Amantadine Hydrochloride
Amantadine Hydrochloride Prescribing Information
Amantadine Hydrochloride is indicated in the treatment of drug-induced extrapyramidal reactions. Although anticholinergic-type side effects have been noted with Amantadine Hydrochloride Tablets when used in patients with drug-induced extrapyramidal reactions, there is a lower incidence of these side effects than that observed with the anticholinergic antiparkinson drugs.
Depending upon creatinine clearance, the following dosage adjustments are recommended:
CREATININE CLEARANCE (mL/min/1.73 m 2) | AMANTADINE HYDROCLORIDE DOSAGE |
| 30 to 50 | 200 mg 1st day and 100 mg each day thereafter |
| 15 to 29 | 200 mg 1st day followed by 100 mg on alternate days |
| < 15 | 200 mg every 7 days |
The recommended dosage for patients on hemodialysis is 200 mg every 7 days.
Amantadine Hydrochloride is contraindicated in patients with known hypersensitivity to amantadine hydrochloride or to any of the other ingredients in Amantadine Hydrochloride Tablets and Capsules.
The adverse reactions reported most frequently at the recommended dose of amantadine hydrochloride (5 to 10%) are: nausea, dizziness (lightheadedness), and insomnia.
Less frequently (1 to 5%) reported adverse reactions are: depression, anxiety and irritability, hallucinations, confusion, anorexia, dry mouth, constipation, ataxia, livedo reticularis, peripheral edema, orthostatic hypotension, headache, somnolence, nervousness, dream abnormality, agitation, dry nose, diarrhea and fatigue.
Infrequently (0.1 to 1%) occurring adverse reactions are: congestive heart failure, psychosis, urinary retention, dyspnea, skin rash, vomiting, weakness, slurred speech, euphoria, thinking abnormality, amnesia, hyperkinesia, hypertension, decreased libido, and visual disturbance, including punctate subepithelial or other corneal opacity, corneal edema, decreased visual acuity, sensitivity to light, and optic nerve palsy.
Rare (less than 0.1%) occurring adverse reactions are: instances of convulsion, leukopenia, neutropenia, eczematoid dermatitis, oculogyric episodes, suicidal attempt, suicide, and suicidal ideation (see WARNINGS).
Other adverse reactions reported during postmarketing experience with amantadine hydrochloride usage include:
Amantadine hydrochloride is designated generically as amantadine hydrochloride and chemically as 1-adamantanamine hydrochloride.
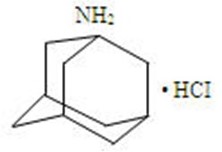 C
C
10H
17N•HCl
Amantadine hydrochloride is a stable white or nearly white crystalline powder, freely soluble in water and soluble in alcohol and in chloroform.
Amantadine hydrochloride has pharmacological actions as both an anti-Parkinson and an antiviral drug.
Each tablet intended for oral administration contains 100 mg amantadine hydrochloride and has the following inactive ingredients: microcrystalline cellulose, povidone, sodium starch glycolate, magnesium stearate, and colloidal silicon dioxide.
Each capsule intended for oral administration contains 100 mg amantadine hydrochloride. Inactive ingredients: magnesium stearate, microcrystalline cellulose, Povidone , Sodium Starch Glycolate, Colloidal Silicon Dioxide. The capsule shells and imprinting ink contain FD&C Blue #1, FD&C Red #40, gelatin, FD&C Yellow #6, sodium lauryl sulfate, and titanium dioxide.
Amantadine hydrochloride is well absorbed orally. Maximum plasma concentrations are directly related to dose for doses up to 200 mg/day. Doses above 200 mg/day may result in a greater than proportional increase in maximum plasma concentrations. It is primarily excreted unchanged in the urine by glomerular filtration and tubular secretion. Eight metabolites of amantadine have been identified in human urine. One metabolite, an N-acetylated compound, was quantified in human urine and accounted for 5 to 15% of the administered dose. Plasma acetylamantadine accounted for up to 80% of the concurrent amantadine plasma concentration in 5 of 12 healthy volunteers following the ingestion of a 200 mg dose of amantadine. Acetylamantadine was not detected in the plasma of the remaining seven volunteers. The contribution of this metabolite to efficacy or toxicity is not known.
There appears to be a relationship between plasma amantadine concentrations and toxicity. As concentration increases, toxicity seems to be more prevalent, however, absolute values of amantadine concentrations associated with adverse effects have not been fully defined.
Amantadine pharmacokinetics were determined in 24 normal adult male volunteers after the oral administration of a single amantadine hydrochloride 100 mg soft gel capsule. The mean ± SD maximum plasma concentration was 0.22 ± 0.03 mcg/mL (range: 0.18 to 0.32 mcg/mL). The time to peak concentration was 3.3 ± 1.5 hours (range: 1.5 to 8 hours). The apparent oral clearance was 0.28 ± 0.11 L/hr/kg (range: 0.14 to 0.62 L/hr/kg). The half-life was 17 ± 4 hours (range: 10 to 25 hours). Across other studies, amantadine plasma half-life has averaged 16 ± 6 hours (range: 9 to 31 hours) in 19 healthy volunteers.
After oral administration of a single dose of 100 mg amantadine syrup to five healthy volunteers, the mean ± SD maximum plasma concentration C
max was 0.24 ± 0.04 mcg/mL and ranged from 0.18 to 0.28 mcg/mL. After 15 days of amantadine 100 mg b.i.d., the Cmax was 0.47 ± 0.11 mcg/mL in four of the five volunteers. The administration of amantadine tablets as a 200 mg single dose to 6 healthy subjects resulted in a C
max of 0.51 ± 0.14 mcg/mL. Across studies, the time to C
max (T
max) averaged about 2 to 4 hours.
Plasma amantadine clearance ranged from 0.2 to 0.3 L/hr/kg after the administration of 5 mg to 25 mg intravenous doses of amantadine to 15 healthy volunteers.
In six healthy volunteers, the ratio of amantadine renal clearance to apparent oral plasma clearance was 0.79 ± 0.17 (mean ± SD).
The volume of distribution determined after the intravenous administration of amantadine to 15 healthy subjects was 3 to 8 L/kg, suggesting tissue binding. Amantadine, after single oral 200 mg doses to 6 healthy young subjects and to 6 healthy elderly subjects has been found in nasal mucus at mean ± SD concentrations of 0.15 ± 0.16, 0.28 ± 0.26, and 0.39 ± 0.34 mcg/g at 1, 4, and 8 hours after dosing, respectively. These concentrations represented 31 ± 33%, 59 ± 61%, and 95 ± 86% of the corresponding plasma amantadine concentrations. Amantadine is approximately 67% bound to plasma proteins over a concentration range of 0.1 to 2 mcg/mL. Following the administration of amantadine 100 mg as a single dose, the mean ± SD red blood cell to plasma ratio ranged from 2.7 ± 0.5 in 6 healthy subjects to 1.4 ± 0.2 in 8 patients with renal insufficiency.
The apparent oral plasma clearance of amantadine is reduced and the plasma half-life and plasma concentrations are increased in healthy elderly individuals age 60 and older. After single dose administration of 25 to 75 mg to 7 healthy, elderly male volunteers, the apparent plasma clearance of amantadine was 0.10 ± 0.04 L/hr/kg (range 0.06 to 0.17 L/hr/kg) and the half-life was 29 ± 7 hours (range 20 to 41 hours). Whether these changes are due to decline in renal function or other age-related factors is not known.
In a study of young healthy subjects (n=20), mean renal clearance of amantadine, normalized for body mass index, was 1.5 fold higher in males compared to females (p<0.032).
Compared with otherwise healthy adult individuals, the clearance of amantadine is significantly reduced in adult patients with renal insufficiency. The elimination half-life increases two to three fold or greater when creatinine clearance is less than 40 mL/min/1.73m
2 and averages eight days in patients on chronic maintenance hemodialysis. Amantadine is removed in negligible amounts by hemodialysis.
The pH of the urine has been reported to influence the excretion rate of amantadine hydrochloride. Since the excretion rate of amantadine hydrochloride increases rapidly when the urine is acidic, the administration of urine acidifying drugs may increase the elimination of the drug from the body.