Acyclovir
Acyclovir Prescribing Information
The frequency and severity of episodes of untreated genital herpes may change over time. After 1 year of therapy, the frequency and severity of the patient's genital herpes infection should be re-evaluated to assess the need for continuation of therapy with acyclovir.
Intravenous acyclovir is indicated for the treatment of varicella-zoster infections in immunocompromised patients.
When therapy is indicated, it should be initiated at the earliest sign or symptom of chickenpox. There is no information about the efficacy of therapy initiated more than 24 hours after onset of signs and symptoms.
Table 3. Dosage Modification for Renal Impairment | |||
Normal Dosage Regimen | Creatinine Clearance (mL/min/1.73 m 2) | Adjusted Dosage Regimen | |
Dose (mg) | Dosing Interval | ||
200 mg every 4 hours | >10 | 200 | every 4 hours, 5x daily |
400 mg every 12 hours | >10 | 400 | every 12 hours |
800 mg every 4 hours | >25 | 800 | every 4 hours, 5x daily |
Acyclovir oral suspension is contraindicated in patients who have had a demonstrated clinically significant hypersensitivity reaction [e.g., anaphylaxis, severe cutaneous adverse reactions (SCARS)] to acyclovir, valacyclovir or any component of the formulation (see
Acyclovir oral suspension is intended for oral ingestion only. Renal failure, in some cases resulting in death, has been observed with acyclovir therapy (see
Severe cutaneous adverse reactions (SCARs), including acute generalized exanthematous pustulosis (AGEP), drug reaction with eosinophilia and systemic symptoms (DRESS), Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and erythema multiforme (EM) have been reported with acyclovir (see
Dosage adjustment is recommended when administering acyclovir to patients with renal impairment (see
Patients should be advised to maintain adequate hydration.
Acyclovir was tested in lifetime bioassays in rats and mice at single daily doses of up to 450 mg/kg administered by gavage. There was no statistically significant difference in the incidence of tumors between treated and control animals, nor did acyclovir shorten the latency of tumors. Maximum plasma concentrations were 3 to 6 times human levels in the mouse bioassay and 1 to 2 times human levels in the rat bioassay.
Acyclovir was tested in 16
Acyclovir did not impair fertility or reproduction in mice (450 mg/kg/day, p.o.) or in rats (25 mg/kg/day, s.c.). In the mouse study, plasma levels were 9 to 18 times human levels, while in the rat study, they were 8 to 15 times human levels. At higher doses (50 mg/kg/day, s.c.) in rats and rabbits (11 to 22 and 16 to 31 times human levels, respectively) implantation efficacy, but not litter size, was decreased. In a rat peri- and post-natal study at 50 mg/kg/day, s.c., there was a statistically significant decrease in group mean numbers of corpora lutea, total implantation sites, and live fetuses.
No testicular abnormalities were seen in dogs given 50 mg/kg/day, IV for 1 month (21 to 41 times human levels) or in dogs given 60 mg/kg/day orally for 1 year (6 to 12 times human levels). Testicular atrophy and aspermatogenesis were observed in rats and dogs at higher dose levels.
There are no adequate and well-controlled studies in pregnant women. A prospective epidemiologic registry of acyclovir use during pregnancy was established in 1984 and completed in April 1999. There were 749 pregnancies followed in women exposed to systemic acyclovir during the first trimester of pregnancy resulting in 756 outcomes. The occurrence rate of birth defects approximates that found in the general population. However, the small size of the registry is insufficient to evaluate the risk for less common defects or to permit reliable or definitive conclusions regarding the safety of acyclovir in pregnant women and their developing fetuses. Acyclovir should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Acyclovir oral suspension is contraindicated in patients who have had a demonstrated clinically significant hypersensitivity reaction [e.g., anaphylaxis, severe cutaneous adverse reactions (SCARS)] to acyclovir, valacyclovir or any component of the formulation (see
Acyclovir oral suspension is intended for oral ingestion only. Renal failure, in some cases resulting in death, has been observed with acyclovir therapy (see
Severe cutaneous adverse reactions (SCARs), including acute generalized exanthematous pustulosis (AGEP), drug reaction with eosinophilia and systemic symptoms (DRESS), Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and erythema multiforme (EM) have been reported with acyclovir (see
Acyclovir oral suspension is intended for oral ingestion only. Renal failure, in some cases resulting in death, has been observed with acyclovir therapy (see
Severe cutaneous adverse reactions (SCARs), including acute generalized exanthematous pustulosis (AGEP), drug reaction with eosinophilia and systemic symptoms (DRESS), Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and erythema multiforme (EM) have been reported with acyclovir (see
Acyclovir, USP is a synthetic nucleoside analogue active against herpesviruses. Acyclovir suspension, USP is formulated for oral administration.
Each teaspoonful (5 mL) of acyclovir oral suspension, USP contains 200 mg of acyclovir and the following inactive ingredients: banana flavor, carboxymethylcellulose sodium, glycerin, methylparaben, microcrystalline cellulose, propylparaben, purified water and sorbitol.
Acyclovir is a white, crystalline powder with the molecular formula C
8H
11N
5O
3and a molecular weight of 225.20. The maximum solubility in water at 37°C is 2.5 mg/mL. The pka's of acyclovir are 2.27 and 9.25.
The chemical name of Acyclovir is 2-amino-1,9-dihydro-9-[(2-hydroxyethoxy)methyl]-6H-purin-6-one; it has the following structural formula:
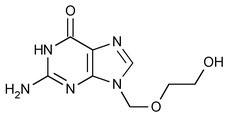
Table 1. Acyclovir Pharmacokinetic Characteristics (Range) | |
Parameter | Range |
Plasma protein binding | 9% to 33% |
Plasma elimination half-life | 2.5 to 3.3 hr |
Average oral bioavailability | 10% to 20%* |
*Bioavailability decreases with increasing dose. | |
In one multiple-dose, crossover study in healthy subjects (n = 23), it was shown that increases in plasma acyclovir concentrations were less than dose proportional with increasing dose, as shown in Table 2. The decrease in bioavailability is a function of the dose and not the dosage form.
Table 2. Acyclovir Peak and Trough Concentrations at Steady State | |||
Parameter | 200 mg | 400 mg | 800 mg |
C | 0.83 mcg/mL | 1.21 mcg/mL | 1.61 mcg/mL |
C | 0.46 mcg/mL | 0.63 mcg/mL | 0.83 mcg/mL |
There was no effect of food on the absorption of acyclovir (n = 6); therefore, acyclovir suspension may be administered with or without food.
The only known urinary metabolite is 9-[(carboxymethoxy)methyl]guanine.
2and 600 mg/m
2in pediatric patients aged 7 months to 7 years was 2.6 hours (range 1.59 to 3.74 hours).
In a study of patients who received acyclovir 400 mg twice daily for 3 years, 45%, 52%, and 63% of patients remained free of recurrences in the first, second, and third years, respectively. Serial analyses of the 3-month recurrence rates for the patients showed that 71% to 87% were recurrence free in each quarter.
In a similar double-blind, placebo-controlled study, acyclovir (800 mg 5 times daily for 7 days) shortened the times to complete lesion scabbing, healing, and cessation of pain; reduced the duration of new lesion formation; and reduced the prevalence of localized zoster-associated neurologic symptoms (paresthesia, dysesthesia, or hyperesthesia).
Treatment was begun within 72 hours of rash onset and was most effective if started within the first 48 hours.
Adults greater than 50 years of age showed greater benefit.