Adrenalin
(Epinephrine)Adrenalin Prescribing Information
Adrenalin injection: clear, colorless solution supplied as 30 mg/30 mL (1 mg/mL) in a multiple dose amber glass vial.
None.
Common adverse reactions to systemically administered epinephrine include anxiety, apprehensiveness, restlessness, tremor, weakness, dizziness, sweating, palpitations, pallor, nausea and vomiting, headache, and respiratory difficulties. These symptoms occur in some persons receiving therapeutic doses of epinephrine, but are more likely to occur in patients with heart disease, hypertension, or hyperthyroidism
The true incidence of adverse reactions associated with the systemic use of epinephrine is difficult to determine. Adverse reactions reported in observational trials, case reports, and studies are listed below by body system:
Rapid rises in blood pressure associated with epinephrine use have produced cerebral hemorrhage, particularly in elderly patients with cardiovascular disease
Patients with Parkinson’s disease may experience psychomotor agitation or a temporary worsening of symptoms
Diabetic patients may experience transient increases in blood sugar.
Injection into the buttock has resulted in cases of gas gangrene
Rare cases of serious skin and soft tissue infections, including necrotizing fasciitis and myonecrosis caused by Clostridia (gas gangrene), have been reported following epinephrine injection in the thigh
Adrenalin (epinephrine injection, USP) is a clear, colorless, sterile solution containing 1 mg/mL epinephrine, packaged as 30 mL of solution in a multiple dose amber glass vial. In the 30 mL vial, each 1 mL of Adrenalin solution contains 1 mg epinephrine, 6.15 mg sodium chloride, 0.457 mg sodium metabisulfite, 0.920 mg sodium hydroxide, 2.25 mg tartaric acid, 0.20 mg disodium edetate dihydrate, 5.18 mg of 4% hydrochloric acid to adjust pH, 5.25 mg chlorobutanol as a preservative and water for injection. The pH range is 2.2-5.0.
Epinephrine is a sympathomimetic catecholamine. The chemical name of epinephrine is: 1,2-Benzenediol, 4-[(1R)-1-hydroxy-2-(methylamino)ethyl]-, or (-)-3,4-Dihydroxy-α-[2-(methylamino)ethyl]benzyl alcohol.
The chemical structure of epinephrine is:
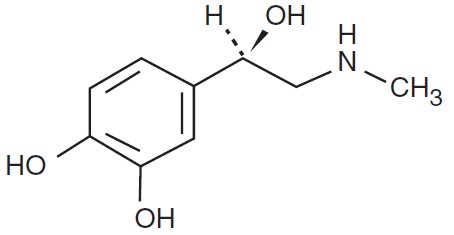
The molecular weight of epinephrine is 183.2.
Epinephrine solution deteriorates rapidly on exposure to air or light, turning pink from oxidation to adrenochrome and brown from the formation of melanin.
Epinephrine acts on both alpha and beta-adrenergic receptors. The mechanism of the rise in blood pressure is 3-fold: a direct myocardial stimulation that increases the strength of ventricular contraction (positive inotropic action), an increased heart rate (positive chronotropic action), and peripheral vasoconstriction.
Long-term studies to evaluate the carcinogenic potential of epinephrine have not been conducted.
Epinephrine and other catecholamines have been shown to have mutagenic potential
The potential for epinephrine to impair reproductive performance has not been evaluated, but epinephrine has been shown to decrease implantation in female rabbits dosed subcutaneously with 1.2 mg/kg/day (15-fold the highest human intramuscular or subcutaneous daily dose) during gestation days 3 to 9.