Ahzantive
(Aflibercept-Mrbb)Ahzantive Prescribing Information
AHZANTIVE is indicated for the treatment of:
1.2 Macular Edema Following Retinal Vein Occlusion (RVO)
1.3 Diabetic Macular Edema (DME)
1.4 Diabetic Retinopathy (DR)
2. DOSAGE AND ADMINISTRATION
For ophthalmic intravitreal injection. AHZANTIVE must only be administered by a qualified physician.
Pre-filled Syringe: A 30-gauge × ½-inch sterile injection needle is needed but not provided.
Vial: A 5-micron sterile filter needle (18-gauge × 1½-inch), a 1-mL sterile Luer lock syringe and a 30‑gauge × ½-inch sterile injection needle are needed, but not provided.
AHZANTIVE is available packaged as follows:
- Pre-filled Syringe. The AHZANTIVE pre-filled syringe cap and plunger are not made with natural rubber latex.
- Vial Only. The AHZANTIVE vial stopper is not made with natural rubber latex.
The recommended dose for AHZANTIVE is 2 mg (0.05 mL of 40 mg/mL solution) administered by intravitreal injection every 4 weeks (approximately every 28 days, monthly) for the first 12 weeks (3 months), followed by 2 mg (0.05 mL of 40 mg/mL solution) via intravitreal injection once every 8 weeks (2 months). Although AHZANTIVE may be dosed as frequently as 2 mg every 4 weeks (approximately every 25 days, monthly), additional efficacy was not demonstrated in most patients when aflibercept was dosed every 4 weeks compared to every 8 weeks
The recommended dose for AHZANTIVE is 2 mg (0.05 mL of 40 mg/mL solution) administered by intravitreal injection once every 4 weeks (approximately every 25 days, monthly)
The recommended dose for AHZANTIVE is 2 mg (0.05 mL of 40 mg/mL solution) administered by intravitreal injection every 4 weeks (approximately every 28 days, monthly) for the first 5 injections, followed by 2 mg (0.05 mL of 40 mg/mL solution) via intravitreal injection once every 8 weeks (2 months). Although AHZANTIVE may be dosed as frequently as 2 mg every 4 weeks (approximately every 25 days, monthly), additional efficacy was not demonstrated in most patients when aflibercept was dosed every 4 weeks compared to every 8 weeks
(5 months).
The recommended dose for AHZANTIVE is 2 mg (0.05 mL of 40 mg/mL solution) administered by intravitreal injection every 4 weeks (approximately every 28 days, monthly) for the first 5 injections, followed by 2 mg (0.05 mL of 40 mg/mL solution) via intravitreal injection once every 8 weeks (2 months). Although AHZANTIVE may be dosed as frequently as 2 mg every 4 weeks (approximately every 25 days, monthly), additional efficacy was not demonstrated in most patients when aflibercept was dosed every 4 weeks compared to every 8 weeks
The AHZANTIVE pre-filled syringe is sterile and for one-time use in one eye only.
The pre-filled syringe should be inspected visually prior to administration.
The intravitreal injection should be performed with a 30-gauge x ½-inch injection needle (not provided).
The pre-filled syringe contains more than the recommended dose of 2 mg aflibercept-mrbb (equivalent to 50 microliters).

Use aseptic technique to carry out the following steps:
1. PREPARE
When ready to administer AHZANTIVE, open the carton and remove sterilized blister pack. Carefully peel open the sterilized blister pack ensuring the sterility of its contents. Keep the syringe in the sterile tray until you are ready for assembly.
2. REMOVE SYRINGE
Using aseptic technique, remove the syringe from the sterilized blister pack.
3. TWIST OFF SYRINGE CAP
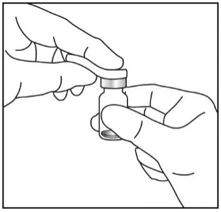
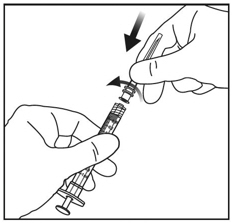
4. ATTACH NEEDLE
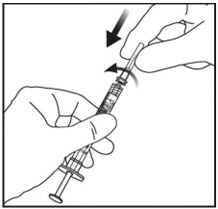
Using aseptic technique, firmly twist a 30-gauge x ½-inch injection needle onto the Luer lock syringe tip (see Figure 3).
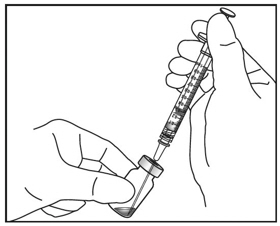
5. DISLODGE AIR BUBBLES
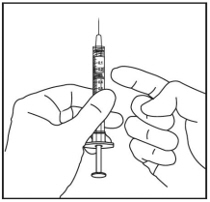
Holding the syringe with the needle pointing up, check the syringe for bubbles. If there are bubbles, gently tap the syringe with your finger until the bubbles rise to the top (see Figure 4).
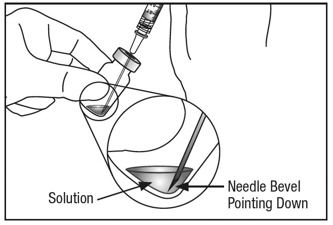
6. EXPEL AIR AND SET THE DOSE
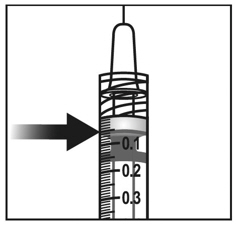
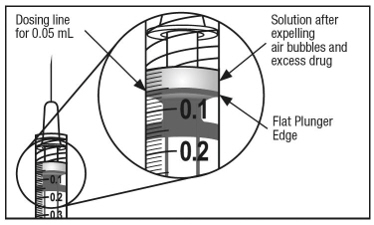
To eliminate all bubbles and to expel excess drug, slowly depress the plunger rod to align the plunger dome edge (see Figure 5a) with the black dosing line on the syringe (equivalent to 50 microliters) (see Figure 5b).
7. The pre-filled syringe is for one-time use in one eye only. After injection any unused product must be discarded.
AHZANTIVE should be inspected visually prior to administration. If particulates, cloudiness, or discoloration are visible, the vial must not be used.
The glass vial is for one-time use in one eye only. Discard unused portion.
Use aseptic technique to carry out the following preparation steps:
Prepare for intravitreal injection with the following medical devices for single use (not provided):
- a 5-micron sterile filter needle (18-gauge × 1½-inch)
- a 1-mL sterile Luer lock syringe (with marking to measure 0.05 mL)
- a sterile injection needle (30-gauge × ½-inch)
1. Remove the protective plastic cap from the vial (see Figure 6).
2. Clean the top of the vial with an alcohol wipe (see Figure 7).
3. Remove the 18-gauge x 1½-inch, 5-micron, filter needle and the 1-mL syringe from their packaging. Attach the filter needle to the syringe by twisting it onto the Luer lock syringe tip (see Figure 8).
4. Push the filter needle into the center of the vial stopper until the needle is completely inserted into the vial and the tip touches the bottom or bottom edge of the vial.
5. Using aseptic technique withdraw all of the AHZANTIVE vial contents into the syringe, keeping the vial in an upright position, slightly inclined to ease complete withdrawal. To deter the introduction of air, ensure the bevel of the filter needle is submerged into the liquid. Continue to tilt the vial during withdrawal keeping the bevel of the filter needle submerged in the liquid (see Figure 9a and Figure 9b).
6. Ensure that the plunger rod is drawn sufficiently back when emptying the vial in order to completely empty the filter needle.
7. Remove the filter needle from the syringe and properly dispose of the filter needle.
8. Remove the 30-gauge x ½-inch injection needle from its packaging and attach the injection needle to the syringe by firmly twisting the injection needle onto the Luer lock syringe tip (see Figure 10).
9. When ready to administer AHZANTIVE, remove the plastic needle shield from the needle.
10. Holding the syringe with the needle pointing up, check the syringe for bubbles. If there are bubbles, gently tap the syringe with your finger until the bubbles rise to the top (see Figure 11).
11. To eliminate all of the bubbles and to expel excess drug, SLOWLY depress the plunger rod so that the plunger edge aligns with the line that marks 0.05 mL on the syringe (see Figure 12a and Figure 12b).

The intravitreal injection procedure should be carried out under controlled aseptic conditions, which include surgical hand disinfection and the use of sterile gloves, a sterile drape, and a sterile eyelid speculum (or equivalent). Adequate anesthesia and a topical broad–spectrum microbicide should be given prior to the injection.
Pre-filled syringe: Inject by pressing the plunger carefully and with constant pressure. Do not apply additional pressure once the plunger has reached the bottom of the syringe. A small residual volume may remain in the syringe after a full dose has been injected. This is normal.
Immediately following the intravitreal injection, patients should be monitored for elevation in intraocular pressure. Appropriate monitoring may consist of a check for perfusion of the optic nerve head or tonometry. If required, a sterile paracentesis needle should be available.
Following intravitreal injection, patients and/or caregivers should be instructed to report any signs and/or symptoms suggestive of endophthalmitis or retinal detachment (e.g., eye pain, redness of the eye, photophobia, blurring of vision) without delay [see Patient Counseling Information (17)].
Each sterile vial should only be used for the treatment of a single eye. If the contralateral eye requires treatment, a new sterile vial should be used and the sterile field, syringe, gloves, drapes, eyelid speculum, filter, and injection needles should be changed before AHZANTIVE is administered to the other eye.
After injection, any unused product must be discarded.
AHZANTIVE is a clear, colorless to pale yellow solution available as:
- Injection: 2 mg (0.05 mL of a 40 mg/mL solution) in a single-dose pre-filled syringe
- Injection: 2 mg (0.05 mL of a 40 mg/mL solution) in a single-dose glass vial
Adequate and well-controlled studies with aflibercept have not been conducted in pregnant women. Aflibercept produced adverse embryofetal effects in rabbits, including external, visceral, and skeletal malformations. A fetal No Observed Adverse Effect Level (NOAEL) was not identified. At the lowest dose shown to produce adverse embryofetal effects, systemic exposures (based on AUC for free aflibercept) were approximately 6 times higher than AUC values observed in humans after a single intravitreal treatment at the recommended clinical dose
[see Animal Data].
Animal reproduction studies are not always predictive of human response, and it is not known whether aflibercept can cause fetal harm when administered to a pregnant woman. Based on the anti-VEGF mechanism of action for aflibercept [see Clinical Pharmacology (12.1)], treatment with aflibercept may pose a risk to human embryofetal development. AHZANTIVE should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. The background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
In two embryofetal development studies, aflibercept produced adverse embryofetal effects when administered every three days during organogenesis to pregnant rabbits at intravenous doses ≥3 mg per kg, or every six days during organogenesis at subcutaneous doses ≥0.1 mg per kg.
Adverse embryofetal effects included increased incidences of postimplantation loss and fetal malformations, including anasarca, umbilical hernia, diaphragmatic hernia, gastroschisis, cleft palate, ectrodactyly, intestinal atresia, spina bifida, encephalomeningocele, heart and major vessel defects, and skeletal malformations (fused vertebrae, sternebrae, and ribs; supernumerary vertebral arches and ribs; and incomplete ossification). The maternal No Observed Adverse Effect Level (NOAEL) in these studies was 3 mg per kg. Aflibercept produced fetal malformations at all doses assessed in rabbits and the fetal NOAEL was not identified. At the lowest dose shown to produce adverse embryofetal effects in rabbits (0.1 mg per kg), systemic exposure (AUC) of free aflibercept was approximately 6 times higher than systemic exposure (AUC) observed in adult patients after a single intravitreal dose of 2 mg.
There is no information regarding the presence of aflibercept products in human milk, the effects of the drug on the breastfed infant, or the effects of the drug on milk production/excretion. Because many drugs are excreted in human milk, and because the potential for absorption and harm to infant growth and development exists, AHZANTIVE is not recommended during breastfeeding.
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for AHZANTIVE and any potential adverse effects on the breastfed child from AHZANTIVE.
Females of reproductive potential are advised to use effective contraception prior to the initial dose, during treatment, and for at least 3 months after the last intravitreal injection of AHZANTIVE.
There are no data regarding the effects of aflibercept on human fertility. Aflibercept adversely affected female and male reproductive systems in cynomolgus monkeys when administered by intravenous injection at a dose approximately 1500 times higher than the systemic level observed in adult patients with an intravitreal dose of 2 mg. A No Observed Adverse Effect Level (NOAEL) was not identified. These findings were reversible within 20 weeks after cessation of treatment [see Nonclinical Toxicology (13.1)].
A pediatric assessment for AHZANTIVE demonstrates that AHZANTIVE is safe and effective for pediatric patients in an indication for which EYLEA (aflibercept) is approved. However, AHZANTIVE is not approved for such indication due to marketing exclusivity for EYLEA (aflibercept).
In the clinical studies, approximately 76% (2049/2701) of patients randomized to treatment with aflibercept were ≥65 years of age and approximately 46% (1250/2701) were ≥75 years of age. No significant differences in efficacy or safety were seen with increasing age in these studies.
AHZANTIVE is contraindicated in patients with ocular or periocular infections.
AHZANTIVE is contraindicated in patients with active intraocular inflammation.
AHZANTIVE is contraindicated in patients with known hypersensitivity to aflibercept or any of the excipients in AHZANTIVE. Hypersensitivity reactions may manifest as rash, pruritus, urticaria, severe anaphylactic/anaphylactoid reactions, or severe intraocular inflammation.
Intravitreal injections, including those with aflibercept products, have been associated with endophthalmitis and retinal detachments [see Adverse Reactions (6.1)] and, more rarely, retinal vasculitis with or without occlusion [see Adverse Reactions (6.2)]. Proper aseptic injection technique must always be used when administering AHZANTIVE. Patients and/or caregivers should be instructed to report any signs and/or symptoms suggestive of endophthalmitis, retinal detachment, or retinal vasculitis without delay and should be managed appropriately [see Dosage and Administration (2.7) and Patient Counseling Information (17)].
Acute increases in intraocular pressure have been seen within 60 minutes of intravitreal injection, including with aflibercept products [see Adverse Reactions (6.1)]. Sustained increases in intraocular pressure have also been reported after repeated intravitreal dosing with vascular endothelial growth factor (VEGF) inhibitors. Intraocular pressure and the perfusion of the optic nerve head should be monitored and managed appropriately [see Dosage and Administration (2.7)].
There is a potential risk of arterial thromboembolic events (ATEs) following intravitreal use of VEGF inhibitors, including aflibercept products. ATEs are defined as nonfatal stroke, nonfatal myocardial infarction, or vascular death (including deaths of unknown cause). The incidence of reported thromboembolic events in wet AMD studies during the first year was 1.8% (32 out of 1824) in the combined group of patients treated with aflibercept compared with 1.5% (9 out of 595) in patients treated with ranibizumab; through 96 weeks, the incidence was 3.3% (60 out of 1824) in the aflibercept group compared with 3.2% (19 out of 595) in the ranibizumab group. The incidence in the DME studies from baseline to week 52 was 3.3% (19 out of 578) in the combined group of patients treated with aflibercept compared with 2.8% (8 out of 287) in the control group; from baseline to week 100, the incidence was 6.4% (37 out of 578) in the combined group of patients treated with aflibercept compared with 4.2% (12 out of 287) in the control group. There were no reported thromboembolic events in the patients treated with aflibercept in the first six months of the RVO studies.