Acetazolamide
Acetazolamide Prescribing Information
For adjunctive treatment of: edema due to congestive heart failure; drug-induced edema; centrencephalic epilepsies (petit mal, unlocalized seizures); chronic simple (open-angle) glaucoma, secondary glaucoma, and preoperatively in acute angle-closure glaucoma where delay of surgery is desired in order to lower intraocular pressure. Acetazolamide tablets are also indicated for the prevention or amelioration of symptoms associated with acute mountain sickness in climbers attempting rapid ascent and in those who are very susceptible to acute mountain sickness despite gradual ascent.
Dosage is 500 mg to 1000 mg daily, in divided doses using tablets or sustained-release capsules as appropriate. In circumstances of rapid ascent, such as in rescue or military operations, the higher dose level of 1000 mg is recommended. It is preferable to initiate dosing 24 to 48 hours before ascent and to continue for 48 hours while at high altitude, or longer as necessary to control symptoms.
Note: The dosage recommendations for glaucoma and epilepsy differ considerably from those for congestive heart failure, since the first two conditions are not dependent upon carbonic anhydrase inhibition in the kidney which requires intermittent dosage if it is to recover from the inhibitory effect of the therapeutic agent.
Hypersensitivity to acetazolamide or any excipients in the formulation. Since acetazolamide is a sulfonamide derivative, cross sensitivity between acetazolamide, sulfonamides and other sulfonamide derivatives is possible.
Acetazolamide therapy is contraindicated in situations in which sodium and/or potassium blood serum levels are depressed, in cases of marked kidney and liver disease or dysfunction, in suprarenal gland failure, and in hyperchloremic acidosis. It is contraindicated in patients with cirrhosis because of the risk of development of hepatic encephalopathy.
Long-term administration of acetazolamide is contraindicated in patients with chronic non-congestive angle-closure glaucoma since it may permit organic closure of the angle to occur while the worsening glaucoma is masked by lowered intraocular pressure.
Body as a whole: Headache, malaise, fatigue, fever, pain at injection site, flushing, growth retardation in children, flaccid paralysis, anaphylaxis
Digestive: Gastrointestinal disturbances such as nausea, vomiting, diarrhea
Hematological/Lymphatic: Blood dyscrasias such as aplastic anemia, agranulocytosis, leukopenia, thrombocytopenia, thrombocytopenic purpura, melena
Hepato-biliary disorders: Abnormal liver function, cholestatic jaundice, hepatic insufficiency, fulminant hepatic necrosis
Metabolic/Nutritional: Metabolic acidosis, electrolyte imbalance, including hypokalemia, hyponatremia, osteomalacia with long-term phenytoin therapy, loss of appetite, taste alteration, hyper/hypoglycemia
Nervous: Drowsiness, paraesthesia (including numbness and tingling of extremities and face), depression, excitement, ataxia, confusion, convulsions, dizziness
Skin: Allergic skin reactions including urticaria, photosensitivity, Stevens-Johnson syndrome, toxic epidermal necrolysis
Special senses: Hearing disturbances, tinnitus, transient myopia. Transient myopia is the result of forward movement of the ciliary body leading to a narrowing of the angle.
Urogenital: Crystalluria, increased risk of nephrolithiasis with long-term therapy, hematuria, glycosuria, renal failure, polyuria
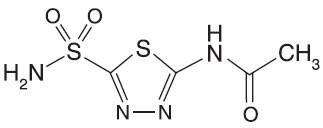
Acetazolamide, an inhibitor of the enzyme carbonic anhydrase is a white to faintly yellowish white crystalline powder, very slightly soluble in water, sparingly soluble in practically boiling water and slightly soluble in alcohol. The chemical name for acetazolamide is
Molecular Weight: 222.25
Molecular Formula: C4H6N4O3S2
Acetazolamide is available as oral tablets containing 125 mg and 250 mg of acetazolamide respectively and the following inactive ingredients: lactose monohydrate, maize starch, magnesium stearate, microcrystalline cellulose, povidone and sodium strach glycolate.
Acetazolamide is a potent carbonic anhydrase inhibitor, effective in the control of fluid secretion (eg, some types of glaucoma), in the treatment of certain convulsive disorders (eg, epilepsy) and in the promotion of diuresis in instances of abnormal fluid retention (eg, cardiac edema).
Acetazolamide is not a mercurial diuretic. Rather, it is a non bacteriostatic sulfonamide possessing a chemical structure and pharmacological activity distinctly different from the bacteriostatic sulfonamides.
Acetazolamide is an enzyme inhibitor that acts specifically on carbonic anhydrase, the enzyme that catalyzes the reversible reaction involving the hydration of carbon dioxide and the dehydration of carbonic acid. In the eye, this inhibitory action of acetazolamide decreases the secretion of aqueous humor and results in a drop in intraocular pressure, a reaction considered desirable incases of glaucoma and even in certain non glaucomatous conditions. Evidence seems to indicate that acetazolamide has utility as an adjuvant in the treatment of certain dysfunctions of the central nervous system (eg, epilepsy). Inhibition of carbonic anhydrase in this area appears to retard abnormal, paroxysmal, excessive discharge from central nervous system neurons. The diuretic effect of acetazolamide is due to its action in the kidney on the reversible reaction involving hydration of carbon dioxide and dehydration of carbonic acid. The result is renal loss of HCO3 ion, which carries out sodium, water, and potassium. Alkalinization of the urine and promotion of diuresis are thus affected. Alteration in ammonia metabolism occurs due to increased reabsorption of ammonia by the renal tubules as a result of urinary alkalinization.
Placebo-controlled clinical trials have shown that prophylactic administration of acetazolamide at a dose of 250 mg every eight to 12 hours (or a 500 mg controlled-release capsule once daily) before and during rapid ascent to altitude results in fewer and/or less severe symptoms (such as headache, nausea, shortness of breath, dizziness, drowsiness, and fatigue) of acute mountain sickness (AMS). Pulmonary function (eg, minute ventilation, expired vital capacity and peak flow) is greater in the acetazolamide treated group, both in subjects with AMS and asymptomatic subjects. The acetazolamide treated climbers also had less difficulty in sleeping.