Amantadine Hcl
(Amantadine Hydrochloride)Amantadine Hcl Prescribing Information
Amantadine Hydrochloride Capsules, USP are indicated for the prophylaxis and treatment of signs and symptoms of infection caused by various strains of influenza A virus. Amantadine Hydrochloride Capsules, USP are also indicated in the treatment of parkinsonism and drug-induced extrapyramidal reactions.
Following vaccination during an influenza A outbreak, Amantadine Hydrochloride Capsules, USP prophylaxis should be considered for the 2- to 4-week time period required to develop an antibody response.
There is no clinical evidence indicating that Amantadine Hydrochloride Capsules, USP are effective in the prophylaxis or treatment of viral respiratory tract illnesses other than those caused by influenza A virus strains.
The following points should be considered before initiating treatment or prophylaxis with Amantadine Hydrochloride Capsules, USP:
- Amantadine Hydrochloride Capsules, USP is not a substitute for early vaccination on an annual basis as recommended by the Centers for Disease Control and Prevention Advisory Committee on Immunization Practices.
- Influenza viruses change over time. Emergence of resistance mutations could decrease drug effectiveness. Other factors (for example, changes in viral virulence) might also diminish clinical benefit of antiviral drugs. Prescribers should consider available information on influenza drug susceptibility patterns and treatment effects when deciding whether to use Amantadine Hydrochloride Capsules, USP.
The dosage of Amantadine Hydrochloride Capsules may need to be reduced in patients with congestive heart failure, peripheral edema, orthostatic hypotension, or impaired renal function (see
| CREATININE CLEARANCE (mL/min/1.73 m2) | Amantadine Hydrochloride Capsules, USP DOSAGE |
| 30 to 50 | 200 mg 1st day and 100 mg each day thereafter |
| 15 to 29 | 200 mg 1st day followed by 100 mg on alternate days |
| < 15 | 200 mg every 7 days |
The recommended dosage for patients on hemodialysis is 200 mg every 7 days.
Amantadine Hydrochloride Capsules are contraindicated in patients with known hypersensitivity to amantadine hydrochloride or to any of the other ingredients in Amantadine Hydrochloride Capsules.
The adverse reactions reported most frequently at the recommended dose of amantadine hydrochloride (5 to 10%) are: nausea, dizziness (lightheadedness), and insomnia.
Less frequently (1 to 5%) reported adverse reactions are: depression, anxiety and irritability, hallucinations, confusion, anorexia, dry mouth, constipation, ataxia, livedo reticularis, peripheral edema, orthostatic hypotension, headache, somnolence, nervousness, dream abnormality, agitation, dry nose, diarrhea and fatigue.
Infrequently (0.1 to 1%) occurring adverse reactions are: congestive heart failure, psychosis, urinary retention, dyspnea, skin rash, vomiting, weakness, slurred speech, euphoria, thinking abnormality, amnesia, hyperkinesia, hypertension, decreased libido, and visual disturbance, including punctate subepithelial or other corneal opacity, corneal edema, decreased visual acuity, sensitivity to light, and optic nerve palsy.
Rare (less than 0.1%) occurring adverse reactions are: instances of convulsion, leukopenia, neutropenia, eczematoid dermatitis, oculogyric episodes, suicidal attempt, suicide, and suicidal ideation (see
Deaths due to drug accumulation (overdose) have been reported in patients with renal impairment, who were prescribed higher than recommended doses of SYMMETREL (amantadine) for their level of renal function (see
Patients receiving amantadine hydrochloride capsules who note central nervous system effects or blurring of vision should be cautioned against driving or working in situations where alertness and adequate motor coordination are important.
Patients with Parkinson's disease improving on amantadine hydrochloride capsules should resume normal activities gradually and cautiously, consistent with other medical considerations, such as the presence of osteoporosis or phlebothrombosis.
Because Amantadine Hydrochloride Capsules has anticholinergic effects and may cause mydriasis, it should not be given to patients with untreated angle closure glaucoma.
Agents with anticholinergic properties may potentiate the anticholinergic-like side effects of amantadine.
Coadministration of thioridazine has been reported to worsen the tremor in elderly patients with Parkinson's disease, however, it is not known if other phenothiazines produce a similar response.
Coadministration of Dyazide
® (triamterene/hydrochlorothiazide) resulted in a higher plasma amantadine concentration in a 61-year-old man receiving amantadine hydrochloride 100 mg TID for Parkinson's disease.
1 It is not known which of the components of Dyazide
® contributed to the observation or if related drugs produce a similar response.
Coadministration of quinine or quinidine with amantadine was shown to reduce the renal clearance of amantadine by about 30%.
The concurrent use of amantadine hydrochloride with live attenuated influenza vaccine (LAIV) intranasal has not been evaluated. However, because of the potential for interference between these products, LAIV should not be administered within 2 weeks before or 48 hours after administration of amantadine hydrochloride, unless medically indicated. The concern about possible interference arises from the potential for antiviral drugs to inhibit replication of live vaccine virus. Trivalent inactivated influenza vaccine can be administered at any time relative to use of amantadine.
Amantadine hydrochloride, USP is designated chemically as 1-adamantanamine hydrochloride.
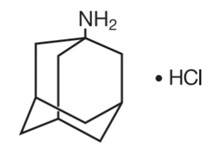
Amantadine hydrochloride is a stable white or nearly white crystalline powder, freely soluble in water and soluble in alcohol and in chloroform.
Amantadine hydrochloride has pharmacological actions as both an anti-Parkinson and an antiviral drug.
Each capsule intended for oral administration contains 100 mg amantadine hydrochloride and has the following inactive ingredients: yellow iron oxide, gelatin, glycerin, hydrogenated vegetable oil, lecithin-bleached, soybean oil, sorbitol, sorbitan, mannitol, titanium dioxide, white beeswax, vegetable shortening, simethicone and iron oxide (black imprint ink).
USP Dissolution Test Pending.