Acyclovir
Acyclovir Prescribing Information
The frequency and severity of episodes of untreated genital herpes may change over time. After 1 year of therapy, the frequency and severity of the patient’s genital herpes infection should be re-evaluated to assess the need for continuation of therapy with acyclovir.
Intravenous acyclovir is indicated for the treatment of varicella-zoster infections in immunocompromised patients.
When therapy is indicated, it should be initiated at the earliest sign or symptom of chickenpox. There is no information about the efficacy of therapy initiated more than 24 hours after onset of signs and symptoms.
| Creatinine | Adjusted Dosage Regimen | ||
| Normal | Clearance | Dose | |
| Dosage Regimen | (mL/min/1.73m2) | (mg) | Dosing Interval |
| 200 mg every 4 hours | >10 | 200 | every 4 hours, 5x daily |
| 0 to 10 | 200 | every 12 hours | |
| 400 mg every 12 hours | >10 | 400 | every 12 hours |
| 0 to 10 | 200 | every 12 hours | |
| 800 mg every 4 hours | >25 | 800 | every 4 hours, 5x daily |
| 10 to 25 | 800 | every 8 hours | |
| 0 to 10 | 800 | every 12 hours | |
Acyclovir oral suspension is contraindicated for patients who develop hypersensitivity to acyclovir or valacyclovir.
Dosage adjustment is recommended when administering acyclovir to patients with renal impairment (see DOSAGE AND ADMINISTRATION). Caution should also be exercised when administering acyclovir to patients receiving potentially nephrotoxic agents since this may increase the risk of renal dysfunction and/or the risk of reversible central nervous system symptoms such as those that have been reported in patients treated with intravenous acyclovir. Adequate hydration should be maintained.
Patients are instructed to consult with their physician if they experience severe or troublesome adverse reactions, they become pregnant or intend to become pregnant, they intend to breastfeed while taking orally administered acyclovir, or they have any other questions.
Patients should be advised to maintain adequate hydration.
See CLINICAL PHARMACOLOGY: Pharmacokinetics.
The data presented below include references to peak steady-state plasma acyclovir concentrations observed in humans treated with 800 mg given orally 5 times a day (dosing appropriate for treatment of herpes zoster) or 200 mg given orally 5 times a day (dosing appropriate for treatment of genital herpes). Plasma drug concentrations in animal studies are expressed as multiples of human exposure to acyclovir at the higher and lower dosing schedules (see CLINICAL PHARMACOLOGY: Pharmacokinetics).
Acyclovir was tested in lifetime bioassays in rats and mice at single daily doses of up to 450 mg/kg administered by gavage. There was no statistically significant difference in the incidence of tumors between treated and control animals, nor did acyclovir shorten the latency of tumors. Maximum plasma concentrations were 3 to 6 times human levels in the mouse bioassay and 1 to 2 times human levels in the rat bioassay.
Acyclovir was tested in 16
Acyclovir did not impair fertility or reproduction in mice (450 mg/kg/day, p.o.) or in rats (25 mg/kg/day, s.c.). In the mouse study, plasma levels were 9 to 18 times human levels, while in the rat study, they were 8 to 15 times human levels. At higher doses (50 mg/kg/day, s.c.) in rats and rabbits (11 to 22 and 16 to 31 times human levels, respectively) implantation efficacy, but not litter size, was decreased. In a rat peri- and post-natal study at 50 mg/kg/day, s.c., there was a statistically significant decrease in group mean numbers of corpora lutea, total implantation sites, and live fetuses.
No testicular abnormalities were seen in dogs given 50 mg/kg/day, IV for 1 month (21 to 41 times human levels) or in dogs given 60 mg/kg/day orally for 1 year (6 to 12 times human levels). Testicular atrophy and aspermatogenesis were observed in rats and dogs at higher dose levels.
Acyclovir administered during organogenesis was not teratogenic in the mouse (450 mg/kg/day, p.o.), rabbit (50 mg/kg/day, s.c. and IV), or rat (50 mg/kg/day, s.c.). These exposures resulted in plasma levels 9 and 18, 16 and 106, and 11 and 22 times, respectively, human levels.
There are no adequate and well-controlled studies in pregnant women. A prospective epidemiologic registry of acyclovir use during pregnancy was established in 1984 and completed in April 1999. There were 749 pregnancies followed in women exposed to systemic acyclovir during the first trimester of pregnancy resulting in 756 outcomes. The occurrence rate of birth defects approximates that found in the general population. However, the small size of the registry is insufficient to evaluate the risk for less common defects or to permit reliable or definitive conclusions regarding the safety of acyclovir in pregnant women and their developing fetuses. Acyclovir should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Acyclovir concentrations have been documented in breast milk in 2 women following oral administration of acyclovir and ranged from 0.6 to 4.1 times corresponding plasma levels. These concentrations would potentially expose the nursing infant to a dose of acyclovir up to 0.3 mg/kg/day. Acyclovir should be administered to a nursing mother with caution and only when indicated.
Safety and effectiveness of oral formulations of acyclovir in pediatric patients younger than 2 years of age have not been established.
Of 376 subjects who received acyclovir in a clinical study of herpes zoster treatment in immunocompetent subjects greater than or equal to 50 years of age, 244 were 65 and over while 111 were 75 and over. No overall differences in effectiveness for time to cessation of new lesion formation or time to healing were reported between geriatric subjects and younger adult subjects. The duration of pain after healing was longer in patients 65 and over. Nausea, vomiting, and dizziness were reported more frequently in elderly subjects. Elderly patients are more likely to have reduced renal function and require dose reduction. Elderly patients are also more likely to have renal or CNS adverse events. With respect to CNS adverse events observed during clinical practice, somnolence, hallucinations, confusion, and coma were reported more frequently in elderly patients (see CLINICAL PHARMACOLOGY, ADVERSE REACTIONS: Observed During Clinical Practice, and DOSAGE AND ADMINISTRATION).
Acyclovir oral suspension is intended for oral ingestion only. Renal failure, in some cases resulting in death, has been observed with acyclovir therapy (see ADVERSE REACTIONS: Observed During Clinical Practice and OVERDOSAGE). Thrombotic thrombocytopenic purpura/hemolytic uremic syndrome (TTP/HUS), which has resulted in death, has occurred in immunocompromised patients receiving acyclovir therapy.
See CLINICAL PHARMACOLOGY: Pharmacokinetics.
Acyclovir is a synthetic nucleoside analogue active against herpes viruses. Acyclovir suspension is formulated for oral administration.
Each teaspoonful (5 mL) of acyclovir oral suspension, USP contains 200 mg of acyclovir and the inactive ingredients carboxymethylcellulose sodium, flavor, glycerin, methylparaben (0.1%), microcrystalline cellulose, propylparaben (0.02%), purified water and sorbitol.
Acyclovir is a white, crystalline powder with the molecular formula C8H11N5O3, and a molecular weight of 225.20. The maximum solubility in water at 37°C is 2.5 mg/mL. The pka’s of acyclovir are 2.27 and 9.25.
The chemical name of acyclovir is 9-[(2-Hydroxyethoxy)methyl]guanine; it has the following structural formula:
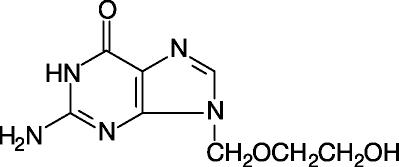
Acyclovir is a synthetic purine nucleoside analogue with
The inhibitory activity of acyclovir is highly selective due to its affinity for the enzyme thymidine kinase (TK) encoded by HSV and VZV. This viral enzyme converts acyclovir into acyclovir monophosphate, a nucleotide analogue. The monophosphate is further converted into diphosphate by cellular guanylate kinase and into triphosphate by a number of cellular enzymes.