Prednisone
Prednisone Prescribing Information
Primary or secondary adrenocortical insufficiency (hydrocortisone or cortisone is the first choice; synthetic analogs may be used in conjunction with mineralocorticoids where applicable; in infancy mineralocorticoid supplementation is of particular importance)
Congenital adrenal hyperplasia
Nonsuppurative thyroiditis
Hypercalcemia associated with cancer
As adjunctive therapy for short-term administration (to tide the patient over an acute episode or exacerbation) in:
Psoriatic arthritis
Rheumatoid arthritis, including juvenile rheumatoid arthritis (selected cases may require low-dose maintenance therapy)
Ankylosing spondylitis
Acute and subacute bursitis
Acute non-specific tenosynovitis
Acute gouty arthritis
Post-traumatic osteoarthritis
Synovitis of osteoarthritis
Epicondylitis
During an exacerbation or as maintenance therapy in selected cases of:
Systemic lupus erythematosus
Acute rheumatic carditis
Pemphigus
Bullous dermatitis herpetiformis
Severe erythema multiforme (Stevens-Johnson syndrome)
Exfoliative dermatitis
Mycosis fungoides
Severe psoriasis
Severe seborrheic dermatitis
Control of severe or incapacitating allergic conditions intractable to adequate trials of conventional treatment: Seasonal or perennial allergic rhinitis
Serum sickness
Bronchial asthma
Contact dermatitis
Atopic dermatitis
Drug hypersensitivity reactions
Severe acute and chronic allergic and inflammatory processes involving the eye and its adnexa such as:
Allergic conjunctivitis
Keratitis
Allergic corneal marginal ulcers
Herpes zoster ophthalmicus
Iritis and iridocyclitis
Chorioretinitis
Anterior segment inflammation
Diffuse posterior uveitis and choroiditis
Optic neuritis
Sympathetic ophthalmia.
Symptomatic sarcoidosis
Loeffler’s syndrome not manageable by other means
Berylliosis
Fulminating or disseminated pulmonary tuberculosis when used concurrently with appropriate antituberculous chemotherapy
Aspiration pneumonitis
Idiopathic thrombocytopenic purpura in adults
Secondary thrombocytopenia in adults
Acquired (autoimmune) hemolytic anemia
Erythroblastopenia (RBC anemia)
Congenital (erythroid) hypoplastic anemia
For palliative management of:
Leukemias and lymphomas in adults
Acute leukemia of childhood
To induce a diuresis or remission of proteinuria in the nephrotic syndrome, without uremia, of the idiopathic type or that due to lupus erythematosus.
To tide the patient over a critical period of the disease in:
Ulcerative colitis
Regional enteritis
Tuberculous meningitis with subarachnoid block or impending block when used concurrently with appropriate anti-tuberculous chemotherapy
Trichinosis with neurologic or myocardial involvement
Dosage of prednisone tablets should be individualized according to the severity of the disease and the response of the patient. For infants and children, the recommended dosage should be governed by the same considerations rather than strict adherence to the ratio indicated by age or body weight.
Hormone therapy is an adjunct to, and not a replacement for, conventional therapy.
Dosage should be decreased or discontinued gradually when the drug has been administered for more than a few days.
The severity, prognosis, expected duration of the disease, and the reaction of the patient to medication are primary factors in determining dosage.
If a period of spontaneous remission occurs in a chronic condition, treatment should be discontinued.
Blood pressure, body weight, routine laboratory studies, including two hour postprandial blood glucose and serum potassium, and a chest X-ray should be obtained at regular intervals during prolonged therapy. Upper GI X-rays are desirable in patients with known or suspected peptic ulcer disease.
The initial dosage of prednisone may vary from 5 mg to 60 mg per day, depending on the specific disease entity being treated. In situations of less severity lower doses will generally suffice, while in selected patients’ higher initial doses may be required. The initial dosage should be maintained or adjusted until a satisfactory response is noted. If after a reasonable period of time there is a lack of satisfactory clinical response, prednisone should be discontinued, and the patient transferred to other appropriate therapy.
Prednisone tablets are contraindicated in systemic fungal infections.
Sodium retention
Fluid retention
Congestive heart failure in susceptible patients
Potassium loss
Hypokalemic alkalosis
Hypertension
Muscle weakness
Steroid myopathy
Loss of muscle mass
Osteoporosis
Tendon rupture, particularly of the Achilles tendon
Vertebral compression fractures
Aseptic necrosis of femoral and humeral heads
Pathologic fracture of long bones
Peptic ulcer with possible perforation and hemorrhage
Pancreatitis
Abdominal distention
Ulcerative esophagitis
Impaired wound healing
Thin fragile skin
Petechiae and ecchymoses
Facial erythema
Increased sweating
May suppress reactions to skin tests
Convulsions
Increased intracranial pressure with papilledema (pseudotumor cerebri) usually after treatment
Vertigo
Headache
Menstrual irregularities
Development of Cushingoid state
Suppression of growth in children;
Secondary adrenocortical and pituitary unresponsiveness, particularly in times of stress, as in trauma, surgery or illness
Decreased carbohydrate tolerance
Manifestations of latent diabetes mellitus
Increased requirements for insulin or oral hypoglycemic agents in diabetics
Posterior subcapsular cataracts
Increased intraocular pressure
Glaucoma
Exophthalmos
Negative nitrogen balance due to protein catabolism.
Urticaria and other allergic, anaphylactic or hypersensitivity reactions
Prednisone, USP is a glucocorticoid. Glucocorticoids are adrenocortical steroids, both naturally occurring and synthetic, that are readily absorbed from the gastrointestinal tract. The chemical formula for prednisone is C21H26O5. Chemically, it is 17,21-dihydroxypregna-1,4-diene- 3,11,20-trione and has the following structure:
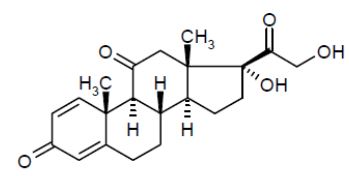
Prednisone, USP is a white or practically white, crystalline powder and has a molecular weight of 358.4 g/mol. It melts at about 234°C. Prednisone, USP is very slightly soluble in water; slightly soluble in alcohol, chloroform, dioxane, and methanol. Prednisone tablets, USP contain 1 mg or 5 mg of prednisone, USP.
The inactive ingredients for prednisone tablets, USP include: lactose monohydrate, magnesium stearate, microcrystalline cellulose, pregelatinized starch, sodium starch glycolate type A and stearic acid.
Meets USP Dissolution Test 2.
Naturally occurring glucocorticoids (hydrocortisone and cortisone), which also have salt-retaining properties, are used as replacement therapy in adrenocortical deficiency states. Their synthetic analogs, such as prednisone, are primarily used for their potent anti-inflammatory effects in disorders of many organ systems.
Glucocorticoids, such as prednisone, cause profound and varied metabolic effects. In addition, they modify the body’s immune response to diverse stimuli.