Alprazolam Prescribing Information
- Concomitant use of benzodiazepines and opioids may result in profound sedation, respiratory depression, coma, and death. Reserve concomitant prescribing of these drugs for patients for whom alternative treatment options are inadequate. Limit dosages and durations to the minimum required. Follow patients for signs and symptoms of respiratory depression and sedation[see Warnings and Precautions , Drug Interactions ].
- The use of benzodiazepines, including alprazolam, exposes users to risks of abuse, misuse, and addiction, which can lead to overdose or death. Abuse and misuse of benzodiazepines commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes. Before prescribing alprazolam and throughout treatment, assess each patient’s risk for abuse, misuse, and addiction[see Warnings and Precautions ].
- The continued use of benzodiazepines, including alprazolam, may lead to clinically significant physical dependence. The risks of dependence and withdrawal increase with longer treatment duration and higher daily dose. Abrupt discontinuation or rapid dosage reduction of alprazolam after continued use may precipitate acute withdrawal reactions, which can be life-threatening. To reduce the risk of withdrawal reactions, use a gradual taper to discontinue alprazolam or reduce the dosage[see Dosage and Administration , Warnings and Precautions ].
Alprazolam tablets are indicated for the:
- acute treatment of generalized anxiety disorder (GAD) in adults.
- treatment of panic disorder (PD), with or without agoraphobia in adults.
Alprazolam tablets, USP are available as follows:
• 0.25 mg: white, round tablet imprinted with 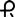 on one side and 027 and bisect on the other side
on one side and 027 and bisect on the other side
• 0.5 mg: peach, round tablet imprinted with 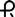 on one side and 029 and bisect on the other side
on one side and 029 and bisect on the other side
• 1 mg: blue, round tablet imprinted with 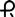 on one side and 031 and bisect on the other side
on one side and 031 and bisect on the other side
• 2 mg: yellow, rectangle shaped, flat faced, beveled edge tablet imprinted with 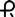 and 039 on one side and multi-scored on both sides
and 039 on one side and multi-scored on both sides
Alprazolam is contraindicated in patients:
- with known hypersensitivity to alprazolam or other benzodiazepines. Angioedema has been reported [see Adverse Reactions ].
- taking strong cytochrome P450 3A (CYP3A) inhibitors (e.g., ketoconazole, itraconazole), except ritonavir [see Dosage and Administration , Warnings and Precautions , Drug Interactions ]
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Risks from Concomitant Use with Opioids [see Warnings and Precautions ]
- Abuse, Misuse, and Addiction [see Warnings and Precautions ]
- Dependence and Withdrawal Reactions [see Warnings and Precautions ]
- Effects on Driving and Operating Machinery [see Warnings and Precautions ]
- Patients with Depression [see Warnings and Precautions ]
- Neonatal Sedation and Withdrawal Syndrome [see Warnings and Precautions ]
- Risks in Patients with Impaired Respiratory Function [see Warnings and Precautions ]
Alprazolam tablets, USP contain alprazolam, USP which is a triazolo analog of the 1,4 benzodiazepine class of central nervous system-active compounds.
The chemical name of alprazolam, USP is 8-Chloro-1-methyl-6-phenyl-4H-s-triazolo [4,3-α] [1,4] benzodiazepine. The structural formula is:
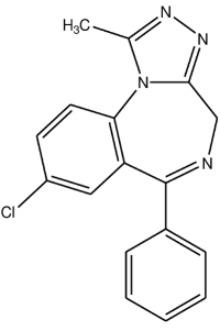
C17H13CIN4 M.W. 308.76
Alprazolam, USP is a white to off-white crystalline powder, which is soluble in methanol or ethanol but which has no appreciable solubility in water at physiological pH.
Each tablet, for oral administration, contains 0.25 mg, 0.5 mg, 1 mg, or 2 mg of alprazolam, USP. The 2 mg tablets are multi-scored and may be divided in half to provide two 1 mg segments, or quarters to provide four 0.5 mg segments.
Inactive ingredients: colloidal silicon dioxide, corn starch, docusate sodium, lactose monohydrate, magnesium stearate, microcrystalline cellulose, and sodium benzoate. The 0.5 mg tablet also contains FD&C Yellow #6 Aluminum Lake (Sunset Yellow Lake). The 1 mg tablet also contains FD&C Blue #2 Aluminum Lake. The 2 mg tablet also contains D&C Yellow #10 Aluminum Lake.