Acyclovir
Acyclovir Prescribing Information
Acyclovir is indicated for the acute treatment of herpes zoster (shingles).
Acyclovir is indicated for the treatment of initial episodes and the management of recurrent episodes of genital herpes.
Acyclovir is indicated for the treatment of chickenpox (varicella).
800 mg every 4 hours orally, 5 times daily for 7 to 10 days.
The frequency and severity of episodes of untreated genital herpes may change over time. After 1 year of therapy, the frequency and severity of the patient’s genital herpes infection should be re-evaluated to assess the need for continuation of therapy with acyclovir.
800 mg 4 times daily for 5 days.
Intravenous acyclovir is indicated for the treatment of varicella-zoster infections in immunocompromised patients.
When therapy is indicated, it should be initiated at the earliest sign or symptom of chickenpox. There is no information about the efficacy of therapy initiated more than 24 hours after onset of signs and symptoms.
In patients with renal impairment, the dose of acyclovir capsules and tablets should be modified as shown in Table 3.
| Normal Dosage Regimen | Creatinine Clearance (mL/min/1.73 m 2 ) | Adjusted Dosage Regimen | |
| Dose (mg) | Dosing Interval | ||
| 200 mg every 4 hours | >10 | 200 | every 4 hours, 5x daily |
| 0-10 | 200 | every 12 hours | |
| 400 mg every 12 hours | >10 | 400 | every 12 hours |
| 0-10 | 200 | every 12 hours | |
| 800 mg every 4 hours | >25 | 800 | every 4 hours, 5x daily |
| 10-25 | 800 | every 8 hours | |
| 0-10 | 800 | every 12 hours | |
For patients who require hemodialysis, the mean plasma half-life of acyclovir during hemodialysis is approximately 5 hours. This results in a 60% decrease in plasma concentrations following a 6-hour dialysis period. Therefore, the patient’s dosing schedule should be adjusted so that an additional dose is administered after each dialysis.
No supplemental dose appears to be necessary after adjustment of the dosing interval.
1 acyclovir 800 mg tablet was shown to be bioequivalent to 4 acyclovir 200 mg capsules (n = 24).
Acyclovir is contraindicated for patients who develop hypersensitivity to acyclovir or valacyclovir.
The most frequent adverse event reported during 3 clinical trials of treatment of herpes zoster (shingles) with 800 mg of oral acyclovir 5 times daily for 7 to 10 days in 323 patients was malaise (11.5%). The 323 placebo recipients reported malaise (11.1%).
The most frequent adverse event reported during 3 clinical trials of treatment of chickenpox with oral acyclovir at doses of 10 to 20 mg/kg 4 times daily for 5 to 7 days or 800 mg 4 times daily for 5 days in 495 patients was diarrhea (3.2%). The 498 patients receiving placebo reported diarrhea (2.2%).
In addition to adverse events reported from clinical trials, the following events have been identified during post-approval use of acyclovir. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. These events have been chosen for inclusion due to either their seriousness, frequency of reporting, potential causal connection to acyclovir, or a combination of these factors.
Musculoskeletal: Myalgia.
Acyclovir is a synthetic nucleoside analogue active against herpesviruses. Acyclovir Capsules, USP and Acyclovir Tablets, USP are formulations for oral administration.
Each capsule contains 200 mg of acyclovir, USP and the inactive ingredients corn starch, lactose monohydrate, magnesium stearate, and sodium lauryl sulfate. The capsule shell consists of gelatin, FD&C Blue No. 1, FD&C Red No. 3, FD&C Yellow No. 6 and titanium dioxide. Printed with edible black ink.
Each 800 mg tablet of acyclovir contains 800 mg of acyclovir, USP and the inactive ingredients magnesium stearate, microcrystalline cellulose PH101, povidone K30, and sodium starch glycolate (Type A)(Starch from Non GMO potatoes).
Each 400 mg tablet of acyclovir contains 400 mg of acyclovir, USP and the inactive ingredients magnesium stearate, microcrystalline cellulose PH101, povidone K30, and sodium starch glycolate (Type A)(Starch from Non GMO potatoes).
Acyclovir, USP is a white, crystalline powder with the molecular formula C
8H
11N
5O
3 and a molecular weight of 225. The maximum solubility in water at 37°C is 2.5mg/mL. The pka’s of acyclovir are 2.27 and 9.25.
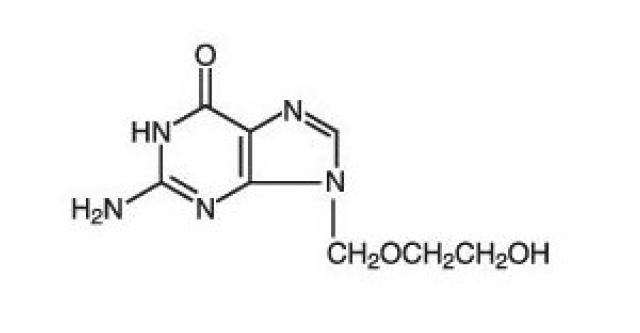
The chemical name of acyclovir, USP is 2-amino-1,9-dihydro-9-[(2-hydroxyethoxy)methyl]-6H-purin-6-one; it has the following structural formula:
Acyclovir is a synthetic purine nucleoside analogue with in vitro and in vivo inhibitory activity against herpes simplex virus types 1 (HSV-1), 2 (HSV-2), and varicella-zoster virus (VZV).
The inhibitory activity of acyclovir is highly selective due to its affinity for the enzyme thymidine kinase (TK) encoded by HSV and VZV. This viral enzyme converts acyclovir into acyclovir monophosphate, a nucleotide analogue. The monophosphate is further converted into diphosphate by cellular guanylate kinase and into triphosphate by a number of cellular enzymes. In vitro, acyclovir triphosphate stops replication of herpes viral DNA. This is accomplished in 3 ways: 1) competitive inhibition of viral DNA polymerase, 2) incorporation into and termination of the growing viral DNA chain, and 3) inactivation of the viral DNA polymerase. The greater antiviral activity of acyclovir against HSV compared with VZV is due to its more efficient phosphorylation by the viral TK.
The quantitative relationship between the in vitro susceptibility of herpes viruses to antivirals and the clinical response to therapy has not been established in humans, and virus sensitivity testing has not been standardized. Sensitivity testing results, expressed as the concentration of drug required to inhibit by 50% the growth of virus in cell culture (IC
50 ), vary greatly depending upon a number of factors. Using plaque-reduction assays, the IC
50 against herpes simplex virus isolates ranges from 0.02 to 13.5 mcg/mL for HSV-1 and from 0.01 to 9.9 mcg/mL for HSV-2. The IC
50 for acyclovir against most laboratory strains and clinical isolates of VZV ranges from 0.12 to 10.8 mcg/mL. Acyclovir also demonstrates activity against the Oka vaccine strain of VZV with a mean IC
50 of 1.35 mcg/mL.
Resistance of HSV and VZV to acyclovir can result from qualitative and quantitative changes in the viral TK and/or DNA polymerase. Clinical isolates of HSV and VZV with reduced susceptibility to acyclovir have been recovered from immunocompromised patients, especially with advanced HIV infection. While most of the acyclovir-resistant mutants isolated thus far from immunocompromised patients have been found to be TK-deficient mutants, other mutants involving the viral TK gene (TK partial and TK altered) and DNA polymerase have been isolated. TK-negative mutants may cause severe disease in infants and immunocompromised adults. The possibility of viral resistance to acyclovir should be considered in patients who show poor clinical response during therapy.
Pharmacokinetics:
The pharmacokinetics of acyclovir after oral administration have been evaluated in healthy volunteers and in immunocompromised patients with herpes simplex or varicella-zoster virus infection. Acyclovir pharmacokinetic parameters are summarized in Table 1.
Table1. Acyclovir Pharmacokinetic Characteristics (Range)
| Parameter | Range |
| Plasma protein binding | 9% to 33% |
| Plasma elimination half-life | 2.5 to 3.3 hr |
| Average oral bioavailability | 10% to 20%* |
| *Bioavailability decreases with increasing dose. | |
In one multiple-dose, crossover study in healthy subjects (n = 23), it was shown that increases in plasma acyclovir concentrations were less than dose proportional with increasing dose, as shown in Table 2. The decrease in bioavailability is a function of the dose and not the dosage form.
Table2. Acyclovir Peak and Trough Concentrations at Steady State
| Parameter | 200 mg | 400 mg | 800 mg |
| C SSmax | 0.83 mcg/mL | 1.21 mcg/mL | 1.61 mcg/mL |
| C SStrough | 0.46 mcg/mL | 0.63 mcg/mL | 0.83 mcg/mL |
There was no effect of food on the absorption of acyclovir (n = 6); therefore, Acyclovir Capsules and Tablets may be administered with or without food.
The only known urinary metabolite is 9-[(carboxymethoxy) methyl] guanine.
2 and 600 mg/m
2 in pediatric patients aged 7 months to 7 years was 2.6 hours (range 1.59 to 3.74 hours).
Coadministration of probenecid with intravenous acyclovir has been shown to increase the mean acyclovir half-life and the area under the concentration-time curve. Urinary excretion and renal clearance were correspondingly reduced.
In a study of patients who received acyclovir 400 mg twice daily for 3 years, 45%, 52%, and 63% of patients remained free of recurrences in the first, second, and third years, respectively. Serial analyses of the 3-month recurrence rates for the patients showed that 71% to 87% were recurrence free in each quarter.
In a similar double-blind, placebo-controlled study, acyclovir (800 mg 5 times daily for 7 days) shortened the times to complete lesion scabbing, healing, and cessation of pain; reduced the duration of new lesion formation; and reduced the prevalence of localized zoster-associated neurologic symptoms (paresthesia, dysesthesia, or hyperesthesia).
Treatment was begun within 72 hours of rash onset and was most effective if started within the first 48 hours.
Adults greater than 50 years of age showed greater benefit.