Amiodarone Hydrochloride
Amiodarone Hydrochloride Prescribing Information
Amiodarone injection is indicated for initiation of treatment and prophylaxis of frequently recurring ventricular fibrillation (VF) and hemodynamically unstable ventricular tachycardia (VT) in patients refractory to other therapy. Amiodarone also can be used to treat patients with VT/VF for whom oral amiodarone is indicated, but who are unable to take oral medication. During or after treatment with amiodarone, patients may be transferred to oral amiodarone therapy
Amiodarone shows considerable interindividual variation in response. Although a starting dose adequate to suppress life-threatening arrhythmias is needed, close monitoring with adjustment of dose is essential. The recommended starting dose of amiodarone is about 1000 mg over the first 24 hours of therapy, delivered by the following infusion regimen:
Loading Infusions | First Rapid: | 150 mg over the FIRST 10 minutes (15 mg/min). Add 3 mL of amiodarone (150 mg) to 100 mL D5W (concentration = 1.5 mg/mL). Infuse 100 mL over 10 minutes. |
Followed by Slow: | 360 mg over the NEXT 6 hours (1 mg/min). Add 18 mL of amiodarone (900 mg) to 500 mL D5W (concentration = 1.8 mg/mL). Infuse 200 mL at a rate of 0.556 mL/min. | |
Maintenance Infusion | 540 mg over the REMAINING 18 hours (0.5 mg/min). Decrease the rate of the slow loading infusion to 0.278 mL/min. |
After the first 24 hours, continue the maintenance infusion rate of 0.5 mg/min (720 mg per 24 hours) utilizing a concentration of 1 mg/mL to 6 mg/mL (Use a central venous catheter for amiodarone concentrations greater than 2 mg/mL). The rate of the maintenance infusion may be increased to achieve effective arrhythmia suppression.
In the event of breakthrough episodes of VF or hemodynamically unstable VT, use 150 mg supplemental infusions of amiodarone (mixed in 100 mL of D5W and infused over 10 minutes to minimize the potential for hypotension).
The first 24-hour dose may be individualized for each patient; however, in controlled clinical trials, mean daily doses above 2100 mg were associated with an increased risk of hypotension. Do not exceed an initial infusion rate of 30 mg/min.
Based on the experience from clinical studies of intravenous amiodarone, a maintenance infusion of up to 0.5 mg/min can be continued for 2 to 3 weeks regardless of the patient's age, renal function, or left ventricular function. There has been limited experience in patients receiving intravenous amiodarone for longer than 3 weeks.
The surface properties of solutions containing injectable amiodarone are altered such that the drop size may be reduced. This reduction may lead to underdosage of the patient by up to 30% if drop counter infusion sets are used. Amiodarone must be delivered by a volumetric infusion pump.
Administer amiodarone, whenever possible, through a central venous catheter dedicated to that purpose. Use an in-line filter during administration.
Intravenous amiodarone loading infusions at much higher concentrations and rates of infusion much faster than recommended have resulted in hepatocellular necrosis and acute renal failure, leading to death
Intravenous amiodarone concentrations greater than 3 mg/mL in D5W have been associated with a high incidence of peripheral vein phlebitis; however, concentrations of 2.5 mg/mL or less appear to be less irritating. Therefore, for infusions longer than 1 hour, do not exceed amiodarone concentrations of 2 mg/mL, unless a central venous catheter is used
Amiodarone infusions exceeding 2 hours must be administered in glass or polyolefin bottles containing D5W. Do not use evacuated glass containers for admixing, as incompatibility with a buffer in the container may cause precipitation.
Amiodarone adsorbs to polyvinyl chloride (PVC) tubing, but all of the clinical experience has been with PVC tubing and the concentrations and rates of infusion provided in DOSAGE AND ADMINISTRATION reflect dosing in these studies.
Amiodarone has been found to leach out plasticizers, including DEHP [di-(2-ethylhexyl)phthalate] from intravenous tubing (including PVC tubing). The degree of leaching increases when infusing amiodarone at higher concentrations and lower flow rates than provided in DOSAGE AND ADMINISTRATION. Polysorbate 80, a component of amiodarone hydrochloride injection, is also known to leach DEHP from PVC
Amiodarone does not need to be protected from light during administration.
NOTE: Inspect parenteral drug products for particulate matter and discoloration prior to administration, whenever solution and container permit – solution should be clear.
CAUTION: Do not use plastic containers in series connections. Such use could result in air embolism due to residual air being drawn from the primary container before the administration of the fluid from the secondary container is complete.
Discard unused portion.
Solution | Concentration (mg/mL) | Container | Comments |
|---|---|---|---|
5% Dextrose in Water (D5W) | 1.0 - 6.0 | PVC | Physically compatible, with amiodarone loss < 10% at 2 hours at room temperature. |
5% Dextrose in Water (D5W) | 1.0 - 6.0 | Polyolefin, Glass | Physically compatible, with no amiodarone loss at 24 hours at room temperature. |
Amiodarone in D5W Injection forms precipitates with the drugs shown in Table 3. If coadministration of the following drugs is necessary, use separate intravenous administration lines.
| D5W = Dextrose 5% in Sterile Water, NS = Normal Saline | |||||||||
Drug | Vehicle | Amiodarone Concentration | |||||||
Aminophylline | D5W; NS | 4 mg/mL | |||||||
Amoxicillin Sodium-Clavulanic Acid | unknown | 12.5 mg/mL | |||||||
Ampicillin Sodium-Sulbactam Sodium | NS | 6 mg/mL | |||||||
Argatroban | D5W | 1.8 mg/mL | |||||||
Bivalirudin | D5W | 4 mg/mL | |||||||
Cefamandole Nafate | D5W | 4 mg/mL | |||||||
Cefazolin Sodium | D5W | 4 mg/mL | |||||||
Ceftazidime | D5W | 6 mg/mL | |||||||
Digoxin | D5W | 6 mg/mL | |||||||
Furosemide (10 mg/mL) | D5W | 6 mg/mL | |||||||
Mezlocillin Sodium | D5W | 4 mg/mL | |||||||
Heparin Sodium | D5W | -- | |||||||
Imipenem-Cilastin Sodium | D5W | 6 mg/mL | |||||||
Magnesium Sulfate (500 mg/mL) | D5W | 6 mg/mL | |||||||
Micafungin | NS | 4 mg/mL | |||||||
Piperacillin Sodium –Tazobactam Sodium | D5W | 6 mg/mL | |||||||
Potassium Phosphates | D5W | 6 mg/mL | |||||||
Sodium Bicarbonate | D5W | 3 mg/mL | |||||||
Sodium Nitroprusside | D5W | 1.5, 6 and 15 mg/mL | |||||||
Sodium Phosphates | D5W | 6 mg/mL | |||||||
Intravenous to Oral Transition
Patients whose arrhythmias have been suppressed by amiodarone may be switched to oral amiodarone. The optimal dose for changing from intravenous to oral administration of amiodarone will depend on the dose of intravenous amiodarone already administered, as well as the bioavailability of oral amiodarone. When changing to oral amiodarone therapy, clinical monitoring is recommended, particularly for elderly patients. See package insert for oral amiodarone.
Since grapefruit juice is known to inhibit CYP3A-mediated metabolism of oral amiodarone in the intestinal mucosa, resulting in increased plasma levels of amiodarone, do not drink grapefruit juice during treatment with oral amiodarone
Table 4 provides suggested doses of oral amiodarone to be initiated after varying durations of amiodarone administration. These recommendations are made on the basis of a similar total body amount of amiodarone delivered by the intravenous and oral routes, based on 50% bioavailability of oral amiodarone.
Duration of Amiodarone Infusion Assuming a 720 mg/day infusion (0.5 mg/min). | Initial Daily Dose of Oral Amiodarone |
|---|---|
< 1 week | 800 mg - 1600 mg |
1 to 3 weeks | 600 mg - 800 mg |
> 3 weeksIntravenous amiodarone is not intended for maintenance treatment. | 400 mg |
- The recommended starting dose is about 1000 mg over the first 24 hours of therapy, delivered by the following infusion regimen :
o Initial Load: 150 mg in 100 mL (in D5W) infused over 10 minutes
o Followed by: 1 mg/min for 6 hours
o Followed by: 0.5 mg/min thereafter
- For breakthrough episodes of VF or hemodynamically unstable VT, repeat the Initial Load
Use amiodarone for acute treatment until the patient's ventricular arrhythmias are stabilized. Most patients will require this therapy for 48 to 96 hours, but amiodarone may be safely administered for longer periods if necessary.
Amiodarone shows considerable interindividual variation in response. Although a starting dose adequate to suppress life-threatening arrhythmias is needed, close monitoring with adjustment of dose is essential. The recommended starting dose of amiodarone is about 1000 mg over the first 24 hours of therapy, delivered by the following infusion regimen:
Loading Infusions | First Rapid: | 150 mg over the FIRST 10 minutes (15 mg/min). Add 3 mL of amiodarone (150 mg) to 100 mL D 5W (concentration = 1.5 mg/mL). Infuse 100 mL over 10 minutes. |
Followed by Slow: | 360 mg over the NEXT 6 hours (1 mg/min). Add 18 mL of amiodarone (900 mg) to 500 mL D 5W (concentration = 1.8 mg/mL). Infuse 200 mL at a rate of 0.556 mL/min. | |
Maintenance Infusion | 540 mg over the REMAINING 18 hours (0.5 mg/min). Decrease the rate of the slow loading infusion to 0.278 mL/min. |
After the first 24 hours, continue the maintenance infusion rate of 0.5 mg/min (720 mg per 24 hours) utilizing a concentration of 1 mg/mL to 6 mg/mL (Use a central venous catheter for amiodarone concentrations greater than 2 mg/mL). The rate of the maintenance infusion may be increased to achieve effective arrhythmia suppression.
In the event of breakthrough episodes of VF or hemodynamically unstable VT, use 150 mg supplemental infusions of amiodarone (mixed in 100 mL of D
5W and infused over 10 minutes to minimize the potential for hypotension).
The first 24-hour dose may be individualized for each patient; however, in controlled clinical trials, mean daily doses above 2100 mg were associated with an increased risk of hypotension. Do not exceed an initial infusion rate of 30 mg/min.
Based on the experience from clinical studies of intravenous amiodarone, a maintenance infusion of up to 0.5 mg/min can be continued for 2 to 3 weeks regardless of the patient's age, renal function, or left ventricular function. There has been limited experience in patients receiving intravenous amiodarone for longer than 3 weeks.
The surface properties of solutions containing injectable amiodarone are altered such that the drop size may be reduced. This reduction may lead to underdosage of the patient by up to 30% if drop counter infusion sets are used. Amiodarone must be delivered by a volumetric infusion pump.
Administer amiodarone, whenever possible, through a central venous catheter dedicated to that purpose. Use an in-line filter during administration.
Intravenous amiodarone loading infusions at much higher concentrations and rates of infusion much faster than recommended have resulted in hepatocellular necrosis and acute renal failure, leading to death
Elevations of blood hepatic enzyme values [alanine aminotransferase (ALT), aspartate aminotransferase (AST), and gamma-glutamyl transferase (GGT)] are commonly seen in patients with immediately life-threatening VT/VF. Interpreting elevated AST activity can be difficult because the values may be elevated in patients who have had recent myocardial infarction, congestive heart failure, or multiple electrical defibrillations. Approximately 54% of patients receiving intravenous amiodarone in clinical studies had baseline liver enzyme elevations, and 13% had clinically significant elevations. In 81% of patients with both baseline and on-therapy data available, the liver enzyme elevations either improved during therapy or remained at baseline levels. Baseline abnormalities in hepatic enzymes are not a contraindication to treatment. Elevated bilirubin levels have been reported in patients administered intravenous amiodarone.
Acute, centrolobular confluent hepatocellular necrosis leading to hepatic coma, acute renal failure, and death has been associated with the administration of intravenous amiodarone
In patients with life-threatening arrhythmias, the potential risk of hepatic injury should be weighed against the potential benefit of amiodarone therapy. Carefully monitor patients receiving amiodarone for evidence of progressive hepatic injury. In such cases, consider reducing the rate of administration or withdrawing amiodarone.
Intravenous amiodarone concentrations greater than 3 mg/mL in D
5W have been associated with a high incidence of peripheral vein phlebitis; however, concentrations of 2.5 mg/mL or less appear to be less irritating. Therefore, for infusions longer than 1 hour, do not exceed amiodarone concentrations of 2 mg/mL, unless a central venous catheter is used
The following adverse reactions have been reported in the post-marketing experience during or in close temporal relationship to intravenous amiodarone administration. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Amiodarone infusions exceeding 2 hours must be administered in glass or polyolefin bottles containing D
5W. Do not use evacuated glass containers for admixing, as incompatibility with a buffer in the container may cause precipitation.
Amiodarone adsorbs to polyvinyl chloride (PVC) tubing, but all of the clinical experience has been with PVC tubing and the concentrations and rates of infusion provided in DOSAGE AND ADMINISTRATION reflect dosing in these studies.
Amiodarone has been found to leach out plasticizers, including DEHP [di-(2-ethylhexyl)phthalate] from intravenous tubing (including PVC tubing). The degree of leaching increases when infusing amiodarone at higher concentrations and lower flow rates than provided in DOSAGE AND ADMINISTRATION. Polysorbate 80, a component of amiodarone hydrochloride injection, is also known to leach DEHP from PVC
Amiodarone Hydrochloride Injection, USP contains amiodarone hydrochloride, USP (C25H29I2NO3•HCl), a class III antiarrhythmic drug. Amiodarone hydrochloride is (2-butyl-3-benzo-furanyl)[4-[2-(diethylamino)ethoxy]-3,5-diiodophenyl]methanone hydrochloride.
Amiodarone hydrochloride has the following structural formula:
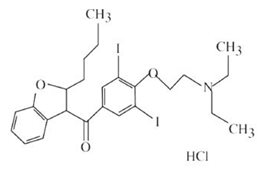
Amiodarone HCl is a white to slightly yellow crystalline powder, and is very slightly soluble in water. It has a molecular weight of 681.78 and contains 37.3% iodine by weight. Amiodarone Hydrochloride Injection, USP is a sterile clear, pale-yellow micellar solution visually free from particulates. Each milliliter of the Amiodarone Hydrochloride Injection, USP formulation contains 50 mg of amiodarone hydrochloride, 20.2 mg of benzyl alcohol, 100 mg of polysorbate 80, and water for injection. Amiodarone Hydrochloride Injection, USP is injection administration.
Amiodarone Hydrochloride Injection, USP contains polysorbate 80, which is known to leach di-(2-ethylhexyl)phthalate (DEHP) from polyvinylchloride (PVC)

Amiodarone does not need to be protected from light during administration.
NOTE: Inspect parenteral drug products for particulate matter and discoloration prior to administration, whenever solution and container permit – solution should be clear.
CAUTION: Do not use plastic containers in series connections. Such use could result in air embolism due to residual air being drawn from the primary container before the administration of the fluid from the secondary container is complete.
Discard unused portion.
Solution | Concentration (mg/mL) | Container | Comments |
|---|---|---|---|
5% Dextrose in Water (D | 1.0 - 6.0 | PVC | Physically compatible, with amiodarone loss < 10% at 2 hours at room temperature. |
5% Dextrose in Water (D | 1.0 - 6.0 | Polyolefin, Glass | Physically compatible, with no amiodarone loss at 24 hours at room temperature. |
Amiodarone in D
5W Injection forms precipitates with the drugs shown in Table 3. If coadministration of the following drugs is necessary, use separate intravenous administration lines.
| D 5W = Dextrose 5% in Sterile Water, NS = Normal Saline | |||||||||
Drug | Vehicle | Amiodarone Concentration | |||||||
Aminophylline | D | 4 mg/mL | |||||||
Amoxicillin Sodium-Clavulanic Acid | unknown | 12.5 mg/mL | |||||||
Ampicillin Sodium-Sulbactam Sodium | NS | 6 mg/mL | |||||||
Argatroban | D | 1.8 mg/mL | |||||||
Bivalirudin | D | 4 mg/mL | |||||||
Cefamandole Nafate | D | 4 mg/mL | |||||||
Cefazolin Sodium | D | 4 mg/mL | |||||||
Ceftazidime | D | 6 mg/mL | |||||||
Digoxin | D | 6 mg/mL | |||||||
Furosemide (10 mg/mL) | D | 6 mg/mL | |||||||
Mezlocillin Sodium | D | 4 mg/mL | |||||||
Heparin Sodium | D | -- | |||||||
Imipenem-Cilastin Sodium | D | 6 mg/mL | |||||||
Magnesium Sulfate (500 mg/mL) | D | 6 mg/mL | |||||||
Micafungin | NS | 4 mg/mL | |||||||
Piperacillin Sodium –Tazobactam Sodium | D | 6 mg/mL | |||||||
Potassium Phosphates | D | 6 mg/mL | |||||||
Sodium Bicarbonate | D | 3 mg/mL | |||||||
Sodium Nitroprusside | D | 1.5, 6 and 15 mg/mL | |||||||
Sodium Phosphates | D | 6 mg/mL | |||||||
Intravenous to Oral Transition
Patients whose arrhythmias have been suppressed by amiodarone may be switched to oral amiodarone. The optimal dose for changing from intravenous to oral administration of amiodarone will depend on the dose of intravenous amiodarone already administered, as well as the bioavailability of oral amiodarone. When changing to oral amiodarone therapy, clinical monitoring is recommended, particularly for elderly patients. See package insert for oral amiodarone.
Since grapefruit juice is known to inhibit CYP3A-mediated metabolism of oral amiodarone in the intestinal mucosa, resulting in increased plasma levels of amiodarone, do not drink grapefruit juice during treatment with oral amiodarone
- Amiodarone is a substrate for CYP3A and CYP2C8, so inhibitors and inducers affect amiodarone exposure
- Amiodarone inhibits p-glycoprotein and CYP1A2, CYP2C9, CYP2D6, and CYP3A, increasing exposure to other drugs
Co-administration of drugs prolonging the QT interval (such as class I and III antiarrhythmics, lithium, certain phenothiazines, tricyclic antidepressants, certain fluoroquinolone and macrolide antibiotics, azole antifungals, halogenated inhalation anesthetic agents) increases the risk of Torsade de Pointes. In general, avoid concomitant use of drugs that prolong the QT interval
Concomitant use of drugs with depressant effects on the sinus and AV nodes (e.g., digoxin, beta blockers, verapamil, diltiazem, ivabradine, clonidine) can potentiate the electrophysiologic and hemodynamic effects of amiodarone, resulting in bradycardia, sinus arrest, and AV block. Monitor heart rate in patients on amiodarone and concomitant drugs that slow heart rate.
Amiodarone is metabolized to the active metabolite desethylamiodarone (DEA) by the cytochrome P450 (CYP450) enzyme group, specifically CYP3A and CYP2C8.
Amiodarone has the potential for interactions with drugs or substances that may be substrates, inhibitors or inducers of CYP450 enzymes (e.g., inhibitors such as
Patients should avoid
Effect of amiodarone on other drugs
Amiodarone and DEA are inhibitors of P-glycoprotein and certain CYP450 enzymes, including CYP1A2, CYP2C9, CYP2D6, and CYP3A [see Clinical Pharmacology (12.3)].
The metabolism of
During transfer to oral amiodarone, reduce the dose levels of previously administered antiarrhythmic agents by 30% to 50% several days after the addition of oral amiodarone. Review the continued need for the other antiarrhythmic agent after the effects of amiodarone have been established, and attempt discontinuation
In patients receiving
Limit the dose of
Potentiation of
Administered in combination with oral amiodarone has been reported to produce persistently elevated plasma concentrations of cyclosporine resulting in elevated creatinine, despite reduction in dose of cyclosporine. Monitor cyclosporine drug levels and renal function in patients taking both drugs.
Increased steady-state levels of
Postmarketing cases of symptomatic bradycardia, some requiring pacemaker insertion and at least one fatal, have been reported when ledipasvir/sofosbuvir or sofosbuvir with simeprevir were initiated in patients on amiodarone. Bradycardia generally occurred within hours to days, but in some cases up to 2 weeks after initiating antiviral treatment. Bradycardia generally resolved after discontinuation of antiviral treatment. The mechanism for this effect is unknown. Monitor heart rate in patients taking or recently discontinuing amiodarone when starting antiviral treatment.
Table 4 provides suggested doses of oral amiodarone to be initiated after varying durations of amiodarone administration. These recommendations are made on the basis of a similar total body amount of amiodarone delivered by the intravenous and oral routes, based on 50% bioavailability of oral amiodarone.
Duration of Amiodarone Infusion Assuming a 720 mg/day infusion (0.5 mg/min). | Initial Daily Dose of Oral Amiodarone |
|---|---|
< 1 week | 800 mg - 1600 mg |
1 to 3 weeks | 600 mg - 800 mg |
> 3 weeks | 400 mg |
Injection, 50 mg/mL
- Pregnancy: Use amiodarone during pregnancy only if the potential benefit to the mother justifies the risk to the fetus ().
8.1 PregnancyTeratogenic EffectsAmiodarone and desethylamiodarone cross the placenta.
Reported risks include:
• neonatal bradycardia, QT prolongation, and periodic ventricular extrasystoles
• neonatal hypothyroidism (with or without goiter) detected antenatally or in the newborn and reported even after a few days of exposure
• neonatal hyperthyroxinemia
• neurodevelopmental abnormalities independent of thyroid function, including speech delay and difficulties with written language and arithmetic, delayed motor development, and ataxia.
• jerk nystagmus with synchronous head titubation
• fetal growth retardation
• premature birthAmiodarone has caused a variety of adverse effects in animals.
Amiodarone was given intravenously to rabbits at dosages of 5 mg/kg per day, 10 mg/kg per day, or 25 mg/kg per day (about 0.1, 0.3, and 0.7 times the human intravenous maintenance dose of 0.5 mg/min on a body surface area basis), during gestation days 8 to 16 (organogenesis). The incidence of maternal deaths increased with increasing dose and occurred in all treated groups, and controls. Mean fetal weights were significantly decreased in the low and middle dose groups and embryotoxicity (as manifested by fewer full- term fetuses and increased resorptions) occurred at dosages of 10 mg/kg and above. There were no significant differences in the number of minor fetal abnormalities and no major fetal abnormalities were observed.
Amiodarone was administered by continuous intravenous infusion to rats at dosages of 25 mg/kg per day, 50 mg/kg per day, or 100 mg/kg per day (about 0.3, 0.7, and 1.3 times the human intravenous maintenance dose of 0.5 mg/min on a body surface area basis) during gestation days 8 to 16 (organogenesis). Maternal toxicity (manifest as reduced weight gain and food consumption) and embryotoxicity (manifest as increased resorptions, decreased live litter size and fetal body weights, and delayed sternal and metacarpal ossification) were observed in the 100 mg/kg group. The delayed ossification was reversible and related to decreased fetal weight. Fetal thyroid tissues appeared normal in all groups.
Nonteratogenic EffectsVery high concentrations of amiodarone and desethylamiodarone may be found in testes. Elevated follicle-stimulating hormone and luteinizing hormone levels, suggestive of testicular dysfunction, have been reported in men on long-term amiodarone treatment.
While planning pregnancy after discontinuation of amiodarone treatment, consider the long half-life of amiodarone and its metabolite DEA.
- Nursing mothers: Advise mothers to discontinue breast feeding ().
8.3 Nursing MothersAmiodarone and one of its major metabolites, desethylamiodarone (DEA), are excreted in human milk, suggesting that breast-feeding could expose the nursing infant to a significant dose of the drug. Nursing offspring of lactating rats administered amiodarone have demonstrated reduced viability and reduced body weight gains. The risk of exposing the infant to amiodarone must be weighed against the potential benefit of arrhythmia suppression in the mother. Advise the mother to discontinue nursing.
- Pediatric use: Safety and efficacy have not been established ().
8.4 Pediatric UseThe safety and effectiveness of amiodarone in pediatric patients have not been established; therefore, the use of amiodarone in pediatric patients is not recommended. In a pediatric trial of 61 patients, aged 30 days to 15 years, hypotension (36%), bradycardia (20%), and AV block (15%) were common dose-related adverse reactions and were severe or life-threatening in some cases. Injection site reactions were seen in 5 (25%) of the 20 patients receiving intravenous amiodarone through a peripheral vein irrespective of dose regimen.
Amiodarone hydrochloride injection contains the preservative benzyl alcohol
[see Description (11)]. There have been reports of fatal "gasping syndrome" in neonates (children less than one month of age) following the administration of intravenous solutions containing the preservative benzyl alcohol. Symptoms include a striking onset of gasping respiration, hypotension, bradycardia, and cardiovascular collapse.
Amiodarone is contraindicated in patients with:
• Known hypersensitivity to any of the components of Amiodarone Hydrochloride Injection, USP, including iodine. Hypersensitivity reactions may involve rash, angioedema, cutaneous/mucosal hemorrhage (bleeding), fever, arthralgias (joint pains), eosinophilia (abnormal blood counts), urticaria (hives), thrombotic thrombocytopenic purpura, or severe periateritis (inflammation around blood vessels)
• Cardiogenic shock
• Marked sinus bradycardia
• Second- or third-degree atrio-ventricular (AV) block unless of a functioning pacemaker is available.
Amiodarone should be administered only by physicians who are experienced in the treatment of life-threatening arrhythmias, who are thoroughly familiar with the risks and benefits of amiodarone therapy, and who have access to facilities adequate for monitoring the effectiveness and side effects of treatment.
Because of the long half-life of amiodarone and its metabolite desethylamiodarone, the potential for adverse reactions or interactions, as well as observed adverse effects, can persist following amiodarone withdrawal.