Acyclovir
Acyclovir Prescribing Information
Acyclovir Ointment 5% is indicated in the management of initial genital herpes and in limited non-life threatening mucocutaneous HSV infections in immunocompromised patients.
Apply sufficient quantity to adequately cover all lesions every 3 hours, 6 times per day for 7 days. The dose size per application will vary depending upon the total lesion area but should approximate a one half inch ribbon of ointment per 4 square inches of surface area. A finger cot or rubber glove should be used when applying acyclovir to prevent autoinoculation of other body sites and transmission of infection to other persons. Therapy should be initiated as early as possible following onset of signs and symptoms.
Acyclovir Ointment 5% is contraindicated in patients who develop hypersensitivity to the components of the formulation.
In the controlled clinical trials, mild pain (including transient burning and stinging) was reported by about 30% of patients in both the active and placebo arms; treatment was discontinued in two of these patients. Local pruritus occurred in 4% of these patients. In all studies, there was no significant difference between the drug and placebo group in the rate or type of reported adverse reactions nor were there any differences in abnormal clinical laboratory findings.
Acyclovir USP is a synthetic nucleoside analogue active against herpes viruses. Acyclovir Ointment USP, 5% is a formulation for topical administration. Each gram of Acyclovir Ointment USP, 5% contains 50 mg of acyclovir, USP in a polyethylene glycol (PEG) base.
Acyclovir, USP is a white, crystalline powder with the molecular formula C8H11N5O3 and a molecular weight of 225. The maximum solubility in water at 37°C is 2.5 mg/mL. The pka's of acyclovir are 2.27 and 9.25.
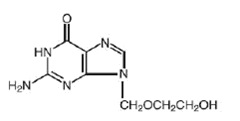
The chemical name of acyclovir is 2-amino-1,9-dihydro-9-[(2-hydroxyethoxy)methyl]-6H-purin-6-one; it has the following structural formula:
The inhibitory activity of acyclovir is highly selective due to its affinity for the enzyme thymidine kinase (TK) encoded by HSV and VZV. This viral enzyme converts acyclovir into acyclovir monophosphate, a nucleotide analogue. The monophosphate is further converted into diphosphate by cellular guanylate kinase and into triphosphate by a number of cellular enzymes. In vitro, acyclovir triphosphate stops replication of herpes viral DNA. This is accomplished in three ways: 1) competitive inhibition of viral DNA polymerase, 2) incorporation into and termination of the growing viral DNA chain, and 3) inactivation of the viral DNA polymerase. The greater antiviral activity of acyclovir against HSV compared to VZV is due to its more efficient phosphorylation by the viral TK.
Two clinical pharmacology studies were performed with acyclovir ointment 5% in immunocompromised adults at risk of developing mucocutaneous HSV infections or with localized varicella-zoster infections. These studies were designed to evaluate the dermal tolerance, systemic toxicity, and percutaneous absorption of acyclovir.
In one of these studies, which included 16 inpatients, the complete ointment or its vehicle were randomly administered in a dose of 1-cm strips (25 mg acyclovir) 4 times a day for 7 days to an intact skin surface area of 4.5 square inches. No local intolerance, systemic toxicity, or contact dermatitis were observed. In addition, no drug was detected in blood and urine by radioimmunoassay (sensitivity, 0.01 mcg/mL).
The other study included 11 patients with localized varicella zoster infections. In this uncontrolled study, acyclovir was detected in the blood of 9 patients and in the urine of all patients tested. Acyclovir levels in plasma ranged from <0.01 to 0.28 mcg/mL in 8 patients with normal renal function, and from <0.01 to 0.78 mcg/mL in one patient with impaired renal function. Acyclovir excreted in the urine ranged from <0.02% to 9.4% of the daily dose. Therefore, systemic absorption of acyclovir after topical application is minimal.
In clinical trials of initial genital herpes infections, acyclovir ointment 5% has shown a decrease in healing time and, in some cases, a decrease in duration of viral shedding and duration of pain. In studies in immunocompromised patients primarily with herpes labialis, there was a decrease in duration of viral shedding and a slight decrease in duration of pain.
In studies of recurrent genital herpes and of herpes labialis in non-immunocompromised patients, there was no evidence of clinical benefit; there was some decrease in duration of viral shedding.