment (Child-Pugh class B and C). (8.6 )
Pregnancy
Risk Summary
There are no adequate and well-controlled studies of PONVORY in pregnant women. In animal studies, administration of ponesimod during pregnancy produced adverse effects on development, including embryo lethality and fetal malformations, in the absence of maternal toxicity. In rats and rabbits, visceral and skeletal malformations occurred at clinically relevant maternal ponesimod exposures (see Data ) . The receptor affected by ponesimod (sphingosine-1-phosphate receptor 1) has been demonstrated to have an important role in embryogenesis, including vascular and neural development.
In the US general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2%–4% and 15%–20%, respectively. The background risk of major birth defects and miscarriage for the indicated population is unknown.
Data
Animal Data
When ponesimod (0, 1, 10, or 40 mg/kg/day) was orally administered to pregnant rats during the period of organogenesis, increased incidences of fetal malformations primarily involving the limbs (syndactyly and ectrodactyly) and cardiovascular system (including ventricular septal defects) were observed at all but the lowest dose tested. A high incidence of embryofetal death was observed at the highest dose tested. Maternal toxicity was not observed, indicating a selective effect on the fetus. Plasma exposure (AUC) at the no-effect dose (1 mg/kg/day) for adverse effects on embryofetal development in rats was lower than that in humans at the recommended human dose (RHD) of 20 mg/day.
When ponesimod (0, 0.25, 1, or 4 mg/kg/day) was orally administered to pregnant rabbits during the period of organogenesis, an increase in post-implantation loss and fetal variations (visceral and skeletal) were noted at the highest dose tested. No maternal toxicity was observed. Plasma exposure at the no-effect dose (1 mg/kg/day) for adverse effects on embryofetal development in rabbits was lower than that in humans at the RHD. In a dose-range finding study in pregnant rabbits, oral administration of ponesimod (0, 6, 20, or 60 mg/kg/day) during organogenesis, an increase in embryofetal death and fetal limb malformation (brachydactyly) were observed at the lowest dose tested; at the higher doses, there were no live fetuses.
When ponesimod (5, 10, or 20 mg/kg) was orally administered to female rats throughout pregnancy and lactation, the offspring exhibited decreased survival, reduced body weight gain, and reduced fertility and reproductive performance (increases in pre- and post-implantation loss) at the highest dose tested, neurobehavioral impairment (increased locomotor activity) at the mid and high doses, and delayed sexual maturation at all doses tested. A no-effect dose for adverse effects on pre- and postnatal development in rats was not identified. Plasma exposure (AUC) in dams at the lowest dose tested was less than that in humans at the RHD.
Lactation
Risk Summary
There are no data on the presence of PONVORY in human milk, the effects on the breastfed infant, or the effects of the drug on milk production. When ponesimod was orally administered to female rats during pregnancy and lactation, ponesimod was detected in the plasma of the offspring, suggesting excretion of ponesimod in milk.
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for PONVORY and any potential adverse effects on the breastfed infant from PONVORY or from the underlying maternal condition.
Females and Males of Reproductive Potential
Contraception
Females
Before initiation of PONVORY treatment, women of childbearing potential should be counseled on the potential for a serious risk to the fetus and the need for effective contraception during treatment with PONVORY [see Use in Specific Populations (8.1) ] . Since it takes approximately one week to eliminate ponesimod from the body after stopping treatment, the potential risk to the fetus may persist, and women should use effective contraception during this period [see Warnings and Precautions (5.7) ] .
Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
Juvenile Animal Toxicity Data
Oral administration of ponesimod (0, 1, 10, 30, or 100 mg/kg/day) to young rats from postnatal day 28 to 91 resulted in lung histopathology (alveolar histiocytosis/edema) and decreased immune function (T-cell dependent antibody response) at the two highest doses tested. Decreased growth (body weight gain and/or long bone length) was observed at all but the low dose, and neurobehavioral impairment (increased locomotor activity) was observed at the highest dose tested. Decreased lymphocyte count and neurobehavioral impairment persisted at the end of a 4-week recovery period.
Geriatric Use
Clinical studies of PONVORY did not include patients 65 years of age and over to determine whether they respond differently from younger subjects. Use of PONVORY in elderly patients should be cautious, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy [see Clinical Pharmacology (12.3) ] .
Hepatic Impairment
No dosage adjustment is necessary in patients with mild hepatic impairment (Child-Pugh class A) [see Clinical Pharmacology (12.3) ] .
PONVORY is not recommended in patients with moderate or severe hepatic impairment (Child-Pugh class B and C, respectively), as the risk of adverse reactions may be greater [see Clinical Pharmacology (12.3) ] .
CONTRAINDICATIONS
PONVORY is contraindicated in patients who:
- In the last 6 months, have experienced myocardial infarction, unstable angina, stroke, transient ischemic attack (TIA), decompensated heart failure requiring hospitalization, or Class III or IV heart failure [see Warnings and Precautions (5.2) ]
- Have presence of Mobitz type II second-degree, third-degree atrioventricular (AV) block, sick sinus syndrome, or sino-atrial block, unless patient has a functioning pacemaker [see Warnings and Precautions (5.2) ]
WARNINGS AND PRECAUTIONS
- Infections : PONVORY may increase the risk of infections. Obtain a complete blood count (CBC) before initiating treatment. Monitor for infection during treatment and for 1–2 weeks after discontinuation. Do not start PONVORY in patients with active infection. (5.1 )
- Bradyarrhythmia and Atrioventricular Conduction Delays : PONVORY may result in a transient decrease in heart rate; titration is required for treatment initiation. Check an electrocardiogram (ECG) to assess for preexisting cardiac conduction abnormalities before starting PONVORY. Consider cardiology consultation for conduction abnormalities or concomitant use with other drugs that decrease heart rate. (5.2 , 7.2 , 7.3 )
- Respiratory Effects : May cause a decline in pulmonary function. Assess pulmonary function (e.g., spirometry) if clinically indicated. (5.3 )
- Liver Injury : Discontinue if significant liver injury is confirmed. Obtain liver function tests before initiating PONVORY. (5.4 )
- Increased Blood Pressure (BP) : Monitor BP during treatment. (5.5 )
- Cutaneous Malignancies: Periodic skin examination is recommended. (5.6 )
- Fetal Risk : Women of childbearing potential should use effective contraception during and for 1 week after stopping PONVORY. (5.7 )
- Macular Edema : An ophthalmic evaluation is recommended before starting treatment and if there is any change in vision while taking PONVORY. Diabetes mellitus and uveitis increase the risk. (5.8 )
Infections
Risk of Infections
PONVORY causes a dose-dependent reduction in peripheral lymphocyte count to 30–40% of baseline values because of reversible sequestration of lymphocytes in lymphoid tissues [see Clinical Pharmacology (12.2) ] . PONVORY may therefore increase the susceptibility to infections. Life-threatening and rare fatal infections have been reported in association with other sphingosine 1-phosphate (S1P) receptor modulators.
In Study 1 [see Clinical Studies (14) ] , the overall rate of infections was comparable between the PONVORY-treated patients and those receiving teriflunomide 14 mg (54.2% vs 52.1%, respectively). PONVORY increased the risk of upper respiratory tract infections. Serious or severe infections occurred in 1.6% of PONVORY-treated patients compared to 0.9% of patients receiving teriflunomide 14 mg.
Before initiating treatment with PONVORY, results from a recent (i.e., within 6 months or after discontinuation of prior therapy) complete blood count including lymphocyte count should be reviewed.
Initiation of treatment with PONVORY should be delayed in patients with active infection until resolution. Lymphocyte counts returned to the normal range in 90% of patients within 1 week of stopping therapy in modeling studies [see Clinical Pharmacology (12.2) ] . In Study 1, peripheral lymphocyte counts returned to normal range within 2 weeks after discontinuation of PONVORY, which was the first timepoint evaluated. Because residual pharmacodynamic effects, such as lowering effects on peripheral lymphocyte count, may persist for 1 to 2 weeks after discontinuation of PONVORY, vigilance for infection should be continued for 1 to 2 weeks after PONVORY is discontinued [see Warnings and Precautions (5.12) ].
In Study 1, the proportion of patients who experienced lymphocyte counts less than 0.2×10 9 /L was 3.2%. Effective diagnostic and therapeutic strategies should be employed in patients with symptoms of infection while on therapy. Consider interruption of treatment with PONVORY if a patient develops a serious infection.
Herpes Viral Infections
Cases of herpes viral infection have been reported in the development program of PONVORY; herpes simplex encephalitis and varicella zoster meningitis have been reported with other S1P receptor modulators.
In Study 1, the rate of herpetic infections was 4.8% for both PONVORY-treated patients and those receiving teriflunomide 14 mg. Patients without a healthcare professional confirmed history of varicella (chickenpox) or without documentation of a full course of vaccination against VZV should be tested for antibodies to VZV before initiating PONVORY (see Vaccinations ) .
Cryptococcal Infections
Cases of fatal cryptococcal meningitis (CM) and disseminated cryptococcal infections have been reported with other S1P receptor modulators. Physicians should be vigilant for clinical symptoms or signs of CM. Patients with symptoms or signs consistent with a cryptococcal infection should undergo prompt diagnostic evaluation and treatment. PONVORY treatment should be suspended until a cryptococcal infection has been excluded. If CM is diagnosed, appropriate treatment should be initiated.
Progressive Multifocal Leukoencephalopathy
Progressive multifocal leukoencephalopathy (PML) is an opportunistic viral infection of the brain caused by the JC virus (JCV) that typically only occurs in patients who are immunocompromised, and that usually leads to death or severe disability. Typical symptoms associated with PML are diverse, progress over days to weeks, and include progressive weakness on one side of the body or clumsiness of limbs, disturbance of vision, and changes in thinking, memory, and orientation leading to confusion and personality changes.
PML has been reported in patients treated with S1P receptor modulators and other multiple sclerosis (MS) therapies and has been associated with some risk factors (e.g., immunocompromised patients, polytherapy with immunosuppressants). Physicians should be vigilant for clinical symptoms or magnetic resonance imaging (MRI) findings that may be suggestive of PML. MRI findings may be apparent before clinical signs or symptoms. If PML is suspected, treatment with PONVORY should be suspended until PML has been excluded.
If PML is confirmed, treatment with PONVORY should be discontinued.
Immune reconstitution inflammatory syndrome (IRIS) has been reported in patients treated with S1P receptor modulators who developed PML and subsequently discontinued treatment. IRIS presents as a clinical decline in the patient's condition that may be rapid, can lead to serious neurological complications or death, and is often associated with characteristic changes on MRI. The time to onset of IRIS in patients with PML was generally within a few months after S1P receptor modulator discontinuation. Monitoring for development of IRIS and appropriate treatment of the associated inflammation should be undertaken.
Prior and Concomitant Treatment with Anti-neoplastic, Immune-Modulating, or Immunosuppressive Therapies
Anti-neoplastic, immune-modulating, or immunosuppressive therapies (including corticosteroids) should be coadministered with caution because of the risk of additive immune system effects [see Drug Interactions (7.1) ] .
Vaccinations
Patients without a healthcare professional confirmed history of chickenpox or without documentation of a full course of vaccination against VZV should be tested for antibodies to VZV before initiating PONVORY treatment. A full course of vaccination for antibody-negative patients with varicella vaccine is recommended prior to commencing treatment with PONVORY, following which initiation of treatment with PONVORY should be postponed for 4 weeks to allow the full effect of vaccination to occur.
No clinical data are available on the efficacy and safety of vaccinations in patients taking PONVORY. Vaccinations may be less effective if administered during PONVORY treatment.
If live attenuated vaccine immunizations are required, administer at least 1 month prior to initiation of PONVORY. Avoid the use of live attenuated vaccines during and for 1 to 2 weeks after treatment with PONVORY.
Bradyarrhythmia and Atrioventricular Conduction Delays
Since initiation of PONVORY treatment results in a transient decrease in heart rate and atrioventricular (AV) conduction delays, an up-titration scheme must be used to reach the maintenance dosage of PONVORY (20 mg) [see Dosage and Administration (2.2) and Clinical Pharmacology (12.2) ] .
Study 1 did not include patients who had:
- A resting heart rate (HR) less than 50 beats per minute (bpm) on baseline electrocardiogram
- Myocardial infarction or unstable ischemic heart disease in the last 6 months
- Cardiac failure (New York Heart Association class III–IV) or presence of any severe cardiac disease
- Cardiac conduction or rhythm disorders (including sino-atrial heart block, symptomatic bradycardia, atrial flutter or atrial fibrillation, ventricular arrhythmia, cardiac arrest) either in history or observed at screening
- Mobitz Type II second degree AV block or higher-grade AV block observed at screening
- QTcF interval greater than 470 ms (females) and greater than 450 ms (males) observed at screening
- History of syncope associated with cardiac disorders
- Uncontrolled systemic arterial hypertension
Reduction in Heart Rate
Initiation of PONVORY may result in a transient decrease in HR. In Study 1, bradycardia at treatment initiation and sinus bradycardia on ECG (defined as HR less than 50 bpm) occurred in 5.8% of PONVORY-treated patients compared to 1.6% of patients receiving teriflunomide 14 mg. After the first titration dose of PONVORY, the decrease in heart rate typically begins within an hour and reaches its nadir within 2–4 hours. The heart rate typically recovers to baseline levels 4–5 hours after administration. The mean decrease in heart rate on Day 1 of dosing was 6 bpm. With up-titration after Day 1, the post-dose decrease in heart rate is less pronounced. Bradycardia resolved in all patients in Study 1 without intervention and did not require discontinuation of PONVORY treatment. On Day 1, 3 patients treated with PONVORY had asymptomatic post-dose HR below or equal to 40 bpm; all 3 patients had baseline HRs below 55 bpm.
Atrioventricular Conduction Delays
Initiation of PONVORY treatment has been associated with transient atrioventricular conduction delays that follow a similar temporal pattern as the observed decrease in heart rate during dose titration. In Study 1, the AV conduction delays manifested as first-degree AV block (prolonged PR interval on ECG), which occurred in 3.4% of PONVORY-treated patients and in 1.2% of patients receiving teriflunomide 14 mg. The conduction abnormalities typically were transient, asymptomatic, resolved within 24 hours, resolved without intervention, and did not require discontinuation of PONVORY treatment. In Study 1, second- and third-degree AV blocks were not reported in patients treated with PONVORY.
If treatment with PONVORY is considered, advice from a cardiologist should be sought for individuals:
- With significant QT prolongation (QTc greater than 500 msec)
- With atrial flutter/fibrillation or arrhythmia treated with Class Ia or Class III anti-arrhythmic drugs [see Drug Interactions (7.2) ]
- With unstable ischemic heart disease, cardiac decompensated failure occurring more than 6 months prior to treatment initiation, history of cardiac arrest, cerebrovascular disease (TIA, stroke occurring more than 6 months prior to treatment initiation), or uncontrolled hypertension
- With a history of Mobitz Type II second degree AV block or higher-grade AV block, sick-sinus syndrome, or sino-atrial heart block [see Contraindications (4) ]
Treatment Initiation Recommendations
- Obtain an ECG in all patients to determine whether preexisting conduction abnormalities are present.
- In all patients, a dose titration is recommended for initiation of PONVORY treatment to help reduce cardiac effects [see Dosage and Administration (2.2) ] .
- In patients with sinus bradycardia, first-or second-degree [Mobitz type I] AV block, or a history of myocardial infarction or heart failure with onset more than 6 months prior to initiation, first-dose monitoring is recommended [see Dosage and Administration (2.1 , 2.3) ] .
- PONVORY is not recommended in patients with a history of cardiac arrest, cerebrovascular disease (e.g., TIA, stroke occurring more than 6 months prior to treatment initiation), uncontrolled hypertension, or severe untreated sleep apnea, since significant bradycardia may be poorly tolerated in these patients. If treatment is considered, advice from a cardiologist should be sought prior to initiation of treatment in order to determine the most appropriate monitoring strategy.
- Use of PONVORY in patients with a history of recurrent syncope or symptomatic bradycardia should be based on an overall benefit-risk assessment. If treatment is considered, advice from a cardiologist should be sought prior to initiation of treatment in order to determine the most appropriate monitoring.
- Experience with PONVORY is limited in patients receiving concurrent therapy with drugs that decrease heart rate (e.g., beta-blockers, non-dihydropyridine calcium channel blockers - diltiazem and verapamil, and other drugs that may decrease heart rate such as digoxin). Concomitant use of these drugs during PONVORY initiation may be associated with severe bradycardia and heart block. If treatment is considered, advice from a cardiologist should be sought prior to initiation of treatment in order to determine the most appropriate monitoring.
- For patients receiving a stable dose of a beta-blocker, the resting heart rate should be considered before introducing PONVORY treatment. If the resting heart rate is greater than 55 bpm under chronic beta-blocker treatment, PONVORY can be introduced. If resting heart rate is less than or equal to 55 bpm, beta-blocker treatment should be interrupted until the baseline heart rate is greater than 55 bpm. Treatment with PONVORY can then be initiated and treatment with a beta-blocker can be reinitiated after PONVORY has been up-titrated to the target maintenance dosage [see Drug Interactions (7.3) ] .
- For patients taking other drugs that decrease heart rate, treatment with PONVORY should generally not be initiated without consultation from a cardiologist because of the potential additive effect on heart rate [see Dosage and Administration (2.3) and Drug Interactions (7.2) ] .
Missed Dose During Treatment Initiation or Maintenance Treatment
If 4 or more consecutive daily doses are missed during treatment initiation or maintenance treatment, reinitiate Day 1 of the dose titration (new starter pack) and follow first-dose monitoring recommendations [see Dosage and Administration (2.4) ] .
Respiratory Effects
Dose-dependent reductions in forced expiratory volume over 1 second (FEV 1 ) and reductions in diffusion lung capacity for carbon monoxide (DL CO ) were observed in PONVORY-treated patients mostly occurring in the first month after treatment initiation. In Study 1, the reduction from baseline in percent predicted FEV 1 at 2 years was 8.3% in PONVORY-treated patients compared to 4.4% in patients receiving teriflunomide 14 mg. In Study 1, 7 patients discontinued PONVORY because of pulmonary adverse events. There is insufficient information to determine the reversibility of the decrease in FEV 1 or FVC after treatment discontinuation. PONVORY should be used with caution in patients with severe respiratory disease (i.e., pulmonary fibrosis, asthma, and chronic obstructive pulmonary disease). Spirometric evaluation of respiratory function should be performed during therapy with PONVORY if clinically indicated.
Liver Injury
Elevations of transaminases may occur in PONVORY-treated patients.
Obtain transaminase and bilirubin levels, if not recently available (i.e., within last 6 months) before initiation of PONVORY.
In Study 1, elevations of ALT to 5-fold the upper limit of normal (ULN) or greater occurred in 4.6% of patients treated with PONVORY compared to 2.5% of patients who received teriflunomide 14 mg. Elevation of ALT to 3-fold the ULN or greater occurred in 17.3% of patients treated with PONVORY and 8.3% of patients treated with teriflunomide 14 mg. The median time to an elevation of 3-fold the ULN was 3 months. The majority (89%) of patients with ALT increases 3-fold or greater the ULN continued treatment with PONVORY with values returning to less than three times the ULN within approximately 2–4 weeks.
In Study 1, the discontinuation rate because of elevations in hepatic enzymes was 2.3% of patients treated with PONVORY and 1.9% of patients who received teriflunomide 14 mg.
Patients who develop symptoms suggestive of hepatic dysfunction, such as unexplained nausea, vomiting, abdominal pain, fatigue, anorexia, rash with eosinophilia, or jaundice and/or dark urine during treatment, should have hepatic enzymes checked. PONVORY should be discontinued if significant liver injury is confirmed.
No dosage adjustment is necessary in patients with mild hepatic impairment (Child-Pugh class A). PONVORY is not recommended in patients with moderate or severe hepatic impairment (Child-Pugh class B and C, respectively) [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3) ] .
Increased Blood Pressure
In Study 1, PONVORY-treated patients had an average increase of 2.9 mm Hg in systolic blood pressure and 2.8 mm Hg in diastolic blood pressure compared to 2.8 mm Hg and 3.1 mm Hg in patients receiving teriflunomide 14 mg, respectively. An increase in blood pressure with PONVORY was first detected after approximately 1 month of treatment initiation and persisted with continued treatment. Hypertensive events were reported as an adverse reaction in 10.1% of PONVORY-treated patients and in 9.0% of patients receiving teriflunomide 14 mg. One patient treated with PONVORY experienced a hypertensive crisis but had evidence of longstanding hypertensive heart disease. Blood pressure should be monitored during treatment with PONVORY and managed appropriately.
Cutaneous Malignancies
Cases of basal cell carcinoma and other skin malignancies have been reported in patients treated with S1P receptor modulators, including PONVORY. In Study 1, the incidence of basal cell carcinoma was 0.4% in PONVORY-treated patients compared to 0.2% in patients receiving teriflunomide 14 mg. Cases of other cutaneous malignancies, including melanoma and squamous cell carcinoma, have also been reported in patients treated with PONVORY and in patients treated with other S1P modulators.
Periodic skin examination is recommended for all patients, particularly those with risk factors for skin cancer. Providers and patients are advised to monitor for suspicious skin lesions. If a suspicious skin lesion is observed, it should be promptly evaluated. As usual for patients with increased risk for skin cancer, exposure to sunlight and ultraviolet light should be limited by wearing protective clothing and using a sunscreen with a high protection factor. Concomitant phototherapy with UV-B radiation or PUVA-photochemotherapy is not recommended in patients taking PONVORY.
Fetal Risk
Based on animal studies, PONVORY may cause fetal harm [see Use in Specific Populations (8.1 , 8.3) ] . Because it takes approximately 1 week to eliminate PONVORY from the body, women of childbearing potential should use effective contraception to avoid pregnancy during and for 1 week after stopping PONVORY treatment.
Macular Edema
S1P receptor modulators, including PONVORY, have been associated with an increased risk of macular edema. In Study 1, macular edema was reported in 1.1% of PONVORY-treated patients compared to none of the patients receiving teriflunomide 14 mg.
An ophthalmic evaluation of the fundus, including the macula, is recommended in all patients before starting treatment and again at any time if a patient reports any change in vision while on PONVORY therapy.
Continuation of PONVORY therapy in patients with macular edema has not been evaluated. A decision on whether PONVORY should be discontinued should take into account the potential benefits and risks for the individual patient.
Macular Edema in Patients with a History of Uveitis or Diabetes Mellitus
Patients with a history of uveitis and patients with diabetes mellitus are at increased risk of macular edema during therapy with S1P receptor modulators, including PONVORY. Therefore, these patients should have regular follow-up examinations of the fundus, including the macula, during treatment with PONVORY.
Posterior Reversible Encephalopathy Syndrome
Rare cases of posterior reversible encephalopathy syndrome (PRES) have been reported in patients receiving a sphingosine 1-phosphate (S1P) receptor modulator. Such events have not been reported for PONVORY-treated patients in the development program. However, should a PONVORY-treated patient develop any unexpected neurological or psychiatric symptoms/signs (e.g., cognitive deficits, behavioral changes, cortical visual disturbances, or any other neurological cortical symptoms/signs), any symptom/sign suggestive of an increase of intracranial pressure, or accelerated neurological deterioration, the physician should promptly schedule a complete physical and neurological examination and should consider an MRI. Symptoms of PRES are usually reversible but may evolve into ischemic stroke or cerebral hemorrhage. Delay in diagnosis and treatment may lead to permanent neurological sequelae. If PRES is suspected, PONVORY should be discontinued.
Unintended Additive Immunosuppressive Effects From Prior Treatment With Immunosuppressive or Immune-Modulating Therapies
When switching from drugs with prolonged immune effects, the half-life and mode of action of these drugs must be considered in order to avoid unintended additive effects on the immune system while at the same time minimizing risk of disease reactivation, when initiating PONVORY.
Initiating treatment with PONVORY after treatment with alemtuzumab is not recommended.
Severe Increase in Disability After Stopping PONVORY
Severe exacerbation of disease, including disease rebound, has been rarely reported after discontinuation of a S1P receptor modulator. The possibility of severe exacerbation of disease should be considered after stopping PONVORY treatment. Patients should be observed for a severe increase in disability upon PONVORY discontinuation and appropriate treatment should be instituted, as required.
After stopping PONVORY in the setting of PML, monitor for development of immune reconstitution inflammatory syndrome (PML-IRIS) [see Warnings and Precautions (5.1) ] .
Immune System Effects After Stopping PONVORY
After stopping PONVORY therapy, ponesimod remains in the blood for up to 1 week. Starting other therapies during this interval will result in concomitant exposure to ponesimod. Lymphocyte counts returned to the normal range in 90% of patients within 1 week of stopping PONVORY therapy in modeling studies [see Clinical Pharmacology (12.2) ] . However, residual pharmacodynamics effects, such as lowering effects on peripheral lymphocyte count, may persist for 1 to 2 weeks after the last dose. Use of immunosuppressants within this period may lead to an additive effect on the immune system, and therefore caution should be applied 1 to 2 weeks after the last dose of PONVORY [see Drug Interactions (7.1) ] .
ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in labeling:
- Infections [see Warnings and Precautions (5.1) ]
- Bradyarrhythmia and Atrioventricular Conduction Delays [see Warnings and Precautions (5.2) ]
- Respiratory Effects [see Warnings and Precautions (5.3) ]
- Liver Injury [see Warnings and Precautions (5.4) ]
- Increased Blood Pressure [see Warnings and Precautions (5.5) ]
- Cutaneous Malignancies [see Warnings and Precautions (5.6) ]
- Fetal Risk [see Warnings and Precautions (5.7) ]
- Macular Edema [see Warnings and Precautions (5.8) ]
- Posterior Reversible Encephalopathy Syndrome [see Warnings and Precautions (5.9) ]
- Unintended Additive Immunosuppressive Effects From Prior Treatment With Immunosuppressive or Immune-Modulating Therapies [see Warnings and Precautions (5.10) ]
- Severe Increase in Disability After Stopping PONVORY [see Warnings and Precautions (5.11) ]
- Immune System Effects After Stopping PONVORY [see Warnings and Precautions (5.12) ]
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
A total of 1438 MS patients have received PONVORY at doses of at least 2 mg daily. These patients were included in Study 1 (2-year active-controlled versus teriflunomide 14 mg) [see Clinical Studies (14) ] and in a Phase 2 (6-month placebo-controlled) study in patients with MS and the uncontrolled extension studies.
In Study 1, 82% of PONVORY-treated patients completed 2 years of study treatment, compared to 82.2% of patients receiving teriflunomide 14 mg. Adverse events led to discontinuation of treatment in 8.7% of PONVORY-treated patients, compared to 6% of patients receiving teriflunomide 14 mg. The most common adverse reactions (incidence at least 10%) in PONVORY-treated patients in Study 1 were upper respiratory tract infection, hepatic transaminase elevation, and hypertension. Table 3 lists adverse reactions that occurred in at least 2% of PONVORY-treated patients and at a higher rate than in patients receiving teriflunomide 14 mg.
| Adverse Reaction | PONVORY | Teriflunomide 14 mg |
|---|---|---|
| N=565 (%) | N=566 (%) | |
| Upper respiratory infection Includes the following terms: nasopharyngitis, upper respiratory tract infection, pharyngitis, respiratory tract infection, bronchitis, respiratory tract infection viral, viral upper respiratory tract infection, tracheitis, and laryngitis. | 37 | 34 |
| Hepatic transaminase elevation Includes the following terms: alanine aminotransferase increased, aspartate aminotransferase increased, hepatic enzyme increased, and transaminases increased. | 23 | 12 |
| Hypertension Includes the following terms: hypertension, hypertensive crisis, blood pressure increased, blood pressure systolic increased, and blood pressure diastolic increased. | 10 | 9 |
| Urinary tract infection | 6 | 5 |
| Dyspnea | 5 | 1 |
| Dizziness | 5 | 3 |
| Cough | 4 | 2 |
| Pain in extremity | 4 | 3 |
| Somnolence | 3 | 2 |
| Pyrexia | 2 | 1 |
| C-reactive protein increased | 2 | 1 |
| Hypercholesterolemia | 2 | 1 |
| Vertigo | 2 | 1 |
In Study 1, the following adverse reactions occurred in less than 2% of PONVORY-treated patients, but at a rate at least 1% higher than in patients receiving teriflunomide 14 mg: viral infection, herpes zoster, hyperkalemia, lymphopenia [see Warnings and Precautions (5.1) , and macular edema [see Warnings and Precautions (5.8) ].
Adverse reactions in patients treated with PONVORY in an additional 6-month placebo-controlled study were generally similar to those in Study 1. The following additional adverse reactions occurred in at least 2% of PONVORY 20 mg-treated patients and at a higher rate than in patients receiving placebo (but did not meet the reporting rate criteria for inclusion in Study 1): rhinitis, fatigue, chest discomfort, peripheral edema, joint swelling, blood cholesterol increased, migraine, insomnia, depression, dyspepsia, dry mouth, bradycardia, back pain, and sinusitis.
Additionally, in uncontrolled extension trials, the adverse reaction of pneumonia was reported.
Seizures
In Study 1, cases of seizures were reported in 1.4% of PONVORY-treated patients, compared to 0.2% in patients receiving teriflunomide 14 mg. It is not known whether these events were related to the effects of MS, to PONVORY, or to a combination of both.
Respiratory Effects
In Study 1, dose-dependent reductions in forced expiratory volume over 1 second (FEV 1 ) were observed in patients treated with PONVORY [see Warnings and Precautions (5.3) ] .
Malignancies
In Study 1, two cases of basal cell carcinoma (0.4%) were reported in PONVORY-treated patients, compared to one case of basal cell carcinoma (0.2%) in patients receiving teriflunomide 14 mg, and a case of malignant melanoma was reported in a PONVORY-treated patient. An increased risk of cutaneous malignancies has been reported in association with other S1P receptor modulators, including PONVORY [see Warnings and Precautions (5.6) ] .
DRUG INTERACTIONS
Anti-Neoplastic, Immune-Modulating, or Immunosuppressive Therapies
PONVORY has not been studied in combination with anti-neoplastic, immune-modulating, or immunosuppressive therapies. Caution should be used during concomitant administration because of the risk of additive immune effects during such therapy and in the weeks following administration [see Warnings and Precautions (5.1) ] .
When switching from drugs with prolonged immune effects, the half-life and mode of action of these drugs must be considered in order to avoid unintended additive effects on the immune system [see Warnings and Precautions (5.10) ] .
Because of the characteristics and duration of alemtuzumab immune suppressive effects, initiating treatment with PONVORY after alemtuzumab is not recommended.
PONVORY can generally be started immediately after discontinuation of beta interferon or glatiramer acetate.
Anti-Arrhythmic Drugs, QT Prolonging Drugs, Drugs that may Decrease Heart Rate
PONVORY has not been studied in patients taking QT prolonging drugs.
Class Ia (e.g., quinidine, procainamide) and Class III (e.g., amiodarone, sotalol) anti-arrhythmic drugs have been associated with cases of Torsades de Pointes in patients with bradycardia. If treatment with PONVORY is considered, advice from a cardiologist should be sought.
Because of the potential additive effects on heart rate, treatment with PONVORY should generally not be initiated in patients who are concurrently treated with QT prolonging drugs with known arrhythmogenic properties, heart rate lowering calcium channel blockers (e.g., verapamil, diltiazem), or other drugs that may decrease heart rate (e.g., digoxin) [see Warnings and Precautions (5.2) and Drug Interactions (7.3) ] . If treatment with PONVORY is considered, advice from a cardiologist should be sought.
Beta-Blockers
Caution should be applied when PONVORY is initiated in patients receiving treatment with a beta-blocker because of the additive effects on lowering heart rate; temporary interruption of the beta-blocker treatment may be needed prior to initiation of PONVORY [see Warnings and Precautions (5.2) ] . Beta-blocker treatment can be initiated in patients receiving stable doses of PONVORY.
Vaccination
During, and for up to 1 to 2 weeks after discontinuation of, treatment with PONVORY, vaccinations may be less effective. The use of live attenuated vaccines may carry the risk of infection and should therefore be avoided during PONVORY treatment and for 1 to 2 weeks after discontinuation of treatment with PONVORY [see Warnings and Precautions (5.1) ] .
Strong CYP3A4 and UGT1A1 Inducers
In vitro assessments and limited clinical data indicated that concomitant use of strong CYP3A4 and UGT1A1 inducers (e.g., rifampin, phenytoin, carbamazepine) may decrease the systemic exposure of ponesimod. It is unclear whether this decrease in ponesimod systemic exposure would be considered of clinical relevance. Coadministration of PONVORY with strong CYP3A4 and UGT1A1 inducers is not recommended.
DESCRIPTION
PONVORY (ponesimod) is a sphingosine 1-phosphate receptor modulator.
The chemical name for ponesimod is (2 Z ,5 Z )-5-[3-chloro-4-[(2 R )-2,3-dihydroxypropoxy]benzylidene]-3-(2-methylphenyl)-2-(propylimino)-1,3-thiazolidin-4-one. It has one chiral center with absolute configuration of (R) . Its molecular formula is C 23 H 25 ClN 2 O 4 S and its molecular weight is 460.97 g/mol. Ponesimod has the following structural formula:
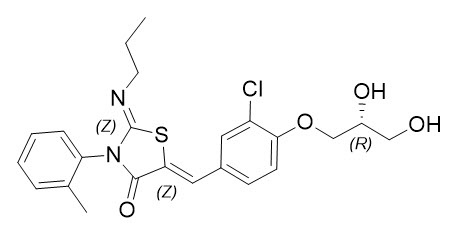
Ponesimod is a white to light yellowish powder that is practically insoluble or insoluble in water.
PONVORY ® (ponesimod) is provided as 2 mg, 3 mg, 4 mg, 5 mg, 6 mg, 7 mg, 8 mg, 9 mg, 10 mg, and 20 mg film-coated tablets for oral administration.
Each tablet contains the following inactive ingredients: croscarmellose sodium, lactose monohydrate, magnesium stearate, microcrystalline cellulose, povidone K30, silica colloidal anhydrous, and sodium lauryl sulfate.
Each tablet coating contains ferrosoferric oxide (included in 4 mg, 5 mg, 8 mg, and 9 mg film-coated tablets), hydroxypropyl methylcellulose 2910, iron oxide red (included in 3 mg, 4 mg, 7 mg, 8 mg, 9 mg, and 10 mg film-coated tablets), iron oxide yellow (included in 3 mg, 5 mg, 7 mg, 9 mg, 10 mg, and 20 mg film-coated tablets), lactose monohydrate, polyethylene glycol 3350, titanium dioxide, and triacetin.
CLINICAL PHARMACOLOGY
Mechanism of Action
Ponesimod is a sphingosine 1-phosphate (S1P) receptor 1 modulator that binds with high affinity to S1P receptor 1.
Ponesimod blocks the capacity of lymphocytes to egress from lymph nodes, reducing the number of lymphocytes in peripheral blood. The mechanism by which ponesimod exerts therapeutic effects in multiple sclerosis is unknown, but may involve reduction of lymphocyte migration into the central nervous system.
Pharmacodynamics
Immune System
In healthy volunteers, PONVORY induces a dose-dependent reduction of the peripheral blood lymphocyte count from a single dose of 5 mg onwards, with the greatest reduction observed 6 hours post-dose, caused by reversible sequestration of lymphocytes in lymphoid tissues. After 7 daily doses of 20 mg, the greatest decrease in absolute mean lymphocyte count was to 26% of baseline (650 cells/µL), observed 6 hours after administration. Peripheral blood B cells [CD19+] and T cells [CD3+], T-helper [CD3+CD4+], and T-cytotoxic [CD3+CD8+] cell subsets are all affected, while NK cells are not. T-helper cells were more sensitive to the effects of ponesimod than T-cytotoxic cells.
PK/PD modeling indicates lymphocyte counts returned to the normal range in greater than 90% of healthy subjects within 1 to 2 weeks of stopping therapy. In Study 1, peripheral lymphocyte counts returned to the normal range within 2 weeks after discontinuation of PONVORY.
Heart Rate and Rhythm
PONVORY causes a transient dose-dependent reduction in heart rate (HR) and AV conduction delays upon treatment initiation [see Warnings and Precautions (5.2) ] . The heart rate decreases plateaued at doses greater than or equal to 40 mg [2 times the recommended maintenance dosage], and bradyarrhythmic events (AV blocks) were detected at a higher incidence under PONVORY treatment, compared to placebo. This effect starts within the first hour of dosing and is maximal at 2–4 hours post-dose. HR generally returns to pre-dose values by 4–5 hours post-dose on Day 1, and the effect diminishes with repeated administration, indicating tolerance.
The decrease in heart rate induced by ponesimod can be reversed by atropine.
Beta-Blockers
The negative chronotropic effect of coadministration of PONVORY and propranolol was evaluated in a dedicated pharmacodynamics safety study. The addition of PONVORY to propranolol at steady state has an additive effect on HR effect [see Drug Interactions (7.3) ] .
Cardiac Electrophysiology
In a thorough QT study, daily administration of ponesimod doses of 40 mg and 100 mg (respectively 2- and 5-fold the recommended maintenance dose) until steady-state conditions were achieved resulted in prolongation of Fridericia-corrected QT (QTcF) intervals, with the maximum mean (upper bound of 90% two-sided confidence interval) at 11.8 ms (40 mg) and 16.2 ms (100 mg). No subject had absolute QTcF greater than 480 ms or ΔQTcF greater than 90 ms for ponesimod treatment.
Pulmonary Function
Dose-dependent reductions in FEV 1 and FVC were observed in PONVORY-treated subjects, and were greater than in subjects taking placebo [see Warnings and Precautions (5.3) ] . These effects can be reversed with administration of a short acting beta2 agonist.
Pharmacokinetics
Following ponesimod oral dosing, C max and AUC increased approximately dose-proportionally in the dose-range studied (1–75 mg). Steady-state levels are approximately 2.0- to 2.6-fold greater than with a single dose, and are achieved following 3 days of administration of the maintenance dose of ponesimod.
The pharmacokinetics of ponesimod are similar in healthy subjects and patients with multiple sclerosis, with 25% inter-subject variability across studies.
Absorption
The time to reach maximum plasma concentration of ponesimod is 2–4 hours post-dose. The absolute oral bioavailability of a 10 mg dose is 84%.
Food Effect
Food does not have a clinically relevant effect on ponesimod pharmacokinetics; therefore, PONVORY may be taken with or without food.
Distribution
Following IV administration in healthy subjects, the steady-state volume of distribution of ponesimod is 160 L.
Ponesimod is highly bound to plasma proteins (> 99%) and is mainly (78.5%) distributed in the plasma fraction of whole blood. Animal studies show that ponesimod readily crosses the blood-brain-barrier.
Metabolism
Ponesimod is extensively metabolized prior to excretion in humans, though unchanged ponesimod was the main circulating component in plasma. Two inactive circulating metabolites, M12 and M13, have also been identified in human plasma. M13 and M12 are respectively about 20% and 6% of total drug-related exposure. Both metabolites are inactive at S1P receptors at concentrations achieved with recommended doses of ponesimod.
Experiments with human liver preparations indicate that metabolism of ponesimod to M13 occurs primarily through a combination of non-Cytochrome P450 (CYP450) enzymatic activities. Multiple CYP450 (CYP2J2, CYP3A4, CYP3A5, CYP4F3A, and CYP4F12) and non-CYP450 enzymes catalyze the oxidation of ponesimod to M12. Ponesimod also undergoes direct glucuronidation (mainly UGT1A1 and UGT2B7).
Excretion
After a single IV administration, the total clearance of ponesimod is 3.8 L/hour. The elimination half-life after oral administration is approximately 33 hours.
Following a single oral administration of 14 C-ponesimod, 57% to 80% of the dose was recovered in feces (16% as unchanged ponesimod), and 10% to 18% in urine (no unchanged ponesimod).
Specific Populations
Renal Impairment
No dose adjustment is necessary in patients with renal impairment. In adult subjects with moderate or severe renal impairment (estimated creatinine clearance [CrCl], as determined by the Cockcroft-Gault, between 30–59 mL/min for moderate and <30 mL/min for severe), there were no significant changes in ponesimod C max and AUC, compared to subjects with normal renal function (CrCl>90 mL/min). The effect of dialysis on the PK of ponesimod has not been studied. Due to the high plasma protein binding (greater than 99%) of ponesimod, dialysis is not expected to alter the total and unbound ponesimod concentration, and no dose adjustments are anticipated based on these considerations.
Hepatic Impairment
In adult subjects with mild, moderate, or severe hepatic impairment (Child-Pugh class A, B and C, respectively), no change in ponesimod C max was observed, but ponesimod AUC 0–∞ was increased by 1.3-, 2.0-, and 3.1-fold, respectively, compared to healthy subjects [see Use in Specific Populations (8.6) ] .
Age
Age (range: 17 to 65 years) was not identified to significantly influence the PK of ponesimod in population pharmacokinetics analyses. The effect of age (65 years of age and older) on the pharmacokinetics of ponesimod is unknown [see Use in Specific Populations (8.5) ] .
Gender
Gender has no clinically significant influence on ponesimod pharmacokinetics.
Race
No clinically relevant pharmacokinetic differences were observed between Japanese and Caucasian subjects.
Drug Interaction Studies
Beta-Blockers
In a drug-drug interaction study, the dose titration regimen of ponesimod [see Dosage and Administration (2.2) ] was administered to subjects receiving propranolol (80 mg) once daily at steady state. No significant changes in pharmacokinetics of ponesimod or propranolol were observed. Compared to ponesimod alone, the combination of propranolol and the first dose of ponesimod (2 mg) led to a mean hourly heart rate decrease of 12.4 bpm (90% CI: -15.6 to -9.1). Compared to ponesimod alone, propranolol administered in combination with the first maintenance dose of ponesimod (20 mg) led to a 7.4 bpm (90% CI: -10.9 to -3.9) mean hourly heart rate decrease.
Effect of Other Drugs on Ponesimod
In vitro studies with human liver preparations indicate that metabolism of ponesimod occurs through multiple distinct enzyme systems, including multiple CYP450 (CYP2J2, CYP3A4, CYP3A5, CYP4F3A, and CYP4F12), UGT (mainly UGT1A1 and UGT2B7), and non-CYP450 oxidative enzymes, without major contribution by any single enzyme.
Ponesimod is not a substrate of P-gp, BCRP, OATP1B1, or OATP1B3 transporters. Drugs that are inhibitors of these transporters are unlikely to impact the PK of ponesimod.
In vitro assessments and limited clinical data indicated that concomitant use of strong CYP3A4 and UGT1A1 inducers (e.g., rifampin, phenytoin, carbamazepine) may decrease the systemic exposure of ponesimod [see Drug Interactions (7.5) ] .
Effect of Ponesimod on Other Drugs
In vitro investigations indicate that at the recommended dose of 20 mg once-daily, ponesimod and its metabolite M13 do not show any clinically relevant drug-drug interaction potential for CYP or UGT enzymes, or transporters.
Oral Contraceptives
Coadministration of ponesimod with an oral hormonal contraceptive (containing 1 mg norethisterone/norethindrone and 35 µg ethinyl estradiol) showed no clinically relevant pharmacokinetic interaction with ponesimod. Therefore, concomitant use of ponesimod is not expected to decrease the efficacy of hormonal contraceptives. No interaction studies have been performed with oral contraceptives containing other progestogens; however, an effect of ponesimod on their exposure is not expected.
NONCLINICAL TOXICOLOGY
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Oral administration of ponesimod to mice (0, 50, 150, or 400 mg/kg/day in males and 30, 100, or 300 mg/kg/day in females) for up to 2 years resulted in incidences in hemangiosarcoma and combined hemangioma and hemangiosarcoma in males at all doses and at the highest dose tested in females. Plasma exposure (AUC) at the lowest dose tested in males (50 mg/kg/day) was approximately 5 times that in humans at the recommended human dose (RHD) of 20 mg.
Oral administration of ponesimod to rats (0, 3, 10, or 30 mg/kg/day in males and 0, 10, 30 or 100 mg/kg/day in females) for up to 2 years did not result in an increase in tumors. Plasma exposure at the highest dose tested in males (30 mg/kg/day) was approximately 4 times that in humans at the recommended human dose (RHD) of 20 mg.
Mutagenesis
Ponesimod was negative in a battery of in vitro (Ames, chromosomal aberration in mammalian cells) and in vivo (micronucleus in rat) assays.
Fertility
In separate studies, oral administration of ponesimod (0, 10, 30, or 100 mg/kg/day) to male and female rats prior to and throughout the mating period and continuing in females to Day 6 of gestation resulted in no effects on fertility. Plasma ponesimod exposures (AUC) at the highest dose tested were approximately 10 (males) and 30 (females) times that in humans at the recommended human dose (RHD) of 20 mg/day.
Animal Toxicology and/or Pharmacology
Increases in lung weight and histopathology (alveolar histiocytosis, edema) were observed in oral toxicity studies in mice, rats, and dogs. At the higher doses tested in short-term studies, alveolar histiocytosis was associated with lung edema, emphysema, or hyalinosis, and with bronchioloalveolar hyperplasia after cessation of dosing in rats and alveolar histiocytosis and hyalinosis in dogs. Effects tended to be absent or less severe after chronic treatment. These findings are considered secondary to increased vascular permeability caused by S1P 1 receptor modulation. The NOAELs for lung findings in the 4-week oral toxicity studies in rats and dogs were associated with plasma exposures (AUC) similar or lower than that expected in humans at the recommended human dose (RHD) of 20 mg/day.
In dogs, coronary arterial lesions (thickening of the vessel wall, hyperplasia/hypertrophy of smooth muscles cells of the tunica media, subendocardial fibrosis) involving the papillary muscle of the left ventricle were observed in oral toxicity studies of 13 to 52 weeks in duration. At the NOAEL (2 mg/kg/day) for these findings, plasma exposures (AUC) were approximately 2 times that expected in humans at the RHD.
CLINICAL STUDIES
The efficacy of PONVORY was demonstrated in Study 1, a randomized, double-blind, parallel group, active-controlled superiority study in patients with relapsing forms of MS (NCT02425644). Patients were treated for 108 weeks. This study included patients who had an Expanded Disability Status Scale (EDSS) score of 0 to 5.5 at baseline, had experienced at least one relapse within the year prior, or two relapses within the prior 2 years, or who had at least one gadolinium-enhancing (Gd-enhancing) lesion on a brain MRI within the prior 6 months or at baseline. Patients with primary progressive MS were excluded.
Patients were randomized to receive either once daily PONVORY, beginning with a 14-day dose titration [see Dosage and Administration (2.2) ] or teriflunomide 14 mg. Neurological evaluations were performed at baseline, every 3 months during the study, and at the time of a suspected relapse. Brain MRI scans were performed at baseline and at Weeks 60 and 108.
The primary endpoint was the annualized relapse rate (ARR) over the study period. Additional outcome measures included: 1) the number of new Gd-enhancing T1 lesions from baseline to Week 108, 2) the number of new or enlarging T2 lesions (without double-counting of lesions) from baseline to Week 108, and 3) the time to 3-month and 6-month confirmed disability progression. A confirmed disability progression was defined as an increase of at least 1.5 in EDSS for patients with a baseline EDSS score of 0, an increase of at least 1.0 in EDSS for patients with a baseline EDSS score of 1.0 to 5.0, or an increase of at least 0.5 in EDSS for patients with a baseline EDSS score at least 5.5, which was confirmed after 3 months and 6 months.
A total of 1133 patients were randomized to either PONVORY (N=567) or teriflunomide 14 mg (N=566); 86.4% of PONVORY-treated patients and 87.5% of teriflunomide 14 mg-treated patients completed the study as per protocol. At baseline, the mean age of patients was 37 years, 97% were White, and 65% were female. The mean disease duration was 7.6 years, the mean number of relapses in the previous year was 1.3, and the mean EDSS score was 2.6; 57% of patients had not received any prior non-steroid treatments for MS. At baseline, 42.6% of patients had one or more Gd-enhancing T1 lesions (mean 2.0) on their baseline MRI scan.
The ARR was statistically significantly lower in patients treated with PONVORY than in patients who received teriflunomide 14 mg. The number of Gd-enhancing T1 lesions and the number of new or enlarging T2 lesions were statistically significantly lower in patients treated with PONVORY than in patients who received teriflunomide 14 mg.
There was no statistically significant difference in the 3-month and 6-month confirmed disability progression outcomes between PONVORY- and teriflunomide 14 mg-treated patients over 108 weeks.
The efficacy results for Study 1 are presented in Table 4.
| Endpoints | PONVORY 20 mg N=567 | Teriflunomide 14 mg N=566 |
|---|---|---|
| All analyses are based on the full analysis set (FAS), which includes all randomized patients. N refers to the number of patients included in the FAS, per treatment group. | ||
| Clinical Endpoints | ||
| Annualized Relapse Rate Defined as confirmed relapses per year through the study period (Negative binomial regression model with stratification variables (EDSS ≤ 3.5 versus EDSS > 3.5; non-steroid treatment for MS within last 2 years prior to randomization [Yes/No]) and the number of relapses in the year prior to study entry (<=1, >=2) as covariates) | 0.202 | 0.290 |
| Relative reduction | 30.5% (p=0.0003) | |
| Percentage of patients without relapse Over the study period of approximately 108 weeks | 70.7% | 60.6% |
| Proportion of Patients with 3-month Confirmed Disability Progression Disability progression defined as 1.5-point increase in EDSS for patients with a baseline EDSS score of 0, 1.0-point increase in EDSS for patients with a baseline EDSS score of 1.0 to 5.0, or 0.5-point increase in EDSS for patients with a baseline EDSS score at least 5.5 confirmed 3 months later. Proportion of patients with 3-month confirmed disability progression refers to Kaplan-Meier estimates at Week 108. | 10.8% | 13.2% |
| Hazard Ratio Defined as time to 3 months confirmed disability progression through the study period (Stratified Cox proportional hazard model, p-value based on the stratified log rank test) | 0.83 (p=0.29) Not statistically significant | |
| MRI Endpoints , Cumulative number of combined unique active lesions (CUALs), defined as new or enlarging T2 lesions or Gd-enhancing T1 lesions (without double counting), mean lesions per year were 1.41 on ponesimod 20 mg (N=539), and 3.16 on teriflunomide 14 mg (N=536), a relative reduction of 56% (p<0.0001). | ||
| Mean number of new or enlarging T2 hyperintense lesions per year | 1.40 | 3.16 |
| Relative reduction | 55.7% (p <.0001) | |
| Mean number of T1 Gd-enhancing lesions per MRI | 0.18 | 0.43 |
| Relative reduction | 58.5% (p <.0001) | |
A similar effect of PONVORY on the ARR and secondary MRI outcomes compared to teriflunomide 14 mg was observed in exploratory subgroups defined by age, gender, prior non-steroid therapy for MS, and baseline disease activity.
HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
PONVORY ® (ponesimod) tablet is available as round, biconvex, film-coated tablets supplied in the following dosage strengths and package configurations.
Starter Pack
| Tablet Strength | Tablet Color | Tablet Size | Tablet Debossing | Pack Size | NDC Code |
|---|---|---|---|---|---|
| 2 mg | White | 5.0 mm | "2" on one side and an arch on the other side. | Child Resistant Starter Pack (14 tablets) | NDC 50458-707-14 |
| 3 mg | Red | 5.0 mm | "3" on one side and an arch on the other side. | ||
| 4 mg | Purple | 5.0 mm | "4" on one side and an arch on the other side. | ||
| 5 mg | Green | 8.6 mm | "5" on one side and an arch and an "A" on the other side. | ||
| 6 mg | White | 8.6 mm | " 6 " on one side and an arch and an "A" on the other side. | ||
| 7 mg | Red | 8.6 mm | "7" on one side and an arch and an "A" on the other side. | ||
| 8 mg | Purple | 8.6 mm | "8" on one side and an arch and an "A" on the other side. | ||
| 9 mg | Brown | 8.6 mm | " 9 " on one side and an arch and an "A" on the other side. | ||
| 10 mg | Orange | 8.6 mm | "10" on one side and an arch and an "A" on the other side. |
Maintenance Dose Bottle
| Tablet Strength | Tablet Color | Tablet Size | Tablet Debossing | Pack Size | NDC Code |
|---|---|---|---|---|---|
| 20 mg | Yellow | 8.6 mm | "20" on one side and an arch and an "A" on the other side. | Bottle of 30 tablets with child-resistant closure. Each bottle contains a desiccant sachet. | NDC 50458-720-30 |
Storage and Handling
Starter Pack
Store at 20ºC to 25ºC (68ºF to 77ºF); excursions permitted from 15ºC to 30ºC (59ºF to 86ºF) [see USP Controlled Room Temperature] .
Store in the original package.
Maintenance Dose Bottle
Store at 20ºC to 25ºC (68ºF to 77ºF); excursions permitted from 15ºC to 30ºC (59ºF to 86ºF) [see USP Controlled Room Temperature] .
Store in the original package. Do not discard desiccant. Protect from moisture.
Keep out of reach of children.
Mechanism of Action
Ponesimod is a sphingosine 1-phosphate (S1P) receptor 1 modulator that binds with high affinity to S1P receptor 1.
Ponesimod blocks the capacity of lymphocytes to egress from lymph nodes, reducing the number of lymphocytes in peripheral blood. The mechanism by which ponesimod exerts therapeutic effects in multiple sclerosis is unknown, but may involve reduction of lymphocyte migration into the central nervous system.