Acetylcysteine
Acetylcysteine Prescribing Information
Acetylcysteine injection is indicated to prevent or lessen hepatic injury after ingestion of a potentially hepatotoxic quantity of acetaminophen in patients with acute ingestion or from repeated supratherapeutic ingestion (RSI).
The following recommendations are related to acute acetaminophen ingestion. For recommendations related to repeated supratherapeutic exposure see
1. Assess the history and timing of acetaminophen ingestion as an overdose.
- The reported history of the quantity of acetaminophen ingested as an overdose is often inaccurate and is not a reliable guide to therapy.
2. Obtain the following laboratory tests to monitor hepatic and renal function and electrolyte and fluid balance: aspartate aminotransferase (AST), alanine aminotransferase (ALT), bilirubin, international normalized ratio (INR), creatinine, blood urea nitrogen (BUN), blood glucose, and electrolytes.
3. Obtain a plasma or serum sample to assay for acetaminophen concentration at least 4 hours after ingestion. Acetaminophen concentrations obtained earlier than 4 hours post-ingestion may be misleading as they may not represent maximum acetaminophen concentrations.
4. If the time of acute acetaminophen ingestion is unknown:
- Administer a loading dose of acetylcysteine injection immediately[seeDosage and Administration (2.4)].
- Obtain an acetaminophen concentration to determine need for continued treatment[seeDosage and Administration (2.2)].
5. If the acetaminophen concentration cannot be obtained (or is unavailable or uninterpretable) within the 8-hour time interval after acetaminophen ingestion or there is clinical evidence of acetaminophen toxicity:
- Administer a loading dose of acetylcysteine injection immediately and continue treatment for a total of three doses over 21 hours[seeDosage and Administration (2.4)].
6. If the patient presents more than 8 hours after ingestion and the time of acute acetaminophen ingestion is known:
- Administer a loading dose of acetylcysteine injection immediately[seeDosage and Administration (2.4)]
- Obtain an acetaminophen concentration to determine need for continued treatment[seeDosage and Administration (2.2)].
7. If the patient presents less than 8 hours after ingestion and the time of acute acetaminophen ingestion is known and the acetaminophen concentration is known:
- Use the Rumack-Matthew nomogram (Figure 1) to determine whether or not to initiate treatment with acetylcysteine injection[seeDosage and Administration (2.2)].
Obtain a plasma or serum sample to assay for acetaminophen concentration at least 4 hours after ingestion.
- If the time of acetaminophen ingestion is unknown:
- Administer a loading dose of acetylcysteine injection immediately.
- Obtain an acetaminophen concentration to determine need for continued treatment.
- If the acetaminophen concentration cannot be obtained (or is unavailable or uninterpretable) within the 8-hour time interval after acetaminophen ingestion or there is clinical evidence of acetaminophen toxicity:
- Administer a loading dose of acetylcysteine injection immediately and continue treatment for a total of three doses over 21 hours.
- If the patient presents more than 8 hours after ingestion and the time of acute acetaminophen ingestion is known:
- Administer a loading dose of acetylcysteine injection immediately
- Obtain acetaminophen concentration to determine need for continued treatment
- If the patient presents less than 8 hours after ingestion and the time of acute acetaminophen ingestion is known and the acetaminophen concentration is known:
- Use the Rumack-Matthew nomogram (Figure 1) to determine whether or not to initiate treatment with acetylcysteine injection ()
2.2 Nomogram for Estimating Potential for Hepatotoxicity from Acute Acetaminophen Ingestion and Need for Acetylcysteine Injection TreatmentAcetylcysteine injection is an antidote for acetaminophen overdose. The critical ingestion-treatment interval for maximal protection against severe hepatic injury is between 0 to 8 hours. Efficacy diminishes progressively after 8 hours and treatment initiation between 15 and 24 hours post-ingestion of acetaminophen yields limited efficacy. However, it does not appear to worsen the condition of patients and it should not be withheld, since the reported time of ingestion may not be correct.
If the timing of the acute acetaminophen ingestion is known and the results of the acetaminophen assay are available within 8 hours:
- Refer to the Rumack-Matthew nomogram (see Figure 1) to determine whether or not to initiate treatment with acetylcysteine injection.
- Initiation of acetylcysteine injection depends on the plasma or serum acetaminophen concentration and also the clinical presentation of the patient.
The nomogram may underestimate the hepatotoxicity risk in patients with chronic alcoholism, malnutrition, or CYP2E1 enzyme inducing drugs (e.g., isoniazid), and consideration should be given to treating these patients even if the acetaminophen concentrations are in the nontoxic range.
Loading doseFor patients whose acetaminophen concentrations are at or above the “possible” toxicity line (dotted line in nomogram):
- Administer a loading dose of acetylcysteine injection[see Dosage and Administration (2.4)].
For patients with an acute overdose from an extended-release acetaminophen, if the acetaminophen concentration at 4 hours post ingestion is below the possible toxicity line then obtain a second sample for acetaminophen concentration 8 to 10 hours after the acute ingestion. If the second value is at or above the “possible” toxicity line (dotted line in nomogram):
- Administer a loading dose of acetylcysteine injection[see Dosage and Administration (2.4)].
For patients whose values are below the “possible” toxicity line, but time of ingestion was unknown or sample was obtained less than 4 hours after ingestion:
- Administer a loading dose of acetylcysteine injection[see Dosage and Administration (2.4)].
For patients whose values are below the “possible” toxicity line and time of ingestion is known and the sample was obtained more than 4 hours after ingestion, do not administer acetylcysteine injection because there is minimal risk of hepatotoxicity.
Figure 1. Rumack-Matthew Nomogram for Estimating Potential for Hepatotoxicity for Acetaminophen Posioning – Plasma or Serum Acetaminophen Concentration versus Time (hours) Post-acetaminophen Ingestion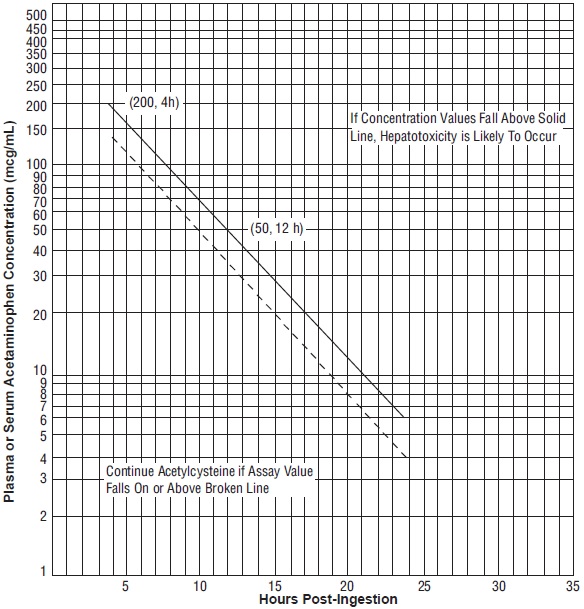
(Adapted from Rumack and Matthew, Pediatrics 1975; 55: 871-876)Maintenance DoseDetermine need for continued treatment with acetylcysteine injection after the loading dose. Choose ONE of the following based on the acetaminophen concentration:
The acetaminophen concentration is above the possible toxicity line according to the nomogram (see Figure 1):
- Continue acetylcysteine injection treatment with the maintenance dose for a total of three separate doses over an infusion period of 21 hours[see Dosage and Administration (2.4)].
- Monitor hepatic and renal function and electrolytes throughout treatment.
The acetaminophen concentration could not be obtained:
- Continue acetylcysteine injection treatment with the maintenance dose for a total of three separate doses over an infusion period of 21 hours[see Dosage and Administration (2.4)].
- Monitor hepatic and renal function and electrolytes throughout treatment.
For patients whose acetaminophen concentration is below the “possible” toxicity line (see Figure 1) and time of ingestion is known and the sample was obtained more than 4 hours after ingestion:
- Discontinue acetylcysteine injection.
The acetaminophen concentration was in the non-toxic range, but time of ingestion was unknown or less than 4 hours:
- Obtain a second sample for acetaminophen concentration and consider the patient’s clinical status to decide whether or not to continue acetylcysteine injection treatment.
- If there is any uncertainty as to patient’s risk of developing hepatotoxicity, it is recommended to administer a complete treatment course.
Continued Therapy After Completion of Loading and Maintenance Doses
In cases of suspected massive overdose, or with concomitant ingestion of other substances, or in patients with preexisting liver disease; the absorption and/or the half-life of acetaminophen may be prolonged. In such cases, consideration should be given to the need for continued treatment with acetylcysteine injection beyond a total of three separate doses over a 21-hour infusion period.
Acetaminophen levels and ALT/AST and INR should be checked after the last maintenance dose. If acetaminophen levels are still detectable, or if the ALT/AST are still increasing or the INR remains elevated; dosing should be continued and the treating physician should contact a US regional poison center at 1-800-222-1222, alternatively, a “special health professional assistance line for acetaminophen overdose” at 1-800-525-6115 for assistance with dosing recommendations, or 1-866-850-2876 for additional information.
Figure 1. Rumack-Matthew Nomogram for Estimating Potential for Hepatotoxicity for Acetaminophen Posioning – Plasma or Serum Acetaminophen Concentration versus Time (hours) Post-acetaminophen Ingestion
- Use the Rumack-Matthew nomogram (Figure 1) to determine whether or not to initiate treatment with acetylcysteine injection (
Acetylcysteine injection is an antidote for acetaminophen overdose. The critical ingestion-treatment interval for maximal protection against severe hepatic injury is between 0 to 8 hours. Efficacy diminishes progressively after 8 hours and treatment initiation between 15 and 24 hours post-ingestion of acetaminophen yields limited efficacy. However, it does not appear to worsen the condition of patients and it should not be withheld, since the reported time of ingestion may not be correct.
If the timing of the acute acetaminophen ingestion is known and the results of the acetaminophen assay are available within 8 hours:
- Refer to the Rumack-Matthew nomogram (see Figure 1) to determine whether or not to initiate treatment with acetylcysteine injection.
- Initiation of acetylcysteine injection depends on the plasma or serum acetaminophen concentration and also the clinical presentation of the patient.
The nomogram may underestimate the hepatotoxicity risk in patients with chronic alcoholism, malnutrition, or CYP2E1 enzyme inducing drugs (e.g., isoniazid), and consideration should be given to treating these patients even if the acetaminophen concentrations are in the nontoxic range.
Loading dose
For patients whose acetaminophen concentrations are at or above the “possible” toxicity line (dotted line in nomogram):
- Administer a loading dose of acetylcysteine injection[see Dosage and Administration (2.4)].
For patients with an acute overdose from an extended-release acetaminophen, if the acetaminophen concentration at 4 hours post ingestion is below the possible toxicity line then obtain a second sample for acetaminophen concentration 8 to 10 hours after the acute ingestion. If the second value is at or above the “possible” toxicity line (dotted line in nomogram):
- Administer a loading dose of acetylcysteine injection[see Dosage and Administration (2.4)].
For patients whose values are below the “possible” toxicity line, but time of ingestion was unknown or sample was obtained less than 4 hours after ingestion:
- Administer a loading dose of acetylcysteine injection[see Dosage and Administration (2.4)].
For patients whose values are below the “possible” toxicity line and time of ingestion is known and the sample was obtained more than 4 hours after ingestion, do not administer acetylcysteine injection because there is minimal risk of hepatotoxicity.

(Adapted from Rumack and Matthew, Pediatrics 1975; 55: 871-876)
Determine need for continued treatment with acetylcysteine injection after the loading dose. Choose ONE of the following based on the acetaminophen concentration:
The acetaminophen concentration is above the possible toxicity line according to the nomogram (see Figure 1):
- Continue acetylcysteine injection treatment with the maintenance dose for a total of three separate doses over an infusion period of 21 hours[see Dosage and Administration (2.4)].
- Monitor hepatic and renal function and electrolytes throughout treatment.
The acetaminophen concentration could not be obtained:
- Continue acetylcysteine injection treatment with the maintenance dose for a total of three separate doses over an infusion period of 21 hours[see Dosage and Administration (2.4)].
- Monitor hepatic and renal function and electrolytes throughout treatment.
For patients whose acetaminophen concentration is below the “possible” toxicity line (see Figure 1) and time of ingestion is known and the sample was obtained more than 4 hours after ingestion:
- Discontinue acetylcysteine injection.
The acetaminophen concentration was in the non-toxic range, but time of ingestion was unknown or less than 4 hours:
- Obtain a second sample for acetaminophen concentration and consider the patient’s clinical status to decide whether or not to continue acetylcysteine injection treatment.
- If there is any uncertainty as to patient’s risk of developing hepatotoxicity, it is recommended to administer a complete treatment course.
In cases of suspected massive overdose, or with concomitant ingestion of other substances, or in patients with preexisting liver disease; the absorption and/or the half-life of acetaminophen may be prolonged. In such cases, consideration should be given to the need for continued treatment with acetylcysteine injection beyond a total of three separate doses over a 21-hour infusion period.
Acetaminophen levels and ALT/AST and INR should be checked after the last maintenance dose. If acetaminophen levels are still detectable, or if the ALT/AST are still increasing or the INR remains elevated; dosing should be continued and the treating physician should contact a US regional poison center at 1-800-222-1222, alternatively, a “special health professional assistance line for acetaminophen overdose” at 1-800-525-6115 for assistance with dosing recommendations, or 1-866-850-2876 for additional information.

- See full prescribing information for instructions on how to use the nomogram to determine the need for dosing.
Because acetylcysteine injection is hyperosmolar (2,600 mOsmol/L), acetylcysteine injection must be diluted in sterile water for injection, 0.45% sodium chloride injection (1/2 normal saline), or 5% dextrose in water prior to intravenous administration
Visually inspect for particular matter and discoloration prior to administration. The color of the diluted solution ranges from colorless to a slight pink or purple once the stopper is punctured (the color change does not affect the quality of the product). The diluted solution can be stored for 24 hours at room temperature. Discard unused portion. If a vial was previously opened, do not use for intravenous administration.
| * Adjust osmolarity to a physiologically safe level (generally not less than 150 mOsmol/L in pediatric patients). | |||
| Acetylcysteine Injection Concentration | Osmolarity | ||
| Sterile Water for Injection | ½ Normal Saline | D5W | |
| 7 mg/mL | 91 mOsmol/L* | 245 mOsmol/L | 343 mOsmol/L |
| 24 mg/mL | 312 mOsmol/L | 466 mOsmol/L | 564 mOsmol/L |
Acetylcysteine injection is hyperosmolar (2,600 mOsmol/L), therefore acetylcysteine injection must be diluted in sterile water for injection, 0.45% sodium chloride injection, or 5% dextrose in water injection prior to intravenous administration. See full prescribing information for examples of osmolarity depending on the type of solution and acetylcysteine injection concentration.
Dosage Regimen
The total recommended dosage of acetylcysteine injection is 300 mg/kg given intravenously as 3 separate, sequential doses (i.e., 3-bag method to administer the loading, second, and third doses). The total recommended infusion time for 3 doses is 21 hours. For the recommended weight-based dosage and weight-based dilution in patients who weigh:
- 5 to 20 kg (see Table 2)
- 21 to 40 kg (see Table 3)
- 41 kg or greater (see Table 4)
| *Dilute acetylcysteine injection in one of the following three solutions: sterile water for injection, 0.45% sodium chloride injection, or 5% dextrose in water. **Recommended dosing for those less than 5 kg has not been studied. | ||||||
| Body Weight | Bag 1 (Loading Dose) 150 mg/kg in 3 mL/kg of diluent* infused over 1 hour | Bag 2 (Second Dose) 50 mg/kg in 7 mL/kg of diluent* infused over 4 hours | Bag 3 (Third Dose) 100 mg/kg diluted in 14 mL/kg of diluent* infused over 16 hours | |||
| Loading Dose | Diluent Volume | Second Dose | Diluent Volume | Third Dose | Diluent Volume | |
| 5 kg** | 750 mg | 15 mL | 250 mg | 35 mL | 500 mg | 70 mL |
| 10 kg | 1,500 mg | 30 mL | 500 mg | 70 mL | 1,000 mg | 140 mL |
| 15 kg | 2,250 mg | 45 mL | 750 mg | 105 mL | 1,500 mg | 210 mL |
| 20 kg | 3,000 mg | 60 mL | 1,000 mg | 140 mL | 2,000 mg | 280 mL |
| * Dilute acetylcysteine injection in one of the following three solutions: sterile water for injection, 0.45% sodium chloride injection, or 5% dextrose in water. | |||
| Body Weight | Bag 1 (Loading Dose) 150 mg/kg in 100 mL of diluent* infused over 1 hour | Bag 2 (Second Dose) 50 mg/kg in 250 mL of diluent* infused over 4 hours | Bag 3 (Third Dose) 100 mg/kg in 500 mL of diluent* infused over 16 hours |
| 21 kg | 3,150 mg | 1,050 mg | 2,100 mg |
| 30 kg | 4,500 mg | 1,500 mg | 3,000 mg |
| 40 kg | 6,000 mg | 2,000 mg | 4,000 mg |
| * Dilute acetylcysteine injection in one of the following three solutions: sterile water for injection, 0.45% sodium chloride injection, or 5% dextrose in water. ** No specific studies have been conducted to evaluate the necessity of dose adjustments in patients weighing over 100 kg. Limited information is available regarding the dosing requirements of patients that weigh more than 100 kg. | |||||||||||||||||||||||||||||||
| Body Weight | Bag 1 (Loading Dose) 150 mg/kg in 200 mL of diluent*infused over 1 hour | Bag 2 (Second Dose) 50 mg/kg in 500 mL of diluent*infused over 4 hours | Bag 3 (Third Dose) 100 mg/kg in 1,000 mL of diluent*infused over 16 hours | ||||||||||||||||||||||||||||
| 41 kg | 6,150 mg | 2,050 mg | 4,100 mg | ||||||||||||||||||||||||||||
| 50 kg | 7,500 mg | 2,500 mg | 5,000 mg | ||||||||||||||||||||||||||||
| 60 kg | 9,000 mg | 3,000 mg | 6,000 mg | ||||||||||||||||||||||||||||
| 70 kg | 10,500 mg | 3,500 mg | 7,000 mg | ||||||||||||||||||||||||||||
| 80 kg | 12,000 mg | 4,000 mg | 8,000 mg | ||||||||||||||||||||||||||||
| 90 kg | 13,500 mg | 4,500 mg | 9,000 mg | ||||||||||||||||||||||||||||
| ≥ 100 kg** | 15,000 mg | 5,000 mg | 10,000 mg | ||||||||||||||||||||||||||||
- Acetylcysteine injection is for intravenous administration only
- Total dosage of acetylcysteine injection is 300 mg/kg given intravenously as 3 separate doses and total recommended infusion time for 3 doses is 21 hours
- See full prescribing information for weight-based dosage and weight-based dilution ()
2.4 Recommended Dosage in Adults and Pediatrics for Acute Acetaminophen IngestionAcetylcysteine Injection is for intravenous administration only.
Dosage Regimen
The total recommended dosage of acetylcysteine injection is 300 mg/kg given intravenously as 3 separate, sequential doses (i.e., 3-bag method to administer the loading, second, and third doses). The total recommended infusion time for 3 doses is 21 hours. For the recommended weight-based dosage and weight-based dilution in patients who weigh:- 5 to 20 kg (see Table 2)
- 21 to 40 kg (see Table 3)
- 41 kg or greater (see Table 4)
Table 2. Recommended Acetylcysteine Injection Dosage and Dilution for Patients 5 kg to 20 kg *Dilute acetylcysteine injection in one of the following three solutions: sterile water for injection, 0.45% sodium chloride injection, or 5% dextrose in water.
**Recommended dosing for those less than 5 kg has not been studied.Body Weight Bag 1 (Loading Dose)150 mg/kg in 3 mL/kg of diluent* infused over 1 hourBag 2 (Second Dose)
50 mg/kg in 7 mL/kg of diluent* infused over 4 hoursBag 3 (Third Dose)
100 mg/kg diluted in 14 mL/kg of diluent* infused over 16 hoursLoading Dose Diluent Volume Second Dose Diluent Volume Third
DoseDiluent
Volume5 kg** 750 mg 15 mL 250 mg 35 mL 500 mg 70 mL 10 kg 1,500 mg 30 mL 500 mg 70 mL 1,000 mg 140 mL 15 kg 2,250 mg 45 mL 750 mg 105 mL 1,500 mg 210 mL 20 kg 3,000 mg 60 mL 1,000 mg 140 mL 2,000 mg 280 mL Table 3. Recommended Acetylcysteine Injection Dosage and Dilution for Patients 21 kg to 40 kg * Dilute acetylcysteine injection in one of the following three solutions: sterile water for injection, 0.45% sodium chloride injection, or 5% dextrose in water. Body Weight Bag 1 (Loading Dose)
150 mg/kg in 100 mL of diluent* infused over 1 hourBag 2 (Second Dose)
50 mg/kg in 250 mL of diluent* infused over 4 hoursBag 3 (Third Dose)
100 mg/kg in 500 mL of diluent* infused over 16 hours21 kg 3,150 mg 1,050 mg 2,100 mg 30 kg 4,500 mg 1,500 mg 3,000 mg 40 kg 6,000 mg 2,000 mg 4,000 mg Table 4. Recommended Acetylcysteine Injection Dosage and Dilution for Patients 41 kg or Greater * Dilute acetylcysteine injection in one of the following three solutions: sterile water for injection, 0.45% sodium chloride injection, or 5% dextrose in water.
** No specific studies have been conducted to evaluate the necessity of dose adjustments in patients weighing over 100 kg. Limited information is available regarding the dosing requirements of patients that weigh more than 100 kg.Body Weight Bag 1 (Loading Dose)150 mg/kg in 200 mL of diluent*infused over 1 hourBag 2 (Second Dose)
50 mg/kg in 500 mL of diluent*infused over 4 hoursBag 3 (Third Dose)
100 mg/kg in 1,000 mL of diluent*infused over 16 hours41 kg 6,150 mg 2,050 mg 4,100 mg 50 kg 7,500 mg 2,500 mg 5,000 mg 60 kg 9,000 mg 3,000 mg 6,000 mg 70 kg 10,500 mg 3,500 mg 7,000 mg 80 kg 12,000 mg 4,000 mg 8,000 mg 90 kg 13,500 mg 4,500 mg 9,000 mg ≥ 100 kg** 15,000 mg 5,000 mg 10,000 mg - See full prescribing information for recommendations for continuing acetylcysteine injection treatment after 21 hours ()
2.2 Nomogram for Estimating Potential for Hepatotoxicity from Acute Acetaminophen Ingestion and Need for Acetylcysteine Injection TreatmentAcetylcysteine injection is an antidote for acetaminophen overdose. The critical ingestion-treatment interval for maximal protection against severe hepatic injury is between 0 to 8 hours. Efficacy diminishes progressively after 8 hours and treatment initiation between 15 and 24 hours post-ingestion of acetaminophen yields limited efficacy. However, it does not appear to worsen the condition of patients and it should not be withheld, since the reported time of ingestion may not be correct.
If the timing of the acute acetaminophen ingestion is known and the results of the acetaminophen assay are available within 8 hours:
- Refer to the Rumack-Matthew nomogram (see Figure 1) to determine whether or not to initiate treatment with acetylcysteine injection.
- Initiation of acetylcysteine injection depends on the plasma or serum acetaminophen concentration and also the clinical presentation of the patient.
The nomogram may underestimate the hepatotoxicity risk in patients with chronic alcoholism, malnutrition, or CYP2E1 enzyme inducing drugs (e.g., isoniazid), and consideration should be given to treating these patients even if the acetaminophen concentrations are in the nontoxic range.
Loading doseFor patients whose acetaminophen concentrations are at or above the “possible” toxicity line (dotted line in nomogram):
- Administer a loading dose of acetylcysteine injection[see Dosage and Administration (2.4)].
For patients with an acute overdose from an extended-release acetaminophen, if the acetaminophen concentration at 4 hours post ingestion is below the possible toxicity line then obtain a second sample for acetaminophen concentration 8 to 10 hours after the acute ingestion. If the second value is at or above the “possible” toxicity line (dotted line in nomogram):
- Administer a loading dose of acetylcysteine injection[see Dosage and Administration (2.4)].
For patients whose values are below the “possible” toxicity line, but time of ingestion was unknown or sample was obtained less than 4 hours after ingestion:
- Administer a loading dose of acetylcysteine injection[see Dosage and Administration (2.4)].
For patients whose values are below the “possible” toxicity line and time of ingestion is known and the sample was obtained more than 4 hours after ingestion, do not administer acetylcysteine injection because there is minimal risk of hepatotoxicity.
Figure 1. Rumack-Matthew Nomogram for Estimating Potential for Hepatotoxicity for Acetaminophen Posioning – Plasma or Serum Acetaminophen Concentration versus Time (hours) Post-acetaminophen Ingestion
(Adapted from Rumack and Matthew, Pediatrics 1975; 55: 871-876)Maintenance DoseDetermine need for continued treatment with acetylcysteine injection after the loading dose. Choose ONE of the following based on the acetaminophen concentration:
The acetaminophen concentration is above the possible toxicity line according to the nomogram (see Figure 1):
- Continue acetylcysteine injection treatment with the maintenance dose for a total of three separate doses over an infusion period of 21 hours[see Dosage and Administration (2.4)].
- Monitor hepatic and renal function and electrolytes throughout treatment.
The acetaminophen concentration could not be obtained:
- Continue acetylcysteine injection treatment with the maintenance dose for a total of three separate doses over an infusion period of 21 hours[see Dosage and Administration (2.4)].
- Monitor hepatic and renal function and electrolytes throughout treatment.
For patients whose acetaminophen concentration is below the “possible” toxicity line (see Figure 1) and time of ingestion is known and the sample was obtained more than 4 hours after ingestion:
- Discontinue acetylcysteine injection.
The acetaminophen concentration was in the non-toxic range, but time of ingestion was unknown or less than 4 hours:
- Obtain a second sample for acetaminophen concentration and consider the patient’s clinical status to decide whether or not to continue acetylcysteine injection treatment.
- If there is any uncertainty as to patient’s risk of developing hepatotoxicity, it is recommended to administer a complete treatment course.
Continued Therapy After Completion of Loading and Maintenance Doses
In cases of suspected massive overdose, or with concomitant ingestion of other substances, or in patients with preexisting liver disease; the absorption and/or the half-life of acetaminophen may be prolonged. In such cases, consideration should be given to the need for continued treatment with acetylcysteine injection beyond a total of three separate doses over a 21-hour infusion period.
Acetaminophen levels and ALT/AST and INR should be checked after the last maintenance dose. If acetaminophen levels are still detectable, or if the ALT/AST are still increasing or the INR remains elevated; dosing should be continued and the treating physician should contact a US regional poison center at 1-800-222-1222, alternatively, a “special health professional assistance line for acetaminophen overdose” at 1-800-525-6115 for assistance with dosing recommendations, or 1-866-850-2876 for additional information.
Figure 1. Rumack-Matthew Nomogram for Estimating Potential for Hepatotoxicity for Acetaminophen Posioning – Plasma or Serum Acetaminophen Concentration versus Time (hours) Post-acetaminophen Ingestion
Repeated supratherapeutic acetaminophen ingestion (RSI) is an ingestion of acetaminophen at dosages higher than those recommended for extended periods of time. The risk of hepatotoxicity and the recommendations for treatment of acute acetaminophen ingestion (i.e., the Rumack-Matthew nomogram) do not apply to patients with RSI. Therefore, obtain the following information to guide acetylcysteine injection treatment for RSI:
- Acetaminophen serum or plasma concentrations. A reported history of the quantity of acetaminophen ingested is often inaccurate and is not a reliable guide to therapy.
- Laboratory tests to monitor hepatic and renal function and electrolyte and fluid balance: AST, ALT, bilirubin, INR, creatinine, BUN, blood glucose, and electrolytes.
For specific acetylcysteine injection dosage and administration information in patients with RSI, consider contacting your regional poison center at 1-800-222-1222, or alternatively, a special health professional assistance line for acetaminophen overdose at 1-800-525-6115.
- Obtain acetaminophen concentration and other laboratory tests to guide treatment; Rumack-Matthew nomogram does not apply.
Injection: 200 mg/mL (6 grams of acetylcysteine in 30 mL) in a single-dose vial.
Limited published case reports and case series of pregnant women exposed to acetylcysteine during various trimesters are not sufficient to inform any drug associated risk. Delaying treatment of acetaminophen overdose may increase the risk of maternal or fetal morbidity and mortality
Acetaminophen and acetylcysteine cross the placenta. Delaying treatment in pregnant women with acetaminophen overdose and potentially toxic acetaminophen plasma levels may increase the risk of maternal and fetal morbidity and mortality.
Data
Animal Data
Reproduction studies have been performed following administration of acetylcysteine during the period of organogenesis in rats at oral doses up to 2,000 mg/kg/day (1.1 times the recommended total human intravenous dose of 300 mg/kg based on body surface area comparison) and in rabbits at oral doses up to 1,000 mg/kg/day (1.1 times the recommended total human intravenous dose of 300 mg/kg based on body surface area comparison). No adverse developmental outcomes due to acetylcysteine were observed.
Acetylcysteine injection is contraindicated in patients with a previous hypersensitivity reaction to acetylcysteine
Serious acute hypersensitivity reactions during acetylcysteine administration including rash, hypotension, wheezing, and/or shortness of breath, have been observed in patients receiving intravenous acetylcysteine for acetaminophen overdose and occurred soon after initiation of the infusion
One patient with asthma developed bronchospasm and died after intravenous administration of acetylcysteine. Acetylcysteine should be used with caution in patients with asthma, or where there is a history of bronchospasm. Patients with asthma should be closely monitored during initiation of acetylcysteine therapy and throughout acetylcysteine therapy.
Acute flushing and erythema of the skin may occur in patients receiving acetylcysteine intravenously. These reactions usually occur 30 to 60 minutes after initiating the infusion and often resolve spontaneously despite continued infusion of acetylcysteine. If a reaction to acetylcysteine involves more than simply flushing and erythema of the skin, it should be treated as a hypersensitivity reaction.
Management of less severe hypersensitivity reactions should be based upon the severity of the reaction and include temporary interruption of the infusion and/or administration of antihistaminic drugs. The acetylcysteine infusion may be carefully restarted after treatment of the hypersensitivity symptoms has been initiated; however, if the hypersensitivity reaction returns upon re-initiation of treatment or increases in severity, acetylcysteine should be discontinued and alternative patient management should be considered.
- Hypersensitivity Reactions, Including Hypotension, Wheezing, Shortness of Breath and Bronchospasm:Observe patients during and after the infusion; immediately discontinue infusion if a serious reaction occurs and initiate appropriate treatment. Acetylcysteine infusion may be carefully restarted after treatment of hypersensitivity has been initiated ().
5.1 Hypersensitivity ReactionsSerious acute hypersensitivity reactions during acetylcysteine administration including rash, hypotension, wheezing, and/or shortness of breath, have been observed in patients receiving intravenous acetylcysteine for acetaminophen overdose and occurred soon after initiation of the infusion
[see Adverse Reactions (6.1)]. If a severe hypersensitivity reaction occurs, immediately stop the infusion of acetylcysteine and initiate appropriate treatment.
One patient with asthma developed bronchospasm and died after intravenous administration of acetylcysteine. Acetylcysteine should be used with caution in patients with asthma, or where there is a history of bronchospasm. Patients with asthma should be closely monitored during initiation of acetylcysteine therapy and throughout acetylcysteine therapy.
Acute flushing and erythema of the skin may occur in patients receiving acetylcysteine intravenously. These reactions usually occur 30 to 60 minutes after initiating the infusion and often resolve spontaneously despite continued infusion of acetylcysteine. If a reaction to acetylcysteine involves more than simply flushing and erythema of the skin, it should be treated as a hypersensitivity reaction.
Management of less severe hypersensitivity reactions should be based upon the severity of the reaction and include temporary interruption of the infusion and/or administration of antihistaminic drugs. The acetylcysteine infusion may be carefully restarted after treatment of the hypersensitivity symptoms has been initiated; however, if the hypersensitivity reaction returns upon re-initiation of treatment or increases in severity, acetylcysteine should be discontinued and alternative patient management should be considered. - Fluid Overload: Total volume administered should be reduced for patients weighing less than 40 kg and for those requiring fluid restriction ().
5.2 Fluid OverloadThe total volume of acetylcysteine administered should be adjusted for patients less than 40 kg and for those requiring fluid restriction. To avoid fluid overload, the volume of diluent should be reduced as needed
[see Dosage and Administration (2)]. If volume is not adjusted fluid overload can occur, potentially resulting in hyponatremia, seizure and death.
Intravenous administration of acetylcysteine can cause fluid overload, potentially resulting in hyponatremia, seizure and death. To avoid fluid overload, use the recommended dilution shown in Tables 2, 3 and 4[see Dosage and Administration (2.4)].