Alyq
(Tadalafil)Alyq Prescribing Information
20 mg, white to off-white, oval shaped, film-coated tablets (not scored) debossed with “J11” on one side and “T” on the other side.
The following serious adverse reactions are discussed elsewhere in the labeling:
- Hypotension [see Warnings and Precautions ()]
5.1 HypotensionALYQ®has vasodilatory properties that may result in transient decreases in blood pressure. Prior to prescribing ALYQ®, carefully consider whether patients with underlying cardiovascular disease could be affected adversely by such vasodilatory effects. Patients with preexisting hypotension, with autonomic dysfunction, with left ventricular outflow obstruction, may be particularly sensitive to the actions of vasodilators.
- Visual Loss [see Warnings and Precautions (and)
5.3 Visual LossWhen used to treat erectile dysfunction, non-arteritic anterior ischemic optic neuropathy (NAION), a cause of decreased vision including permanent loss of vision, has been reported postmarketing in temporal association with the use of phosphodiesterase type 5 (PDE-5) inhibitors, including tadalafil. Most, but not all, of these patients had underlying anatomic or vascular risk factors for development of NAION, including but not necessarily limited to: low cup to disc ratio (“crowded disc”), age over 50, diabetes, hypertension, coronary artery disease, hyperlipidemia, and smoking. Based on published literature, the annual incidence of NAION is 2.5 to 11.8 cases per 100,000 in males aged ≥50 in the general population. An observational case-crossover study evaluated the risk of NAION when PDE5 inhibitor use, as a class, typical of erectile dysfunction treatment, occurred immediately before NAION onset (within 5 half-lives), compared to PDE5 inhibitor use in a prior time period. The results suggest an approximate 2-fold increase in the risk of NAION, with a risk estimate of 2.15 (95% CI 1.06, 4.34). A similar study reported a consistent result, with a risk estimate of 2.27 (95% CI 0.99, 5.20). Other risk factors for NAION, such as the presence of “crowded” optic disc, may have contributed to the occurrence of NAION in these studies.
Patients with known hereditary degenerative retinal disorders, including retinitis pigmentosa, were not included in the clinical trials, and use in these patients is not recommended.
Patient Counseling Information ()]PATIENT INFORMATIONALYQ®(ah-LIQ)(tadalafil tablets, USP)Read this patient information before you start taking ALYQ®and each time you get a refill. There may be new information. This information does not take the place of talking with your healthcare provider about your medical condition or treatment.
What is the most important information I should know about ALYQ®?Never take ALYQ®with any nitrate or guanylate cyclase stimulator medicines:
- Your blood pressure could drop quickly to an unsafe level
- You could get dizzy, faint and even have a heart attack or stroke.
Nitrates include:
- Medicines that treat chest pain (angina)
- Nitroglycerin in any form including tablets, patches, sprays, and ointments
- Other nitrate medicines (isosorbide mononitrate or dinitrate)
- Street drugs that are inhaled, called “poppers” (amyl nitrate, butyl nitrate or nitrite)
Guanylate cyclase stimulators include:
- Riociguat (Adempas®) a medicine that treats pulmonary arterial hypertension and chronic-thromboembolic pulmonary hypertension
Ask your healthcare provider or pharmacist if you are not sure if you take a nitrate or guanylate cyclase stimulator medicine.
What is ALYQ®?ALYQ®is a prescription medicine used to treat pulmonary arterial hypertension (PAH, high blood pressure in your lungs) to improve your ability to exercise.
It is not known if ALYQ®is safe or effective in children.
Who should not take ALYQ®?Do not take ALYQ®if you- take any medicines called nitrates.
- use recreational drugs called “poppers” like amyl nitrate, butyl nitrate or nitrite.
- take any medicines called guanylate cyclase stimulators
- are allergic to tadalafil or any other ingredient in ALYQ®. See“What are the ingredients in ALYQ®?”at the end of this leaflet.
See
“What is the most important information I should know about ALYQ®?”What should I tell my healthcare provider before taking ALYQ®?Before taking ALYQ®, tell your healthcare provider about all of your medical conditions, including if you:
- are allergic to ALYQ®or Cialis®or any of its ingredients. See the end of this leaflet for a complete list of ingredients in ALYQ®.
- have pulmonary veno-occlusive disease (PVOD)
- have heart problems
- have low blood pressure
- have liver problems
- have kidney problems or get dialysis
- have retinitis pigmentosa, a rare genetic eye disease
- have ever had any sudden vision loss, including any damage to your optic nerve or NAION.
- have ever had hearing problems such as ringing in the ears, dizziness, or loss of hearing
- have a deformed penis shape or Peyronie's disease
- have had an erection that lasted more than 4 hours
- have blood cell problems such as sickle cell anemia, multiple myeloma, or leukemia
- are pregnant or planning to become pregnant. Talk to your healthcare provider if you are pregnant or plan to become pregnant.
- are breastfeeding or plan to breast feed. If you breastfeed while taking ALYQ®, it is likely that ALYQ®will pass into your breast milk. You and your healthcare provider should decide if you will take ALYQ®or breastfeed. You should not do both.
Tell your healthcare provider about all the medicines you take,including prescription and non-prescription medicines, vitamins, and herbal supplements. ALYQ®and other medicines may affect each other.Especially tell your healthcare provider if you take any of these medicines:
- nitrates or guanylate cyclase stimulators (see“What is the most important information I should know about ALYQ®?”)
- alpha blockers, used to treat prostate disease and high blood pressure. Your blood pressure could suddenly drop. You could get dizzy or faint.
- protease inhibitors, used to treat HIV infection, such as ritonavir (Norvir®, Kaletra®)
- ketoconazole (Extina®, Xolegel®, Ketozole®, Nizoral A-D®, Nizoral®) itraconazole (Sporanox®)
- erythromycin (several brand names exist. Please consult your healthcare provider to determine if you are taking this medicine)
- rifampin (Rifadin®, Rifamate®, Rifater®, Rimactane®)
- bosentan (Tracleer®)
- phenobarbital, phenytoin (Dilantin®), carbamazepine (Tegretol®)
- CIALIS®or other medicines or treatments for erectile dysfunction (impotence).
- Tadalafil is also marketed as CIALIS®for the treatment of male erectile dysfunction (ED, impotence) and for the signs and symptoms of benign prostatic hyperplasia (BPH, enlarged prostate). Do not take both ALYQ®and CIALIS®. Do not take ALYQ®and other medicines or treatments for erectile dysfunction.
Ask your healthcare provider or pharmacist for a list of these medicines, if you are not sure.
Know the medicines you take. Keep a list of them and show it to your healthcare provider and pharmacist when you get a new medicine.
How should I take ALYQ®?- Take ALYQ®exactly as your healthcare provider tells you.
- Take ALYQ®tablets at the same time every day. You should take both tablets at the same time, one after the other, every day. Do not split your dose.
- ALYQ®can be taken with or without food.
- Do not change your dose or stop taking ALYQ®without speaking to your healthcare provider.
- If you take too much ALYQ®, call your healthcare provider or go to an emergency department right away.
What should I avoid while taking ALYQ®?Do not have more than 4 alcohol-containing drinks in a short period of time while you take ALYQ®. Drinking too much alcohol can lower your blood pressure. You could get dizzy or faint.
What are the possible side effects of ALYQ®?The following side effects were reported rarely in patients taking tadalafil:- Decreased eyesight or loss of vision in one or both eyes (NAION). If you notice a sudden decrease or loss of vision in one or both eyes, contact a healthcare provider right away.
- Sudden decrease or loss of hearing, sometimes with ringing in the ears and dizziness. If you notice a sudden decrease or loss of hearing, contact a healthcare provider right away.
- In men, an erection that lasts more than 4 hours (with or without pain).Talk to your healthcare provider or go to the emergency department right away.An erection that lasts more than 4 hoursmust be treated as soon as possible or you can have lasting damage to your penis, including the inability to have erections.
See
“What is the most important information I should know about ALYQ®?”The most common side effects with ALYQ®include:- headache
- muscle pain
- getting red or hot in the face (flushing)
- nausea
- pain in the arms, legs, or back
- upset stomach
- stuffy or congested nose
Tell your healthcare provider about any side effect that bothers you or does not go away.
These are not all the possible side effects of ALYQ®. For more information, ask your healthcare provider or pharmacist. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store ALYQ®?Store ALYQ®at room temperature between 59° and 86°F (15° and 30°C).
Keep ALYQ®and all medicines out of the reach of children.General information about the safe and effective use of ALYQ®Medicines are sometimes prescribed for conditions that are not mentioned in patient information leaflets. Do not use ALYQ®for a condition for which it was not prescribed. Do not give ALYQ®to other people, even if they have the same symptoms you have. It may harm them.
This patient information leaflet summarizes the most important information about ALYQ®. If you would like more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about ALYQ®that is written for healthcare professionals. For more information, call Teva at 1-888-838-2872.
What are the ingredients in ALYQ®?Active Ingredient: tadalafil
Inactive Ingredients: colloidal silicon dioxide, croscarmellose sodium, hypromellose, lactose monohydrate, magnesium stearate, pregelatinized (corn) starch, sodium lauryl sulfate, titanium dioxide, and triacetin.
Rx onlyBrands listed are the trademarks of their respective owners.
Manufactured In India By:
Watson Pharma Private LimitedVerna, Salcette Goa 403 722 IndiaManufactured For:
Teva PharmaceuticalsParsippany, NJ 07054Rev. A 4/2023
- Hearing loss [see Warnings and Precautions ()]
5.4 Hearing ImpairmentCases of sudden decrease or loss of hearing, which may be accompanied by tinnitus and dizziness, have been reported in patients taking tadalafil. It is not possible to determine whether these events are related directly to the use of PDE5 inhibitors or to other factors
[see Adverse Reactions ]. - Priapism [see Warnings and Precautions ()]
5.6 Prolonged ErectionThere have been reports of prolonged erections greater than 4 hours and priapism (painful erections greater than 6 hours in duration) for this class of compounds. Patients with conditions that might predispose them to priapism (such as sickle cell anemia, multiple myeloma, or leukemia), or in patients with anatomical deformation of the penis (such as angulation, cavernosal fibrosis, or Peyronie's disease) are at an increased risk. Priapism, if not treated promptly, can result in irreversible damage to the erectile tissue. Patients who have an erection lasting greater than 4 hours, whether painful or not, should seek emergency medical attention.
ALYQ® (tadalafil, USP), an oral treatment for pulmonary arterial hypertension, is a selective inhibitor of cyclic guanosine monophosphate (cGMP)-specific phosphodiesterase type 5 (PDE5). Tadalafil, USP has the molecular formula C22H19N3O4 representing a molecular weight of 389.40. The structural formula is:
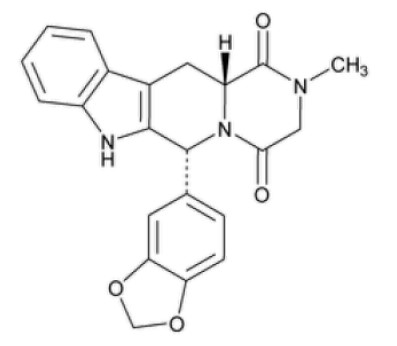
The chemical designation is pyrazino[1´,2´:1,6]pyrido[3,4-b]indole-1,4-dione, 6-(1,3-benzodioxol-5-yl)-2,3,6,7,12,12a-hexahydro-2-methyl-, (6R,12aR)-. It is a white or almost white powder that is practically insoluble in water, slightly soluble in methylene chloride, and freely soluble in dimethylsulfoxide.
ALYQ® (tadalafil tablets, USP) is available as white to off-white, oval shaped, film-coated tablets for oral administration. Each tablet contains 20 mg of tadalafil, USP and the following inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, hypromellose, lactose monohydrate, magnesium stearate, pregelatinized (corn) starch, sodium lauryl sulfate, titanium dioxide, and triacetin.
ALYQ® (tadalafil tablets, USP) is supplied as follows:
20 mg white to off-white, oval shaped, film-coated tablets (not scored) debossed with “J11” on one side and “T” on the other side.
Bottles of 60 NDC 0480-9277-06
Tadalafil is an inhibitor of phosphodiesterase type 5 (PDE5), the enzyme responsible for the degradation of cyclic guanosine monophosphate (cGMP). Pulmonary arterial hypertension is associated with impaired release of nitric oxide by the vascular endothelium and consequent reduction of cGMP concentrations in the pulmonary vascular smooth muscle. PDE5 is the predominant phosphodiesterase in the pulmonary vasculature. Inhibition of PDE5 by tadalafil increases the concentrations of cGMP resulting in relaxation of pulmonary vascular smooth muscle cells and vasodilation of the pulmonary vascular bed.
Studies