Amantadine
Amantadine Prescribing Information
Amantadine hydrochloride capsules are indicated for the prophylaxis and treatment of signs and symptoms of infection caused by various strains of influenza A virus. Amantadine hydrochloride capsules are also indicated in the treatment of parkinsonism and drug-induced extrapyramidal reactions.
Amantadine hydrochloride capsules are indicated for chemoprophylaxis against signs and symptoms of influenza A virus infection. Because amantadine does not completely prevent the host immune response to influenza A infection, individuals who take this drug may still develop immune responses to natural disease or vaccination and may be protected when later exposed to antigenically related viruses. Following vaccination during an influenza A outbreak, amantadine prophylaxis should be considered for the 2- to 4-week time period required to develop an antibody response.
Amantadine hydrochloride capsules are also indicated in the treatment of uncomplicated respiratory tract illness caused by influenza A virus strains especially when administered early in the course of illness. There are no well-controlled clinical studies demonstrating that treatment with amantadine hydrochloride capsules will avoid the development of influenza A virus pneumonitis or other complications in high risk patients.
There is no clinical evidence indicating that amantadine hydrochloride capsules are effective in the prophylaxis or treatment of viral respiratory tract illnesses other than those caused by influenza A virus strains.
The following points should be considered before initiating treatment or prophylaxis with amantadine hydrochloride capsules.
· Amantadine hydrochloride capsules are not a substitute for early vaccination on an annual basis as recommended by the Centers for Disease Control and Prevention Advisory Committee onImmunization Practices.
· Influenza viruses change over time. Emergence of resistance mutations could decrease drug effectiveness. Other factors (for example, changes in viral virulence) might also diminish clinical benefit of antiviral drugs. Prescribers should consider available information on influenza drug susceptibility patterns and treatment effects when deciding whether to use amantadine hydrochloride capsules.
Amantadine hydrochloride capsules are indicated in the treatment of idiopathic Parkinson’s disease (Paralysis Agitans), postencephalitic parkinsonism and symptomatic parkinsonism which may follow injury to the nervous system by carbon monoxide intoxication. It is indicated in those elderly patients believed to develop parkinsonism in association with cerebral arteriosclerosis. In the treatment of Parkinson’s disease, amantadine is less effective than levodopa, (-)-3-(3,4-dihydroxyphenyl)-L-alanine, and its efficacy in comparison with the anticholinergic antiparkinson drugs has not yet been established.
Amantadine hydrochloride is indicated in the treatment of drug-induced extrapyramidal reactions. Although anticholinergic-type side effects have been noted with amantadine when used in patients with drug-induced extrapyramidal reactions, there is a lower incidence of these side effects than that observed with the anticholinergic antiparkinson drugs.
The dose of amantadine hydrochloride capsules may need reduction in patients with congestive heart failure, peripheral edema, orthostatic hypotension, or impaired renal function (see
Adult
The adult daily dosage of amantadine hydrochloride capsules is 200 mg; two 100 mg capsules as a single daily dose. The daily dosage may be split into one capsule of 100 mg twice a day. If central nervous system effects develop in once-a-day dosage, a split dosage schedule may reduce such complaints. In persons 65 years of age or older, the daily dosage of amantadine hydrochloride capsules is 100 mg.
A 100 mg daily dose has also been shown in experimental challenge studies to be effective as prophylaxis in healthy adults who are not at high risk for influenza-related complications. However, it has not been demonstrated that a 100 mg daily dose is as effective as a 200 mg daily dose for prophylaxis, nor has the 100 mg daily dose been studied in the treatment of acute influenza illness. In recent clinical trials, the incidence of central nervous system (CNS) side effects associated with the 100 mg daily dose was at or near the level of placebo. The 100 mg dose is recommended for persons who have demonstrated intolerance to 200 mg of amantadine hydrochloride daily because of CNS or other toxicities.
Pediatric Patients
The total daily dose should be calculated on the basis of 2 to 4 mg/lb/day (4.4 to 8.8 mg/kg/day), but not to exceed 150 mg per day.
The total daily dose is 200 mg given as one capsule of 100 mg twice a day. The 100 mg daily dose has not been studied in this pediatric population. Therefore, there are no data which demonstrate that this dose is as effective as or is safer than the 200 mg daily dose in this patient population.
Prophylactic dosing should be started in anticipation of an influenza A outbreak and before or aftercontact with individuals with influenza A virus respiratory tract illness.
Amantadine hydrochloride capsules should be continued daily for at least 10 days following a known exposure. If amantadine is used chemoprophylactically in conjunction with inactivated influenza A virus vaccine until protective antibody responses develop, then it should be administered for 2 to 4 weeks after the vaccine has been given. When inactivated influenza A virus vaccine is unavailable or contraindicated, amantadine hydrochloride capsules should be administered for the duration of known influenza A in the community because of repeated and unknown exposure.
Treatment of influenza A virus illness should be started as soon as possible, preferably within 24 to 48 hours after onset of signs and symptoms, and should be continued for 24 to 48 hours after the disappearance of signs and symptoms.
Adult
The usual dose of amantadine hydrochloride capsules is 100 mg twice a day when used alone. Amantadine has an onset of action usually within 48 hours.
The initial dose of amantadine hydrochloride capsules is 100 mg daily for patients with serious associated medical illnesses or who are receiving high doses of other antiparkinson drugs. After one to several weeks at 100 mg once daily, the dose may be increased to 100 mg twice daily, if necessary.
Occasionally, patients whose responses are not optimal with amantadine hydrochloride capsules at 200 mg daily may benefit from an increase up to 400 mg daily in divided doses. However, such patients should be supervised closely by their physicians.
Patients initially deriving benefit from amantadine hydrochloride capsules not uncommonly experience a fall-off of effectiveness after a few months. Benefit may be regained by increasing the dose to 300 mg daily. Alternatively, temporary discontinuation of amantadine hydrochloride capsules for several weeks, followed by reinitiation of the drug, may result in regaining benefit in some patients. A decision to use other antiparkinson drugs may be necessary.
Some patients who do not respond to anticholinergic antiparkinson drugs may respond to amantadine hydrochloride capsules. When amantadine hydrochloride capsules or anticholinergic antiparkinson drugs are each used with marginal benefit, concomitant use may produce additional benefit.
When amantadine and levodopa are initiated concurrently, the patient can exhibit rapid therapeutic benefits. Amantadine hydrochloride capsules should be held constant at 100 mg daily or twice daily while the daily dose of levodopa is gradually increased to optimal benefit.
When amantadine is added to optimal well-tolerated doses of levodopa, additional benefit may result, including smoothing out the fluctuations in improvement which sometimes occur in patients on levodopa alone. Patients who require a reduction in their usual dose of levodopa because of development of side effects may possibly regain lost benefit with the addition of amantadine hydrochloride capsules.
Adult
The usual dose of amantadine hydrochloride capsules is 100 mg twice a day. Occasionally, patients whose responses are not optimal with amantadine hydrochloride capsules at 200 mg daily may benefit from an increase up to 300 mg daily in divided doses.
Depending upon creatinine clearance, the following dosage adjustments are recommended:
CREATININE CLEARANCE (mL/min/1.73m2) | AMANTADINE HYDROCHLORIDE CAPSULES DOSAGE |
| 30 to 50 | 200 mg 1st day and 100 mg each day thereafter |
| 15 to 29 | 200 mg 1st day followed by 100 mg on alternate days |
| <15 | 200 mg every 7 days |
The recommended dosage for patients on hemodialysis is 200 mg every 7 days.
Amantadine hydrochloride capsules are contraindicated in patients with known hypersensitivity to amantadine hydrochloride or to any of the other ingredients in amantadine hydrochloride capsules.
The adverse reactions reported most frequently at the recommended dose of amantadine (5 to 10%) are: nausea, dizziness (lightheadedness), and insomnia.
Less frequently (1 to 5%) reported adverse reactions are: depression, anxiety and irritability, hallucinations, confusion, anorexia, dry mouth, constipation, ataxia, livedo reticularis, peripheral edema, orthostatic hypotension, headache, somnolence, nervousness, dream abnormality, agitation, dry nose, diarrhea and fatigue.
Infrequently (0.1 to 1%) occurring adverse reactions are: congestive heart failure, psychosis, urinary retention, dyspnea, skin rash, vomiting, weakness, slurred speech, euphoria, thinking abnormality, amnesia, hyperkinesia, hypertension, decreased libido, and visual disturbance, including punctate subepithelial or other corneal opacity, corneal edema, decreased visual acuity, sensitivity to light, and optic nerve palsy.
Rare (less than 0.1%) occurring adverse reactions are: instances of convulsion, leukopenia, neutropenia, eczematoid dermatitis, oculogyric episodes, suicidal attempt, suicide, and suicidal ideation (see
Other adverse reactions reported during postmarketing experience with amantadine usage include:
coma, stupor, delirium, hypokinesia, hypertonia, delusions, aggressive behavior, paranoid reaction, manic reaction, involuntary muscle contractions, gait abnormalities, paresthesia, EEG changes, and tremor. Abrupt discontinuation may also precipitate delirium, agitation, delusions, hallucinations, paranoid reaction, stupor, anxiety, depression and slurred speech;
cardiac arrest, arrhythmias including malignant arrhythmias, hypotension, and tachycardia;
acute respiratory failure, pulmonary edema, and tachypnea;
dysphagia;
leukocytosis, agranulocytosis;
keratitis, mydriasis, and corneal edema;
pruritus and diaphoresis;
neuroleptic malignant syndrome (see
elevated: CPK, BUN, serum creatinine, alkaline phosphatase, LDH, bilirubin, GGT, SGOT, and SGPT.
Careful observation is required when amantadine is administered concurrently with central nervous system stimulants. Agents with anticholinergic properties may potentiate the anticholinergic-like side effects of amantadine.
Coadministration of thioridazine has been reported to worsen the tremor in elderly patients with Parkinson’s disease, however, it is not known if other phenothiazines produce a similar response. Coadministration of triamterene and hydrochlorothiazide capsules resulted in a higher plasma amantadine concentration in a 61-year-old man receiving amantadine (hydrochloride capsules) 100 mg t.i.d. for Parkinson’s disease1. It is not known which of the components of triamterene and hydrochlorothiazide capsules contributed to the observation or if related drugs produce a similar response. Coadministration of quinine or quinidine with amantadine was shown to reduce the renal clearance of amantadine by about 30%.
The concurrent use of amantadine with live attenuated influenza vaccine (LAIV) intranasal has not been evaluated. However, because of the potential for interference between these products, LAIV should not be administered within 2 weeks before or 48 hours after administration of amantadine, unless medically indicated. The concern about possible interference arises from the potential for antiviral drugs to inhibit replication of live vaccine virus. Trivalent inactivated influenza vaccine can be administered at any time relative to use of amantadine.
Amantadine hydrochloride is designated chemically as 1-adamantanamine hydrochloride. Its molecular weight is 187.71 with a molecular formula C10H18NCl. It has the following structural formula:
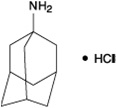
Amantadine hydrochloride is a stable white or almost white, crystalline powder, freely soluble in water and soluble in alcohol and in chloroform.
Amantadine hydrochloride has pharmacological actions as both an anti-Parkinson and an antiviral drug.
Amantadine hydrochloride is available as 100 mg capsules for oral administration. Inactive ingredients: microcrystalline cellulose, hydroxypropyl cellulose, croscarmellose sodium, colloidal silicon dioxide, magnesium stearate, gelatin, titanium dioxide, FD&C Blue #1, FD&C Red #40. The non-volatile components of imprinting ink are shellac, propylene glycol, potassium hydroxide and titanium oxide.
Meets USP Dissolution Test 2.