Get your patient on Soolantra (Ivermectin)
Soolantra prior authorization resources
Most recent state uniform prior authorization forms
Soolantra patient education
Patient toolkit
Dosage & administration
DOSAGE AND ADMINISTRATION
Apply to the affected areas of the face once daily. Use a pea-size amount for each area of the face (forehead, chin, nose, each cheek) that is affected. Spread as a thin layer, avoiding the eyes and lips.
SOOLANTRA cream is not for oral, ophthalmic, or intravaginal use.

By using PrescriberAI, you agree to the AI Terms of Use.
Soolantra prescribing information
INDICATIONS AND USAGE
SOOLANTRA cream is indicated for the treatment of inflammatory lesions of rosacea.
DOSAGE AND ADMINISTRATION
Apply to the affected areas of the face once daily. Use a pea-size amount for each area of the face (forehead, chin, nose, each cheek) that is affected. Spread as a thin layer, avoiding the eyes and lips. SOOLANTRA cream is not for oral, ophthalmic, or intravaginal use.
DOSAGE FORMS AND STRENGTHS
Cream, 1%. Each gram of SOOLANTRA cream contains 10 mg of ivermectin in a white to pale yellow cream base. SOOLANTRA cream is supplied in tubes of 30 g, 45 g and 60 g.
USE IN SPECIFIC POPULATIONS
Pregnancy
Risk Summary The available data on the use of ivermectin, including SOOLANTRA cream, in pregnant women are insufficient to establish a drug- associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. In animal reproduction studies, ivermectin induced adverse developmental outcomes when orally administered to pregnant rats and rabbits during the period of organogenesis at doses 1909 or 354 times the maximum recommended human dose (MRHD), respectively. These orally administered doses were maternally toxic to pregnant rats and rabbits. In a pre-and postnatal developmental study in rats, neonatal toxicity and adverse effects on behavioral development were observed when ivermectin was orally administered to pregnant females during gestation and lactation (see Data). The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data Human Data No adequate and well-controlled trials of Soolantra cream have been conducted in pregnant women. Retrospective observational studies evaluated pregnancy outcomes in over 700 women in various stages of pregnancy who received oral ivermectin for the treatment of soil-transmitted helminths in rural Africa. In an additional, randomized open-label trial, 397 pregnant women in their second trimester received a single dose of oral ivermectin, or ivermectin plus albendazole, for soil-transmitted helminths. When compared with a pregnant, untreated population, no differences in pregnancy outcomes were observed between the treated and untreated populations. These studies cannot definitively establish or exclude any drug-associated risk during pregnancy, because either the timing of administration during gestation was not accurately ascertained or the administration occurred only during the second trimester.
Animal Data Systemic embryofetal development studies were conducted in rats and rabbits. Oral doses of 1.5, 4, and 12mg/kg/day ivermectin were administered during the period of organogenesis to pregnant female rats. Maternal death occurred at 12 mg/kg/day [1909 times the MRHD based on area under the curve (AUC) comparison]. Cleft palate occurred in the fetuses from the 12 mg/kg/day (1909 times the MRHD based on AUC comparison) group. No treatment related embryofetal toxicity or malformations were noted at 4 mg/kg/day (708 times the MRHD based on AUC comparison). Oral doses of 0.5, 1.5, 2.5, 3.5 and 4.5 mg/kg/day ivermectin were administered during the period of organogenesis to pregnant female rabbits. Maternal death occurred at doses ≥ 2.5 mg/kg/day (72 times the MRHD based on AUC comparison). Carpal flexure occurred in the fetuses from the 4.5 mg/kg/day (354 times the MRHD based on AUC comparison) group. Fetal weight decrease was noted at 3.5 mg/kg/day (146 times the MRHD based on AUC comparison). No treatment related embryofetal toxicity or malformations were noted at 2.5 mg/kg/day (72 times the MRHD based on AUC comparison). A pre- and postnatal development study was conducted in rats. Oral doses of 1, 2 and 4 mg/kg/day ivermectin were administered to pregnant female rats during gestational days 6-20 and lactation days 2-20. Neonatal death occurred at doses ≥ 2 mg/kg/day. Behavior development of newborn rats was adversely affected at all doses.
Lactation
Risk Summary The presence of ivermectin in human milk following topical administration of ivermectin has not been evaluated. There are no data available regarding the effects of ivermectin on milk production. Published literature suggests that ivermectin was detectable in human milk in 4 lactating women after a single 150 mcg/kg oral dose of ivermectin. However, there is insufficient information from this report to determine the effects of ivermectin on the breastfed infant. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for Soolantra cream and any potential adverse effects on the breastfed infant from Soolantra cream or from the underlying maternal conditions.
Pediatric Use
Safety and effectiveness of SOOLANTRA cream in pediatric patients have not been established.
Geriatric Use
Of the 1371 subjects in the two pivotal clinical studies of SOOLANTRA cream, 170 (12.4%) were 65 and over, while 37 (2.7%) were 75 and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
CONTRAINDICATIONS
None.
ADVERSE REACTIONS
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. During clinical trials, 2047 subjects with inflammatory lesions of rosacea received SOOLANTRA cream once daily. A total of 1555 subjects were treated once daily for more than 12 weeks, and 519 for approximately one year. Adverse reactions, reported in ≤ 1% of subjects treated with SOOLANTRA cream for at least 3 months in vehicle-controlled clinical trials, included skin burning sensation and skin irritation.
Postmarketing Experience
Because adverse reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. Local adverse reactions: contact dermatitis and allergic dermatitis.
DRUG INTERACTIONS
In vitro studies have shown that SOOLANTRA cream, at therapeutic concentrations, neither inhibits nor induces cytochrome P450 (CYP450) enzymes.
DESCRIPTION
SOOLANTRA (ivermectin) cream, 1% is a white to pale yellow hydrophilic cream intended for topical use. Each gram of SOOLANTRA cream contains 10 mg of ivermectin.
Ivermectin is a semi-synthetic derivative isolated from the fermentation of Streptomyces avermitilis that belongs to the avermectin family of macrocyclic lactones.
Ivermectin is a mixture containing not less than 95.0% and not more than 102.0% of 5-O-demethyl-22,23-dihydroavermectin A 1a plus 5-O-demethyl-25-de(1-methylpropyl)-25-(1-methylethyl)-22,23-dihydroavermectin A 1a , generally referred to as 22,23-dihydroavermectin B 1a and B 1b or H 2 B 1a and H 2 B 1b , respectively; and the ratio (calculated by area percentage) of component H 2 B 1a /(H 2 B 1a + H 2 B 1b ) is not less than 90.0%. The respective empirical formulas of H 2 B 1a and H 2 B 1b are C 48 H 74 O 14 and C 47 H 72 O 14 with molecular weights of 875.10 and 861.07 respectively.
The structural formulas are:
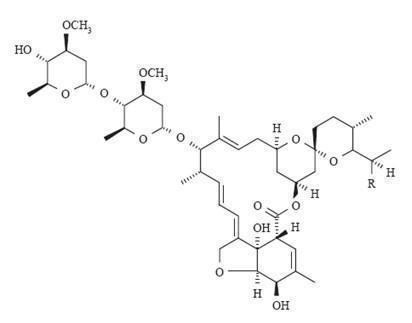
Component H 2 B 1a : R = C 2 H 5 , Component H 2 B 1b : R = CH 3 .
SOOLANTRA cream contains the following inactive ingredients: carbomer copolymer type B, cetyl alcohol, citric acid monohydrate, dimethicone, edetate disodium, glycerin, isopropyl palmitate, methylparaben, oleyl alcohol, phenoxyethanol, polyoxyl 20 cetostearyl ether, propylene glycol, propylparaben, purified water, sodium hydroxide, sorbitan monostearate, and stearyl alcohol.
CLINICAL PHARMACOLOGY
Mechanism of Action
The mechanism of action of SOOLANTRA cream in treating rosacea lesions is unknown.
Pharmacodynamics
Cardiac Electrophysiology At therapeutic doses, SOOLANTRA cream is not expected to prolong QTc interval.
Pharmacokinetics
Absorption The absorption of ivermectin from SOOLANTRA cream was evaluated in a clinical trial in 15 adult male and female subjects with severe papulopustular rosacea applying 1 g SOOLANTRA cream, 1% once daily. At steady state (after 2 weeks of treatment), the highest mean ± standard deviation plasma concentrations of ivermectin peaked (T max ) at 10 ± 8 hours post dose, the maximum concentration (C max ) was 2.10 ± 1.04 ng/mL (range: 0.69 - 4.02 ng/mL) and the area under the concentration curve (AUC 0-24hr ) was 36.14 ± 15.56 ng.hr/mL (range: 13.69-75.16 ng.hr/mL). In addition, systemic exposure assessment in longer treatment duration (Phase 3 studies) showed that there was no plasma accumulation of ivermectin over the 52-week treatment period.
Distribution An in vitro study demonstrated that ivermectin is greater than 99% bound to plasma proteins and is bound primarily to human serum albumin. No significant binding of ivermectin to erythrocytes was observed.
Metabolism In vitro studies using human hepatic microsomes and recombinant CYP450 enzymes have shown that ivermectin is primarily metabolized by CYP3A4. In vitro studies show that ivermectin at therapeutic concentrations does not inhibit the CYP450 isoenzymes 1A2, 2A6, 2B6, 2C8, 2C9, 2C19, 2D6, 2E1, 3A4 or 4A11, or induce 1A2, 2B6, 2C9 or 3A4.
Excretion The apparent terminal half-life averaged 6.5 days (mean ± standard deviation: 155± 40 hours, range 92-238 hours) in patients receiving a once daily cutaneous application of SOOLANTRA cream for 28 days.
NONCLINICAL TOXICOLOGY
Carcinogenesis, Mutagenesis, Impairment of Fertility
In a 2-year dermal mouse carcinogenicity study, ivermectin was administered to CD-1 mice at topical doses of 1, 3, and 10 mg/kg/day (0.1%, 0.3% and 1% ivermectin cream applied at 2 ml/kg/day). No drug-related tumors were noted in this study up to the highest dose evaluated in this study of 10 mg/kg/day (747 times the MRHD based on AUC comparison).
In a 2-year oral rat carcinogenicity study, ivermectin was administered to Wistar rats at gavage doses of 1, 3, and 9 mg/kg/day. A statistically significant increase in the incidence of hepatocellular adenoma was noted in males treated with 9 mg/kg/day (1766 times the MRHD based on AUC comparison) ivermectin. The clinical relevance of this finding is unknown. No drug-related tumors were noted in females up to the highest dose evaluated in this study of 9 mg/kg/day (1959 times the MRHD based on AUC comparison). No drug-related tumors were noted in males at doses ≤ 3 mg/kg/day (599 times the MRHD based on AUC comparison).
Ivermectin revealed no evidence of genotoxic potential based on the results of two in vitro genotoxicity tests (the Ames test and the L5178Y/TK +/- mouse lymphoma assay) and one in vivo genotoxicity test (rat micronucleus assay).
In a fertility study, oral doses of 0.1, 1 and 9 mg/kg/day ivermectin were administered to male and female rats. Mortality occurred at 9 mg/kg/day (1027 times the MRHD based on AUC comparison). The precoital period was generally prolonged at 9 mg/kg/day. No treatment-related effects on fertility or mating performance were noted at doses ≤ 1 mg/kg/day (68 times the MRHD based on AUC comparison).
CLINICAL STUDIES
SOOLANTRA cream applied once daily at bedtime was evaluated in the treatment of inflammatory lesions of rosacea in two randomized, double-blind, vehicle controlled clinical trials, which were identical in design. The trials were conducted in 1371 subjects aged 18 years and older who were treated once daily for 12 weeks with either SOOLANTRA cream or vehicle cream.
Overall, 96% of subjects were Caucasian and 67% were female. Using the 5-point Investigator Global Assessment (IGA) scale (0=clear, 1=almost clear, 2=mild, 3=moderate, 4=severe), 79% of subjects were scored as moderate (IGA=3) and 21% scored as severe (IGA= 4) at baseline.
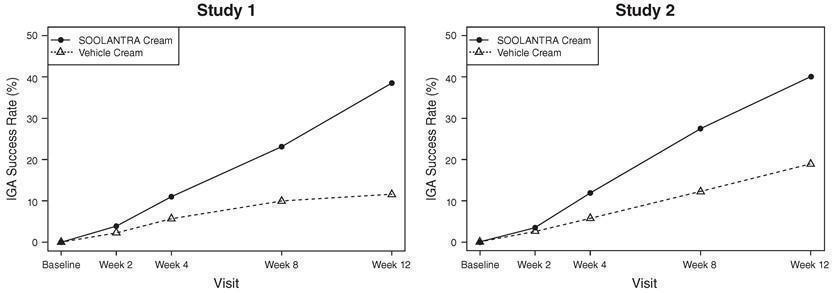
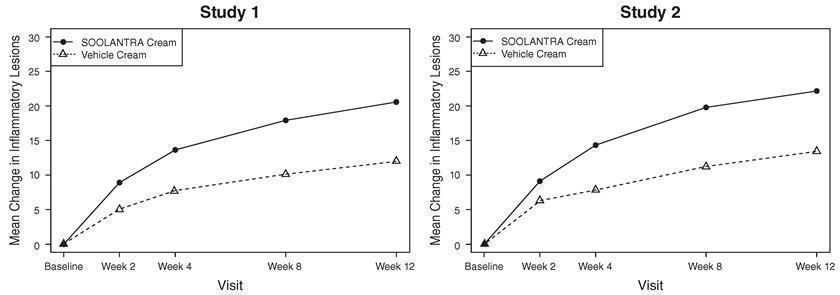
The co-primary efficacy endpoints in both pivotal trials were the success rate based on the IGA outcome (percentage of subjects “clear” and “almost clear”) and absolute change from baseline in inflammatory lesion counts at Week 12. Table 1 presents the co-primary efficacy results at Week 12. SOOLANTRA cream was more effective than vehicle cream on the co-primary efficacy endpoints starting from 4 weeks of treatment in both studies, see Figures 1 through 4.
| Study 1 | Study 2 | |
SOOLANTRA Vehicle Cream (N=451) Cream (N=232) | SOOLANTRA Vehicle Cream (N=459) Cream (N=229) | |
Investigator Global Assessment: Number (%) of Subjects Clear or Almost Clear | 173 (38.4%) 27 (11.6%) | 184 (40.1%) 43 (18.8%) |
| Inflammatory Lesion Counts : Mean Absolute (%) Change from Baseline | 20.5 (64.9%) 12.0 (41.6%) | 22.2 (65.7%) 13.4 (43.4%) |
Figures 1 and 2: IGA Success Rates Over Time
Figures 3 and 4: Mean Absolute Change in Inflammatory Lesion Counts from Baseline Over Time
HOW SUPPLIED/STORAGE AND HANDLING
SOOLANTRA (ivermectin) cream, 1% is a white to pale yellow cream, supplied in a laminated tube with a child resistant cap in the following sizes: 30 gram NDC 0299-3823-30 45 gram NDC 0299-3823-45 60 gram NDC 0299-3823-60
Storage Store at 20°C to 25°C (68°F to 77°F), excursions permitted between 15°C and 30°C (59°F and 86°F) [See USP Controlled Room Temperature].
Mechanism of Action
The mechanism of action of SOOLANTRA cream in treating rosacea lesions is unknown.