Acyclovir
Acyclovir Prescribing Information
800 mg every 4 hours orally, 5 times daily for 7 to 10 days.
| Normal Dosage Regimen | Creatinine Clearance (mL/min/1.73m 2) | Adjusted Dosage Regimen Dose (mg) | Dosing Interval |
| 200 mg every 4 hours | >10 0-10 | 200 200 | every 4 hours, 5x daily every 12 hours |
| 400 mg every 12 hours | >10 0-10 | 400 200 | every 12 hours every 12 hours |
| 800 mg every 4 hours | >25 10-25 0-10 | 800 800 800 | every 4 hours, 5x daily every 8 hours every 12 hours |
For patients who require hemodialysis, the mean plasma half-life of acyclovir during hemodialysis is approximately 5 hours. This results in a 60% decrease in plasma concentrations following a 6-hour dialysis period. Therefore, the patient’s dosing schedule should be adjusted so that an additional dose is administered after each dialysis.
Acyclovir is contraindicated for patients who develop hypersensitivity to acyclovir or valacyclovir.
Acyclovir is a synthetic nucleoside analogue active against herpes viruses. Acyclovir capsules are formulations for oral administration.
Each capsule contains 200 mg of acyclovir and the inactive ingredients: lactose monohydrate, sodium lauryl sulfate, sodium starch glycolate, and magnesium stearate. The capsule shell consists of FD&C Blue #1, gelatin, and titanium dioxide. The imprinting ink contains, FD&C Blue #1, FD&C Blue #2, FD&C Red #40, D&C Yellow #10, iron oxide black, pharmaceutical shellac glaze and propylene glycol.
Acyclovir is a white, crystalline powder with the molecular formula C
8H
11N
5O
3 and a molecular weight of 225. The maximum solubility in water at 37degree C is 2.5 mg/mL. The pka's of acyclovir are 2.27 and 9.25.
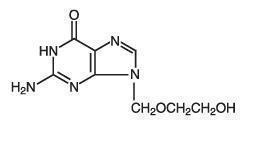
The chemical name of acyclovir is 2-amino-1,9-dihydro-9-[(2-hydroxyethoxy)methyl]-6
The pharmacokinetics of acyclovir after oral administration have been evaluated in healthy volunteers and in immunocompromised patients with herpes simplex or varicella-zoster virus infection. Acyclovir pharmacokinetic parameters are summarized in Table 1.
Parameter | Range |
Plasma protein binding | 9% to 33% |
Plasma elimination half-life | 2.5 to 3.3 hr |
Average oral bioavailability | 10% to 20%* |
*Bioavailability decreases with increasing dose. | |
In one multiple-dose, crossover study in healthy subjects (n = 23), it was shown that increases in plasma acyclovir concentrations were less than dose proportional with increasing dose, as shown in Table 2. The decrease in bioavailability is a function of the dose and not the dosage form.
Parameter | 200 mg | 400 mg | 800 mg |
C | 0.83 mcg/mL | 1.21 mcg/mL | 1.61 mcg/mL |
C | 0.46 mcg/mL | 0.63 mcg/mL | 0.83 mcg/mL |
There was no effect of food on the absorption of acyclovir (n = 6); therefore, acyclovir capsules may be administered with or without food.
The only known urinary metabolite is 9-[(carboxymethoxy)methyl]guanine.
DOSAGE AND ADMINISTRATION).
Of 376 subjects who received acyclovir in a clinical study of herpes zoster treatment in immunocompetent subjects ≥ 50 years of age, 244 were 65 and over while 111 were 75 and over. No overall differences in effectiveness for time to cessation of new lesion formation or time to healing were reported between geriatric subjects and younger adult subjects. The duration of pain after healing was longer in patients 65 and over. Nausea, vomiting, and dizziness were reported more frequently in elderly subjects. Elderly patients are more likely to have reduced renal function and require dose reduction. Elderly patients are also more likely to have renal or CNS adverse events. With respect to CNS adverse events observed during clinical practice, somnolence, hallucinations, confusion, and coma were reported more frequently in elderly patients (see CLINICAL PHARMACOLOGY, ADVERSE REACTIONS: Observed During Clinical Practice, and DOSAGE AND ADMINISTRATION).
Double-blind, placebo-controlled studies have demonstrated that orally administered acyclovir significantly reduced the duration of acute infection and duration of lesion healing. The duration of pain and new lesion formation was decreased in some patient groups.
Acyclovir Capsules USP, 200 mg Oval capsule, light blue opaque cap and aqua blue opaque body imprinted with “QP146” are supplied in bottles of 100 (NDC 69076-146-01)