Amlodipine Besylate And Atorvastatin Calcium
Amlodipine Besylate And Atorvastatin Calcium Prescribing Information
Warnings and Precautions, Myopathy and Rhabdomyolysis (
Warnings and Precautions, Immune-Mediated Necrotizing Myopathy (
Amlodipine besylate and atorvastatin calcium tablets are indicated in patients for whom treatment with both amlodipine and atorvastatin is appropriate.
Amlodipine besylate and atorvastatin calcium
Dosage of amlodipine besylate and atorvastatin calcium must be individualized on the basis of both effectiveness and tolerance for each individual component in the treatment of hypertension/angina and hyperlipidemia. Select doses of amlodipine and atorvastatin independently.
Amlodipine besylate and atorvastatin calcium may be substituted for its individually titrated components. Patients may be given the equivalent dose of amlodipine besylate and atorvastatin calcium or a dose of amlodipine besylate and atorvastatin calcium with increased amounts of amlodipine, atorvastatin, or both for additional antianginal effects, blood pressure lowering, or lipid-lowering effect.
Amlodipine besylate and atorvastatin calcium may be used to provide additional therapy for patients already on one of its components. Amlodipine besylate and atorvastatin calcium may be used to initiate treatment in patients with hyperlipidemia and either hypertension or angina.
Amlodipine
The usual initial antihypertensive oral dose of amlodipine is 5 mg once daily, and the maximum dose is 10 mg once daily.
Pediatric (age > 6 years), small adult, fragile, or elderly patients, or patients with hepatic insufficiency may be started on 2.5 mg once daily and this dose may be used when adding amlodipine to other antihypertensive therapy.
Adjust dosage according to blood pressure goals. In general, wait 7 to 14 days between titration steps. Titration may proceed more rapidly, however, if clinically warranted, provided the patient is assessed frequently.
In PREVENT, 825 patients with angiographically documented CAD were randomized to amlodipine (5 to 10 mg once daily) or placebo and followed for 3 years. Although the study did not show significance on the primary objective of change in coronary luminal diameter as assessed by quantitative coronary angiography, the data suggested a favorable outcome with respect to fewer hospitalizations for angina and revascularization procedures in patients with CAD.
CAMELOT enrolled 1318 patients with CAD recently documented by angiography, without left main coronary disease and without heart failure or an ejection fraction <40%. Patients (76% males, 89% Caucasian, 93% enrolled at U.S. sites, 89% with a history of angina, 52% without PCI, 4% with PCI and no stent, and 44% with a stent) were randomized to double-blind treatment with either amlodipine (5 to 10 mg once daily) or placebo in addition to standard care that included aspirin (89%), statins (83%), beta-blockers (74%), nitroglycerin (50%), anticoagulants (40%), and diuretics (32%), but excluded other calcium channel blockers. The mean duration of follow-up was 19 months. The primary endpoint was the time to first occurrence of one of the following events: hospitalization for angina pectoris, coronary revascularization, myocardial infarction, cardiovascular death, resuscitated cardiac arrest, hospitalization for heart failure, stroke/TIA, or peripheral vascular disease. A total of 110 (16.6%) and 151 (23.1%) first events occurred in the amlodipine and placebo groups, respectively, for a hazard ratio of 0.691 (95% CI: 0.540– 0.884, p = 0.003). The primary endpoint is summarized in Figure 1 below. The outcome of this study was largely derived from the prevention of hospitalizations for angina and the prevention of revascularization procedures (see Table 8). Effects in various subgroups are shown in Figure 2.
In an angiographic substudy (n=274) conducted within CAMELOT, there was no significant difference between amlodipine and placebo on the change of atheroma volume in the coronary artery as assessed by intravascular ultrasound.
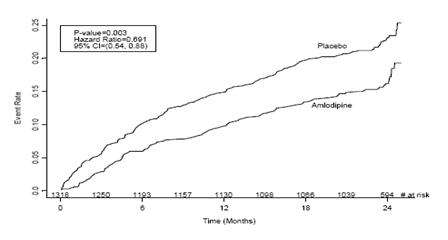
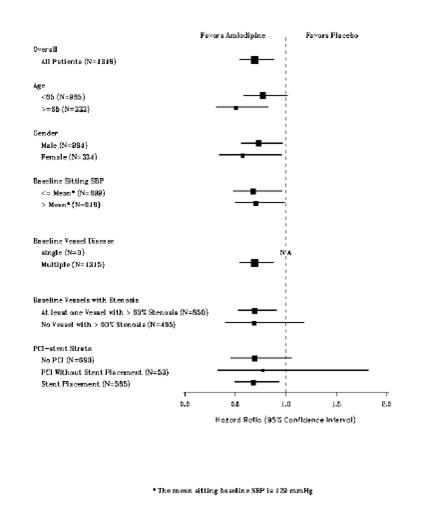
Table 8 below summarizes the significant composite endpoint and clinical outcomes from the composites of the primary endpoint. The other components of the primary endpoint including cardiovascular death, resuscitated cardiac arrest, myocardial infarction, hospitalization for heart failure, stroke/TIA, or peripheral vascular disease did not demonstrate a significant difference between amlodipine and placebo.
Clinical Outcomes N (%) | Amlodipine (N=663) | Placebo (N=655) | Risk Reduction (p-value) |
Composite CV | 110 | 151 | 31% |
Endpoint | (16.6) | (23.1) | (0.003) |
| Hospitalization for | 51 | 84 | 42% |
| Angina* | (7.7) | (12.8) | (0.002) |
| Coronary | 78 | 103 | 27% |
| Revascularization* | (11.8) | (15.7) | (0.033) |
* Total patients with these events.
Absorption
Amlodipine besylate and atorvastatin calcium: Following oral administration of amlodipine besylate and atorvastatin calcium, peak plasma concentrations of amlodipine and atorvastatin are seen at 6 to 12 hours and 1 to 2 hours post dosing, respectively. The rate and extent of absorption (bioavailability) of amlodipine and atorvastatin from amlodipine besylate and atorvastatin calcium are not significantly different from the bioavailability of amlodipine and atorvastatin administered separately (see above).
The bioavailability of amlodipine from amlodipine besylate and atorvastatin calcium was not affected by food. Food decreases the rate and extent of absorption of atorvastatin from amlodipine besylate and atorvastatin calcium by approximately 32% and 11%, respectively, as it does with atorvastatin when given alone. LDL-C reduction is similar whether atorvastatin is given with or without food.
Distribution
Metabolism
Excretion
Specific Populations
Pediatric
Gender
Renal Impairment
Hemodialysis
While studies have not been conducted in patients with end-stage renal disease, hemodialysis is not expected to clear atorvastatin or amlodipine since both drugs are extensively bound to plasma proteins.
Hepatic Impairment
Atorvastatin is contraindicated in patients with active liver disease.
Heart Failure
Effects of Other Drugs on Amlodipine besylate and atorvastatin calcium
Co-administered cimetidine, magnesium-and aluminum hydroxide antacids, sildenafil, and grapefruit juice have no impact on the exposure to amlodipine.
Atorvastatin is a substrate of the hepatic transporters, OATP1B1 and OATP1B3 transporter. Metabolites of atorvastatin are substrates of OATP1B1. Atorvastatin is also identified as a substrate of the efflux transporter BCRP, which may limit the intestinal absorption and biliary clearance of atorvastatin
Table 6 shows effects of other drugs on the pharmacokinetics of atorvastatin
Co-administered drug and dosing regimen | Atorvastatin | ||
Dose (mg) | Ratio of AUC& | Ratio of Cmax& | |
| #Cyclosporine 5.2 mg/kg/day, stable dose | 10 mg QDafor 28 days | 8.69 | 10.66 |
| #Tipranavir 500 mg BIDb/ritonavir 200 mg BIDb, 7 days | 10 mg, SDc | 9.36 | 8.58 |
| #Glecaprevir 400 mg QDa/pibrentasvir 120 mg QDa, 7 days | 10 mg QDafor 7 days | 8.28 | 22.00 |
| #Telaprevir 750 mg q8hf, 10 days | 20 mg, SDc | 7.88 | 10.60 |
| #, ‡Saquinavir 400 mg BIDb/ritonavir 400mg BID, 15 days | 40 mg QDafor 4 days | 3.93 | 4.31 |
| #Elbasvir 50 mg QDa/grazoprevir 200 mg QDa, 13 days | 10 mg SDc | 1.95 | 4.34 |
| #Simeprevir 150 mg QDa, 10 days | 40 mg SDc | 2.12 | 1.70 |
| #Clarithromycin 500 mg BIDb, 9 days | 80 mg QDafor 8 days | 4.54 | 5.38 |
| #Darunavir 300 mg BIDb/ritonavir 100 mg BID, 9 days | 10 mg QDafor 4 days | 3.45 | 2.25 |
| #Itraconazole 200 mg QDa, 4 days | 40 mg SDc | 3.32 | 1.20 |
| #Letermovir 480 mg QDa, 10 days | 20 mg SDc | 3.29 | 2.17 |
| #Fosamprenavir 700 mg BIDb/ritonavir 100 mg BIDb, 14 days | 10 mg QDafor 4 days | 2.53 | 2.84 |
| #Fosamprenavir 1400 mg BIDb, 14 days | 10 mg QDafor 4 days | 2.30 | 4.04 |
| #Nelfinavir 1250 mg BIDb, 14 days | 10 mg QDafor 28 days | 1.74 | 2.22 |
| #Grapefruit Juice, 240 mL QDa,* | 40 mg, SDc | 1.37 | 1.16 |
| Diltiazem 240 mg QDa, 28 days | 40 mg, SDc | 1.51 | 1.00 |
| Erythromycin 500 mg QIDe, 7 days | 10 mg, SDc | 1.33 | 1.38 |
| Amlodipine 10 mg, single dose | 80 mg, SDc | 1.18 | 0.91 |
| Cimetidine 300 mg QIDe, 2 weeks | 10 mg QDafor 2 weeks | 1.00 | 0.89 |
| Colestipol 10 g BIDb, 24 weeks | 40 mg QDafor 8 weeks | NA | 0.74** |
| Maalox TC®30 mL QDa, 17 days | 10 mg QDafor 15 days | 0.66 | 0.67 |
| Efavirenz 600 mg QDa, 14 days | 10 mg for 3 days | 0.59 | 1.01 |
| #Rifampin 600 mg QDa, 7 days (coadministered)† | 40 mg SDc | 1.12 | 2.90 |
| #Rifampin 600 mg QDa, 5 days (doses separated)† | 40 mg SDc | 0.20 | 0.60 |
| #Gemfibrozil 600 mg BIDb, 7 days | 40 mg SDc | 1.35 | 1.00 |
| #Fenofibrate 160 mg QDa, 7 days | 40 mg SDc | 1.03 | 1.02 |
| Boceprevir 800 mg TIDd, 7 days | 40 mg SDc | 2.32 | 2.66 |
&
#See Sections 5.1 and 7 for clinical significance.
* Greater increases in AUC (ratio of AUC up to 2.5) and/or Cmax(ratio of Cmaxup to 1.71) have been reported with excessive grapefruit consumption (≥ 750 mL to 1.2 liters per day).
** Ratio based on a single sample taken 8 to 16 h post dose.
† Due to the dual interaction mechanism of rifampin, simultaneous co-administration of atorvastatin with rifampin is recommended, as delayed administration of atorvastatin after administration of rifampin has been associated with a significant reduction in atorvastatin plasma concentrations.
‡ The dose of saquinavir plus ritonavir in this study is not the clinically used dose. The increase in atorvastatin exposure when used clinically is likely to be higher than what was observed in this study. Therefore, caution should be applied and the lowest dose necessary should be used.
aOnce daily
bTwice daily
cSingle dose
dThree times daily
eFour times daily
fEvery 8 hours
Effects of Amlodipine besylate and atorvastatin calcium on Other Drugs
Amlodipine is a weak inhibitor of CYP3A and may increase exposure to CYP3A substrates.
Co-administered amlodipine does not affect the exposure to atorvastatin, digoxin, ethanol and the warfarin prothrombin response time.
Table 7 shows the effects of atorvastatin on the pharmacokinetics of other drugs.
Atorvastatin | Co-administered drug and dosing regimen | ||
Drug/Dose (mg) | Ratio of AUC | Ratio of Cmax | |
| 80 mg QDafor 15 days | Antipyrine, 600 mg SDc | 1.03 | 0.89 |
| 80 mg QDafor 10 days | #Digoxin 0.25 mg QDa, 20 days | 1.15 | 1.20 |
| 40 mg QDafor 22 days | Oral contraceptive QDa, 2 months – norethindrone 1 mg – ethinyl estradiol 35 mcg | 1.281.19 | 1.231.30 |
| 10 mg, SDc | Tipranavir 500 mg BIDb/ritonavir 200 mg BIDb, 7 days | 1.08 | 0.96 |
| 10 mg QDafor 4 days | Fosamprenavir 1400 mg BIDb, 14 days | 0.73 | 0.82 |
| 10 mg QDafor 4 days | Fosamprenavir 700 mg BIDb/ritonavir 100 mg BIDb, 14 days | 0.99 | 0.94 |
# See Section 7 for clinical significance.
aOnce daily
bTwice daily
cSingle dose
Atorvastatin had no clinically significant effect on prothrombin time when administered to patients receiving chronic warfarin treatment.
Adult Patients
The antihypertensive efficacy of amlodipine has been demonstrated in a total of 15 double-blind, placebo-controlled, randomized studies involving 800 patients on amlodipine and 538 on placebo. Once daily administration produced statistically significant placebo-corrected reductions in supine and standing blood pressures at 24 hours postdose, averaging about 12/6 mmHg in the standing position and 13/7 mmHg in the supine position in patients with mild to moderate hypertension. Maintenance of the blood pressure effect over the 24-hour dosing interval was observed, with little difference in peak and trough effect. Tolerance was not demonstrated in patients studied for up to 1 year. The 3 parallel, fixed dose, dose response studies showed that the reduction in supine and standing blood pressures was dose related within the recommended dosing range. Effects on diastolic pressure were similar in young and older patients. The effect on systolic pressure was greater in older patients, perhaps because of greater baseline systolic pressure. Effects were similar in black patients and in white patients.
Pediatric Patients
Two hundred sixty-eight hypertensive patients aged 6 to 17 years were randomized first to amlodipine 2.5 or 5 mg once daily for 4 weeks and then randomized again to the same dose or to placebo for another 4 weeks. Patients receiving 2.5 mg or 5 mg at the end of 8 weeks had significantly lower systolic blood pressure than those secondarily randomized to placebo. The magnitude of the treatment effect is difficult to interpret, but it is probably less than 5 mmHg systolic on the 5 mg dose and 3.3 mmHg systolic on the 2.5 mg dose. Adverse events were similar to those seen in adults.
Atorvastatin (Hyperlipidemia)
Data from a drug-drug interaction study involving 10 mg of amlodipine and 80 mg of atorvastatin in healthy subjects indicate that the pharmacokinetics of amlodipine are not altered when the drugs are co-administered. The effect of amlodipine on the pharmacokinetics of atorvastatin showed no effect on the Cmax: 91% (90% confidence interval: 80 to 103%), but the AUC of atorvastatin increased by 18% (90% confidence interval: 109 to 127%) in the presence of amlodipine, which is not clinically meaningful.
No drug interaction studies have been conducted with amlodipine besylate and atorvastatin calcium and other drugs, although studies have been conducted in the individual amlodipine and atorvastatin components, as described below:
Amlodipine
Increased Risk of Myopathy and Rhabdomyolysis
| Cyclosporine, tipranavir plus ritonavir, glecaprevir plus pibrentasvir | Avoid atorvastatin |
| Clarithromycin, itraconazole, saquinavir plus ritonavir, darunavir plus ritonavir, fosamprenavir, fosamprenavir plus ritonavir, elbasvir plus grazoprevir,letermovir | Do not exceed 20 mg atorvastatin daily |
| Nelfinavir | Do not exceed 40 mg atorvastatin daily |
| Lopinavir plus ritonavir, simeprevir, fibric acid derivatives, erythromycin, azole antifungals, lipid-modifying doses of niacin, colchicine | Consider the risk/benefit of concomitant use with atorvastatin |
- Other Lipid-Lowering Medications: Increased risk of myopathy (7).
- Rifampin should be simultaneously co-administered with atorvastatin .
- Oral Contraceptives: Norethindrone and ethinyl estradiol may be increased .
- Digoxin: Patients should be monitored appropriately .
Co-administration with CYP3A inhibitors (moderate and strong) results in increased systemic exposure to amlodipine and may require dose reduction. Monitor for symptoms of hypotension and edema when amlodipine is co-administered with CYP3A inhibitors to determine the need for dose adjustment [see
No information is available on the quantitative effects of CYP3A inducers on amlodipine. Blood pressure should be closely monitored when amlodipine is co-administered with CYP3A inducers.
Monitor for hypotension when sildenafil is co-administered with amlodipine [see
Amlodipine may increase the systemic exposure of cyclosporine or tacrolimus when co-administered. Frequent monitoring of trough blood levels of cyclosporine and tacrolimus is recommended and adjust the dose when appropriate [see
The risk of myopathy during treatment with statins is increased with concurrent administration of fibric acid derivatives, lipid-modifying doses of niacin, cyclosporine, or strong CYP3A4 inhibitors (e.g., clarithromycin, HIV and HCV protease inhibitors, and itraconazole) [see
Atorvastatin is a substrate of CYP3A4 and transporters (e.g., OATP1B1/1B3, P-gp, or BCRP). Atorvastatin plasma levels can be significantly increased with concomitant administration of inhibitors of CYP3A4 and transporters. Table 3 includes a list of drugs that may increase exposure to atorvastatin and may increase the risk of myopathy and rhabdomyolysis when used concomitantly and instructions for preventing or managing them [see
Cyclosporine or Gemfibrozil | |
Clinical Impact: | Atorvastatin plasma levels were significantly increased with concomitant administration of atorvastatin and cyclosporine, an inhibitor of CYP3A4 and OATP1B1 [ seeClinical Pharmacology ]. Cases of myopathy and rhabdomyolysis have been reported with concomitant use of ledipasvir plus sofosbuvir with atorvastatin. |
Intervention : |
|
Examples: | Tipranavir plus ritonavir, glecaprevir plus pibrentasvir, lopinavir plus ritonavir, simeprevir, saquinavir plus ritonavir, darunavir plus ritonavir, fosamprenavir, fosamprenavir plus ritonavir, elbasvir plus grazoprevir, letermovir, nelfinavir, and ledipasvir plus sofosbuvir. |
Select Azole Antifungals or Macrolide Antibiotics | |
Clinical Impact: | Atorvastatin plasma levels were significantly increased with concomitant administration of atorvastatin with select azole antifungals or macrolide antibiotics, due to inhibition of CYP3A4 and/or transporters [ seeClinical Pharmacology ]. |
Intervention: | In patients taking clarithromycin or itraconazole, do not exceed atorvastatin 20 mg [ seeDosage and Administration ]. Consider the risk/benefit of concomitant use of other azole antifungals or macrolide antibiotics with atorvastatin. Monitor all patients for signs and symptoms of myopathy particularly during initiation of therapy and during upward dose titration of either drug. |
Examples: | Erythromycin, clarithromycin, itraconazole, ketoconazole, posaconazole, and voriconazole. |
Niacin | |
Clinical Impact: | Cases of myopathy and rhabdomyolysis have been observed with concomitant use of lipid modifying dosages of niacin (>1 gram/day niacin) with atorvastatin. |
Intervention: | Consider if the benefit of using lipid modifying dosages of niacin concomitantly with atorvastatin outweighs the increased risk of myopathy and rhabdomyolysis. If concomitant use is decided, monitor patients for signs and symptoms of myopathy particularly during initiation of therapy and during upward dose titration of either drug. |
Fibrates (other than Gemfibrozil) | |
Clinical Impact: | Fibrates may cause myopathy when given alone. The risk of myopathy and rhabdomyolysis is increased with concomitant use of fibrates with atorvastatin. |
Intervention: | Consider if the benefit of using fibrates concomitantly with atorvastatin outweighs the increased risk of myopathy and rhabdomyolysis. If concomitant use is decided, monitor patients for signs and symptoms of myopathy particularly during initiation of therapy and during upward dose titration of either drug. |
Colchicine | |
Clinical Impact: | Cases of myopathy and rhabdomyolysis have been reported with concomitant use of colchicine with atorvastatin. |
Intervention : | Consider the risk/benefit of concomitant use of colchicine with atorvastatin. If concomitant use is decided, monitor patients for signs and symptoms of myopathy particularly during initiation of therapy and during upward dose titration of either drug. |
Grapefruit Juice | |
Clinical Impact: | Grapefruit juice consumption, especially excessive consumption, more than 1.2 liters/daily, can raise the plasma levels of atorvastatin and may increase the risk of myopathy and rhabdomyolysis. |
Intervention: | Avoid intake of large quantities of grapefruit juice, more than 1.2 liters daily, when taking atorvastatin. |
Table 4 presents drug interactions that may decrease exposure to atorvastatin and instructions for preventing or managing them.
Rifampin | |
Clinical Impact: | Concomitant administration of atorvastatin with rifampin, an inducer of cytochrome P450 3A4 and inhibitor of OATP1B1, can lead to variable reductions in plasma concentrations of atorvastatin. Due to the dual interaction mechanism of rifampin, delayed administration of atorvastatin after administration of rifampin has been associated with a significant reduction in atorvastatin plasma concentrations. |
Intervention : | Administer atorvastatin and rifampin simultaneously. |
Table 5 presents atorvastatin effect on other drugs and instructions for preventing or managing them.
Oral Contraceptives | |
Clinical Impact: | Co-administration of atorvastatin and an oral contraceptive increased plasma concentrations of norethindrone and ethinyl estradiol [ seeClinical Pharmacology ( 12.3)]. |
Intervention: | Consider this when selecting an oral contraceptive for patients taking atorvastatin. |
Digoxin | |
Clinical Impact : | When multiple doses of atorvastatin and digoxin were co-administered, steady state plasma digoxin concentrations increased [ seeClinical Pharmacology ]. |
Intervention: | Monitor patients taking digoxin appropriately. |
Absorption
Amlodipine besylate and atorvastatin calcium: Following oral administration of amlodipine besylate and atorvastatin calcium, peak plasma concentrations of amlodipine and atorvastatin are seen at 6 to 12 hours and 1 to 2 hours post dosing, respectively. The rate and extent of absorption (bioavailability) of amlodipine and atorvastatin from amlodipine besylate and atorvastatin calcium are not significantly different from the bioavailability of amlodipine and atorvastatin administered separately (see above).
The bioavailability of amlodipine from amlodipine besylate and atorvastatin calcium was not affected by food. Food decreases the rate and extent of absorption of atorvastatin from amlodipine besylate and atorvastatin calcium by approximately 32% and 11%, respectively, as it does with atorvastatin when given alone. LDL-C reduction is similar whether atorvastatin is given with or without food.
Distribution
Metabolism
Excretion
Specific Populations
Pediatric
Gender
Renal Impairment
Hemodialysis
While studies have not been conducted in patients with end-stage renal disease, hemodialysis is not expected to clear atorvastatin or amlodipine since both drugs are extensively bound to plasma proteins.
Hepatic Impairment
Atorvastatin is contraindicated in patients with active liver disease.
Heart Failure
Effects of Other Drugs on Amlodipine besylate and atorvastatin calcium
Co-administered cimetidine, magnesium-and aluminum hydroxide antacids, sildenafil, and grapefruit juice have no impact on the exposure to amlodipine.
Atorvastatin is a substrate of the hepatic transporters, OATP1B1 and OATP1B3 transporter. Metabolites of atorvastatin are substrates of OATP1B1. Atorvastatin is also identified as a substrate of the efflux transporter BCRP, which may limit the intestinal absorption and biliary clearance of atorvastatin
Table 6 shows effects of other drugs on the pharmacokinetics of atorvastatin
Co-administered drug and dosing regimen | Atorvastatin | ||
Dose (mg) | Ratio of AUC& | Ratio of Cmax& | |
| #Cyclosporine 5.2 mg/kg/day, stable dose | 10 mg QDafor 28 days | 8.69 | 10.66 |
| #Tipranavir 500 mg BIDb/ritonavir 200 mg BIDb, 7 days | 10 mg, SDc | 9.36 | 8.58 |
| #Glecaprevir 400 mg QDa/pibrentasvir 120 mg QDa, 7 days | 10 mg QDafor 7 days | 8.28 | 22.00 |
| #Telaprevir 750 mg q8hf, 10 days | 20 mg, SDc | 7.88 | 10.60 |
| #, ‡Saquinavir 400 mg BIDb/ritonavir 400mg BID, 15 days | 40 mg QDafor 4 days | 3.93 | 4.31 |
| #Elbasvir 50 mg QDa/grazoprevir 200 mg QDa, 13 days | 10 mg SDc | 1.95 | 4.34 |
| #Simeprevir 150 mg QDa, 10 days | 40 mg SDc | 2.12 | 1.70 |
| #Clarithromycin 500 mg BIDb, 9 days | 80 mg QDafor 8 days | 4.54 | 5.38 |
| #Darunavir 300 mg BIDb/ritonavir 100 mg BID, 9 days | 10 mg QDafor 4 days | 3.45 | 2.25 |
| #Itraconazole 200 mg QDa, 4 days | 40 mg SDc | 3.32 | 1.20 |
| #Letermovir 480 mg QDa, 10 days | 20 mg SDc | 3.29 | 2.17 |
| #Fosamprenavir 700 mg BIDb/ritonavir 100 mg BIDb, 14 days | 10 mg QDafor 4 days | 2.53 | 2.84 |
| #Fosamprenavir 1400 mg BIDb, 14 days | 10 mg QDafor 4 days | 2.30 | 4.04 |
| #Nelfinavir 1250 mg BIDb, 14 days | 10 mg QDafor 28 days | 1.74 | 2.22 |
| #Grapefruit Juice, 240 mL QDa,* | 40 mg, SDc | 1.37 | 1.16 |
| Diltiazem 240 mg QDa, 28 days | 40 mg, SDc | 1.51 | 1.00 |
| Erythromycin 500 mg QIDe, 7 days | 10 mg, SDc | 1.33 | 1.38 |
| Amlodipine 10 mg, single dose | 80 mg, SDc | 1.18 | 0.91 |
| Cimetidine 300 mg QIDe, 2 weeks | 10 mg QDafor 2 weeks | 1.00 | 0.89 |
| Colestipol 10 g BIDb, 24 weeks | 40 mg QDafor 8 weeks | NA | 0.74** |
| Maalox TC®30 mL QDa, 17 days | 10 mg QDafor 15 days | 0.66 | 0.67 |
| Efavirenz 600 mg QDa, 14 days | 10 mg for 3 days | 0.59 | 1.01 |
| #Rifampin 600 mg QDa, 7 days (coadministered)† | 40 mg SDc | 1.12 | 2.90 |
| #Rifampin 600 mg QDa, 5 days (doses separated)† | 40 mg SDc | 0.20 | 0.60 |
| #Gemfibrozil 600 mg BIDb, 7 days | 40 mg SDc | 1.35 | 1.00 |
| #Fenofibrate 160 mg QDa, 7 days | 40 mg SDc | 1.03 | 1.02 |
| Boceprevir 800 mg TIDd, 7 days | 40 mg SDc | 2.32 | 2.66 |
&
#See Sections 5.1 and 7 for clinical significance.
* Greater increases in AUC (ratio of AUC up to 2.5) and/or Cmax(ratio of Cmaxup to 1.71) have been reported with excessive grapefruit consumption (≥ 750 mL to 1.2 liters per day).
** Ratio based on a single sample taken 8 to 16 h post dose.
† Due to the dual interaction mechanism of rifampin, simultaneous co-administration of atorvastatin with rifampin is recommended, as delayed administration of atorvastatin after administration of rifampin has been associated with a significant reduction in atorvastatin plasma concentrations.
‡ The dose of saquinavir plus ritonavir in this study is not the clinically used dose. The increase in atorvastatin exposure when used clinically is likely to be higher than what was observed in this study. Therefore, caution should be applied and the lowest dose necessary should be used.
aOnce daily
bTwice daily
cSingle dose
dThree times daily
eFour times daily
fEvery 8 hours
Effects of Amlodipine besylate and atorvastatin calcium on Other Drugs
Amlodipine is a weak inhibitor of CYP3A and may increase exposure to CYP3A substrates.
Co-administered amlodipine does not affect the exposure to atorvastatin, digoxin, ethanol and the warfarin prothrombin response time.
Table 7 shows the effects of atorvastatin on the pharmacokinetics of other drugs.
Atorvastatin | Co-administered drug and dosing regimen | ||
Drug/Dose (mg) | Ratio of AUC | Ratio of Cmax | |
| 80 mg QDafor 15 days | Antipyrine, 600 mg SDc | 1.03 | 0.89 |
| 80 mg QDafor 10 days | #Digoxin 0.25 mg QDa, 20 days | 1.15 | 1.20 |
| 40 mg QDafor 22 days | Oral contraceptive QDa, 2 months – norethindrone 1 mg – ethinyl estradiol 35 mcg | 1.281.19 | 1.231.30 |
| 10 mg, SDc | Tipranavir 500 mg BIDb/ritonavir 200 mg BIDb, 7 days | 1.08 | 0.96 |
| 10 mg QDafor 4 days | Fosamprenavir 1400 mg BIDb, 14 days | 0.73 | 0.82 |
| 10 mg QDafor 4 days | Fosamprenavir 700 mg BIDb/ritonavir 100 mg BIDb, 14 days | 0.99 | 0.94 |
# See Section 7 for clinical significance.
aOnce daily
bTwice daily
cSingle dose
Atorvastatin had no clinically significant effect on prothrombin time when administered to patients receiving chronic warfarin treatment.
Atorvastatin is a substrate of CYP3A4 and transporters (e.g., OATP1B1/1B3, P-gp, or BCRP). Atorvastatin plasma levels can be significantly increased with concomitant administration of inhibitors of CYP3A4 and transporters. Table 3 includes a list of drugs that may increase exposure to atorvastatin and may increase the risk of myopathy and rhabdomyolysis when used concomitantly and instructions for preventing or managing them [see
Cyclosporine or Gemfibrozil | |
Clinical Impact: | Atorvastatin plasma levels were significantly increased with concomitant administration of atorvastatin and cyclosporine, an inhibitor of CYP3A4 and OATP1B1 [ seeClinical Pharmacology ]. Cases of myopathy and rhabdomyolysis have been reported with concomitant use of ledipasvir plus sofosbuvir with atorvastatin. |
Intervention : |
|
Examples: | Tipranavir plus ritonavir, glecaprevir plus pibrentasvir, lopinavir plus ritonavir, simeprevir, saquinavir plus ritonavir, darunavir plus ritonavir, fosamprenavir, fosamprenavir plus ritonavir, elbasvir plus grazoprevir, letermovir, nelfinavir, and ledipasvir plus sofosbuvir. |
Select Azole Antifungals or Macrolide Antibiotics | |
Clinical Impact: | Atorvastatin plasma levels were significantly increased with concomitant administration of atorvastatin with select azole antifungals or macrolide antibiotics, due to inhibition of CYP3A4 and/or transporters [ seeClinical Pharmacology ]. |
Intervention: | In patients taking clarithromycin or itraconazole, do not exceed atorvastatin 20 mg [ seeDosage and Administration ]. Consider the risk/benefit of concomitant use of other azole antifungals or macrolide antibiotics with atorvastatin. Monitor all patients for signs and symptoms of myopathy particularly during initiation of therapy and during upward dose titration of either drug. |
Examples: | Erythromycin, clarithromycin, itraconazole, ketoconazole, posaconazole, and voriconazole. |
Niacin | |
Clinical Impact: | Cases of myopathy and rhabdomyolysis have been observed with concomitant use of lipid modifying dosages of niacin (>1 gram/day niacin) with atorvastatin. |
Intervention: | Consider if the benefit of using lipid modifying dosages of niacin concomitantly with atorvastatin outweighs the increased risk of myopathy and rhabdomyolysis. If concomitant use is decided, monitor patients for signs and symptoms of myopathy particularly during initiation of therapy and during upward dose titration of either drug. |
Fibrates (other than Gemfibrozil) | |
Clinical Impact: | Fibrates may cause myopathy when given alone. The risk of myopathy and rhabdomyolysis is increased with concomitant use of fibrates with atorvastatin. |
Intervention: | Consider if the benefit of using fibrates concomitantly with atorvastatin outweighs the increased risk of myopathy and rhabdomyolysis. If concomitant use is decided, monitor patients for signs and symptoms of myopathy particularly during initiation of therapy and during upward dose titration of either drug. |
Colchicine | |
Clinical Impact: | Cases of myopathy and rhabdomyolysis have been reported with concomitant use of colchicine with atorvastatin. |
Intervention : | Consider the risk/benefit of concomitant use of colchicine with atorvastatin. If concomitant use is decided, monitor patients for signs and symptoms of myopathy particularly during initiation of therapy and during upward dose titration of either drug. |
Grapefruit Juice | |
Clinical Impact: | Grapefruit juice consumption, especially excessive consumption, more than 1.2 liters/daily, can raise the plasma levels of atorvastatin and may increase the risk of myopathy and rhabdomyolysis. |
Intervention: | Avoid intake of large quantities of grapefruit juice, more than 1.2 liters daily, when taking atorvastatin. |
In a double-blind, placebo-controlled study followed by an open-label phase, 187 boys and post-menarchal girls 10 years to 17 years of age (mean age 14.1 years) with HeFH or severe hypercholesterolemia, were randomized to atorvastatin (n=140) or placebo (n=47) for 26 weeks and then all received atorvastatin for 26 weeks. Inclusion in the study required 1) a baseline LDL-C level ≥ 190 mg/dL or 2) a baseline LDL-C level ≥ 160 mg/dL and positive family history of FH or documented premature cardiovascular disease in a first or second-degree relative. The mean baseline LDL-C value was 218.6 mg/dL (range: 138.5 to 385 mg/dL) in the atorvastatin group compared to 230 mg/dL (range: 160 to 324.5 mg/dL) in the placebo group. The dosage of atorvastatin (once daily) was 10 mg for the first 4 weeks and uptitrated to 20 mg if the LDL-C level was > 130 mg/dL. The number of atorvastatin-treated patients who required uptitration to 20 mg after Week 4 during the double-blind phase was 78 (55.7%).
Atorvastatin significantly decreased plasma levels of total-C, LDL-C, TG, and apolipoprotein B during the 26-week double-blind phase (see Table 14).
DOSAGE | N | Total-C | LDL-C | HDL-C | TG | Apo B |
Placebo | 47 | -1.5 | -0.4 | -1.9 | 1 | 0.7 |
Atorvastatin | 140 | -31.4 | -39.6 | 2.8 | -12 | -34 |
The mean achieved LDL-C value was 130.7 mg/dL (range: 70 to 242 mg/dL) in the atorvastatin group compared to 228.5 mg/dL (range: 152 to 385 mg/dL) in the placebo group during the 26-week double-blind phase.
Atorvastatin was also studied in a three year open-label, uncontrolled trial that included 163 patients with HeFH who were 10 years to 15 years old (82 boys and 81 girls). All patients had a clinical diagnosis of HeFH confirmed by genetic analysis (if not already confirmed by family history). Approximately 98% were Caucasian, and less than 1% were Black or Asian. Mean LDL-C at baseline was 232 mg/dL. The starting atorvastatin dosage was 10 mg once daily and doses were adjusted to achieve a target of <130 mg/dL LDL-C. The reductions in LDL-C from baseline were generally consistent across age groups within the trial as well as with previous clinical studies in both adult and pediatric placebo-controlled trials.
Atorvastatin is indicated:
- As an adjunct to diet to reduce elevated total cholesterol (total-C), low-density lipoprotein cholesterol (LDL-C), apolipoprotein B (apo B), and triglycerides (TG) levels and to increase HDL-C in adult patients with primary hypercholesterolemia (heterozygous familial and nonfamilial) and mixed dyslipidemia (FredricksonTypes IIa and IIb)
- As an adjunct to diet for the treatment of adult patients with elevated serum TG levels (FredricksonType IV);
- For the treatment of adult patients with primary dysbetalipoproteinemia (FredricksonType III) who do not respond adequately to diet
- To reduce total-C and LDL-C in patients with homozygous familial hypercholesterolemia (HoFH) as an adjunct to other lipid-lowering treatments (e.g., LDL apheresis) or if such treatments are unavailable
- As an adjunct to diet to reduce total-C, LDL-C, and apo B levels in pediatric patients, 10 years to 17 years of age, with heterozygous familial hypercholesterolemia (HeFH) if after an adequate trial of diet therapy the following findings are present:
a. LDL-C remains ≥ 190 mg/dL or
b. LDL-C remains ≥ 160 mg/dL and:
- there is a positive family history of premature CVD or
- two or more other CVD risk factors are present in the pediatric patient
Amlodipine besylate and atorvastatin calcium is a combination of two drugs, a dihydropyridine calcium channel blocker (amlodipine) and an HMG-CoA reductase inhibitor (atorvastatin). The amlodipine component of amlodipine besylate and atorvastatin calcium inhibits the transmembrane influx of calcium ions into vascular smooth muscle and cardiac muscle. The atorvastatin component of amlodipine besylate and atorvastatin calcium is a selective, competitive inhibitor of HMG-CoA reductase, the rate-limiting enzyme that converts 3-hydroxy-3-methylglutaryl-coenzyme A to mevalonate, a precursor of sterols, including cholesterol.
Amlodipine
Amlodipine binds to both dihydropyridine and nondihydropyridine binding sites. The contractile processes of cardiac muscle and vascular smooth muscle are dependent upon the movement of extracellular calcium ions into these cells through specific ion channels. Amlodipine inhibits calcium ion influx across cell membranes selectively, with a greater effect on vascular smooth muscle cells than on cardiac muscle cells. Negative inotropic effects can be detected
Amlodipine is a peripheral arterial vasodilator that acts directly on vascular smooth muscle to cause a reduction in peripheral vascular resistance and reduction in blood pressure. The precise mechanisms by which amlodipine relieves angina have not been fully delineated, but are thought to include the following:
Atorvastatin
Atorvastatin is a selective, competitive inhibitor of HMG-CoA reductase, the rate-limiting enzyme that converts 3-hydroxy-3-methylglutaryl-coenzyme A to mevalonate, a precursor of sterols, including cholesterol. In animal models, atorvastatin lowers plasma cholesterol and lipoprotein levels by inhibiting HMG-CoA reductase and cholesterol synthesis in the liver and by increasing the number of hepatic LDL receptors on the cell surface to enhance uptake and catabolism of LDL; atorvastatin also reduces LDL production and the number of LDL particles.
Amlodipine
Following administration of therapeutic doses to patients with hypertension, amlodipine produces vasodilation resulting in a reduction of supine and standing blood pressures. These decreases in blood pressure are not accompanied by a significant change in heart rate or plasma catecholamine levels with chronic dosing. Although the acute intravenous administration of amlodipine decreases arterial blood pressure and increases heart rate in hemodynamic studies of patients with chronic stable angina, chronic oral administration of amlodipine in clinical trials did not lead to clinically significant changes in heart rate or blood pressures in normotensive patients with angina.
With chronic once daily oral administration, antihypertensive effectiveness is maintained for at least 24 hours. Plasma concentrations correlate with effect in both young and elderly patients. The magnitude of reduction in blood pressure with amlodipine is also correlated with the height of pretreatment elevation; thus, individuals with moderate hypertension (diastolic pressure 105 to 114 mmHg) had about a 50% greater response than patients with mild hypertension (diastolic pressure 90 to 104 mmHg). Normotensive subjects experienced no clinically significant change in blood pressures (+1/–2 mmHg).
In hypertensive patients with normal renal function, therapeutic doses of amlodipine resulted in a decrease in renal vascular resistance and an increase in glomerular filtration rate and effective renal plasma flow without change in filtration fraction or proteinuria.
As with other calcium channel blockers, hemodynamic measurements of cardiac function at rest and during exercise (or pacing) in patients with normal ventricular function treated with amlodipine have generally demonstrated a small increase in cardiac index without significant influence on dP/dt or on left ventricular end diastolic pressure or volume. In hemodynamic studies, amlodipine has not been associated with a negative inotropic effect when administered in the therapeutic dose range to intact animals and man, even when co-administered with beta-blockers to man. Similar findings, however, have been observed in normal or well-compensated patients with heart failure with agents possessing significant negative inotropic effects.
Amlodipine does not change sinoatrial nodal function or atrioventricular conduction in intact animals or man. In patients with chronic stable angina, intravenous administration of 10 mg did not significantly alter A-H and H-V conduction and sinus node recovery time after pacing. Similar results were obtained in patients receiving amlodipine and concomitant beta-blockers. In clinical studies in which amlodipine was administered in combination with beta-blockers to patients with either hypertension or angina, no adverse effects on electrocardiographic parameters were observed. In clinical trials with angina patients alone, amlodipine therapy did not alter electrocardiographic intervals or produce higher degrees of AV blocks.
Atorvastatin
Atorvastatin, as well as some of its metabolites, are pharmacologically active in humans. The liver is the primary site of action and the principal site of cholesterol synthesis and LDL clearance. Drug dosage, rather than systemic drug concentration, correlates better with LDL-C reduction. Individualization of drug dosage should be based on therapeutic response [see
Absorption
Amlodipine besylate and atorvastatin calcium: Following oral administration of amlodipine besylate and atorvastatin calcium, peak plasma concentrations of amlodipine and atorvastatin are seen at 6 to 12 hours and 1 to 2 hours post dosing, respectively. The rate and extent of absorption (bioavailability) of amlodipine and atorvastatin from amlodipine besylate and atorvastatin calcium are not significantly different from the bioavailability of amlodipine and atorvastatin administered separately (see above).
The bioavailability of amlodipine from amlodipine besylate and atorvastatin calcium was not affected by food. Food decreases the rate and extent of absorption of atorvastatin from amlodipine besylate and atorvastatin calcium by approximately 32% and 11%, respectively, as it does with atorvastatin when given alone. LDL-C reduction is similar whether atorvastatin is given with or without food.
Distribution
Metabolism
Excretion
Specific Populations
Pediatric
Gender
Renal Impairment
Hemodialysis
While studies have not been conducted in patients with end-stage renal disease, hemodialysis is not expected to clear atorvastatin or amlodipine since both drugs are extensively bound to plasma proteins.
Hepatic Impairment
Atorvastatin is contraindicated in patients with active liver disease.
Heart Failure
Effects of Other Drugs on Amlodipine besylate and atorvastatin calcium
Co-administered cimetidine, magnesium-and aluminum hydroxide antacids, sildenafil, and grapefruit juice have no impact on the exposure to amlodipine.
Atorvastatin is a substrate of the hepatic transporters, OATP1B1 and OATP1B3 transporter. Metabolites of atorvastatin are substrates of OATP1B1. Atorvastatin is also identified as a substrate of the efflux transporter BCRP, which may limit the intestinal absorption and biliary clearance of atorvastatin
Table 6 shows effects of other drugs on the pharmacokinetics of atorvastatin
Co-administered drug and dosing regimen | Atorvastatin | ||
Dose (mg) | Ratio of AUC& | Ratio of Cmax& | |
| #Cyclosporine 5.2 mg/kg/day, stable dose | 10 mg QDafor 28 days | 8.69 | 10.66 |
| #Tipranavir 500 mg BIDb/ritonavir 200 mg BIDb, 7 days | 10 mg, SDc | 9.36 | 8.58 |
| #Glecaprevir 400 mg QDa/pibrentasvir 120 mg QDa, 7 days | 10 mg QDafor 7 days | 8.28 | 22.00 |
| #Telaprevir 750 mg q8hf, 10 days | 20 mg, SDc | 7.88 | 10.60 |
| #, ‡Saquinavir 400 mg BIDb/ritonavir 400mg BID, 15 days | 40 mg QDafor 4 days | 3.93 | 4.31 |
| #Elbasvir 50 mg QDa/grazoprevir 200 mg QDa, 13 days | 10 mg SDc | 1.95 | 4.34 |
| #Simeprevir 150 mg QDa, 10 days | 40 mg SDc | 2.12 | 1.70 |
| #Clarithromycin 500 mg BIDb, 9 days | 80 mg QDafor 8 days | 4.54 | 5.38 |
| #Darunavir 300 mg BIDb/ritonavir 100 mg BID, 9 days | 10 mg QDafor 4 days | 3.45 | 2.25 |
| #Itraconazole 200 mg QDa, 4 days | 40 mg SDc | 3.32 | 1.20 |
| #Letermovir 480 mg QDa, 10 days | 20 mg SDc | 3.29 | 2.17 |
| #Fosamprenavir 700 mg BIDb/ritonavir 100 mg BIDb, 14 days | 10 mg QDafor 4 days | 2.53 | 2.84 |
| #Fosamprenavir 1400 mg BIDb, 14 days | 10 mg QDafor 4 days | 2.30 | 4.04 |
| #Nelfinavir 1250 mg BIDb, 14 days | 10 mg QDafor 28 days | 1.74 | 2.22 |
| #Grapefruit Juice, 240 mL QDa,* | 40 mg, SDc | 1.37 | 1.16 |
| Diltiazem 240 mg QDa, 28 days | 40 mg, SDc | 1.51 | 1.00 |
| Erythromycin 500 mg QIDe, 7 days | 10 mg, SDc | 1.33 | 1.38 |
| Amlodipine 10 mg, single dose | 80 mg, SDc | 1.18 | 0.91 |
| Cimetidine 300 mg QIDe, 2 weeks | 10 mg QDafor 2 weeks | 1.00 | 0.89 |
| Colestipol 10 g BIDb, 24 weeks | 40 mg QDafor 8 weeks | NA | 0.74** |
| Maalox TC®30 mL QDa, 17 days | 10 mg QDafor 15 days | 0.66 | 0.67 |
| Efavirenz 600 mg QDa, 14 days | 10 mg for 3 days | 0.59 | 1.01 |
| #Rifampin 600 mg QDa, 7 days (coadministered)† | 40 mg SDc | 1.12 | 2.90 |
| #Rifampin 600 mg QDa, 5 days (doses separated)† | 40 mg SDc | 0.20 | 0.60 |
| #Gemfibrozil 600 mg BIDb, 7 days | 40 mg SDc | 1.35 | 1.00 |
| #Fenofibrate 160 mg QDa, 7 days | 40 mg SDc | 1.03 | 1.02 |
| Boceprevir 800 mg TIDd, 7 days | 40 mg SDc | 2.32 | 2.66 |
&
#See Sections 5.1 and 7 for clinical significance.
* Greater increases in AUC (ratio of AUC up to 2.5) and/or Cmax(ratio of Cmaxup to 1.71) have been reported with excessive grapefruit consumption (≥ 750 mL to 1.2 liters per day).
** Ratio based on a single sample taken 8 to 16 h post dose.
† Due to the dual interaction mechanism of rifampin, simultaneous co-administration of atorvastatin with rifampin is recommended, as delayed administration of atorvastatin after administration of rifampin has been associated with a significant reduction in atorvastatin plasma concentrations.
‡ The dose of saquinavir plus ritonavir in this study is not the clinically used dose. The increase in atorvastatin exposure when used clinically is likely to be higher than what was observed in this study. Therefore, caution should be applied and the lowest dose necessary should be used.
aOnce daily
bTwice daily
cSingle dose
dThree times daily
eFour times daily
fEvery 8 hours
Effects of Amlodipine besylate and atorvastatin calcium on Other Drugs
Amlodipine is a weak inhibitor of CYP3A and may increase exposure to CYP3A substrates.
Co-administered amlodipine does not affect the exposure to atorvastatin, digoxin, ethanol and the warfarin prothrombin response time.
Table 7 shows the effects of atorvastatin on the pharmacokinetics of other drugs.
Atorvastatin | Co-administered drug and dosing regimen | ||
Drug/Dose (mg) | Ratio of AUC | Ratio of Cmax | |
| 80 mg QDafor 15 days | Antipyrine, 600 mg SDc | 1.03 | 0.89 |
| 80 mg QDafor 10 days | #Digoxin 0.25 mg QDa, 20 days | 1.15 | 1.20 |
| 40 mg QDafor 22 days | Oral contraceptive QDa, 2 months – norethindrone 1 mg – ethinyl estradiol 35 mcg | 1.281.19 | 1.231.30 |
| 10 mg, SDc | Tipranavir 500 mg BIDb/ritonavir 200 mg BIDb, 7 days | 1.08 | 0.96 |
| 10 mg QDafor 4 days | Fosamprenavir 1400 mg BIDb, 14 days | 0.73 | 0.82 |
| 10 mg QDafor 4 days | Fosamprenavir 700 mg BIDb/ritonavir 100 mg BIDb, 14 days | 0.99 | 0.94 |
# See Section 7 for clinical significance.
aOnce daily
bTwice daily
cSingle dose
Atorvastatin had no clinically significant effect on prothrombin time when administered to patients receiving chronic warfarin treatment.
Amlodipine besylate and atorvastatin calcium tablets are formulated for oral administration in the following strength combinations:
Amlodipine besylate and atorvastatin calcium tablets 2.5 mg/10 mg are white to off-white, round, film coated tablets debossed ‘R’ on one side and ‘407’ on other side.
Combinations of atorvastatin with 2.5 mg and 5 mg amlodipine are film-coated white, and combinations of atorvastatin with 10 mg amlodipine are film-coated blue.
•
•
Amlodipine besylate and atorvastatin calcium tablets are contraindicated in women who are pregnant.
Atorvastatin is contraindicated for use in pregnant women since safety in pregnant women has not been established and there is no apparent benefit of lipid lowering drugs during pregnancy. Because HMG-CoA reductase inhibitors decrease cholesterol synthesis and possibly the synthesis of other biologically active substances derived from cholesterol, atorvastatin may cause fetal harm when administered to a pregnant woman. Amlodipine besylate and atorvastatin calcium tablets should be discontinued as soon as pregnancy is recognized [see
The limited available data based on post-marketing reports with amlodipine use in pregnant women are not sufficient to inform a drug-associated risk for major birth defects and miscarriage. There are risks to the mother and fetus associated with poorly controlled hypertension in pregnancy (
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Hypertension in pregnancy increases the maternal risk for pre-eclampsia, gestational diabetes, premature delivery, and delivery complications (e.g., need for cesarean section and post-partum hemorrhage). Hypertension increases the fetal risk for intrauterine growth restriction and intrauterine death. Pregnant women with hypertension should be carefully monitored and managed accordingly.
Limited published data on atorvastatin calcium from observational studies, meta-analyses and case reports have not shown an increased risk of major congenital malformations or miscarriage. Rare reports of congenital anomalies have been received following intrauterine exposure to other HMG-CoA reductase inhibitors. In a review of approximately 100 prospectively followed pregnancies in women exposed to simvastatin or lovastatin, the incidences of congenital anomalies, spontaneous abortions, and fetal deaths/stillbirths did not exceed what would be expected in the general population. The number of cases is adequate to exclude a ≥3 to 4-fold increase in congenital anomalies over the background incidence. In 89% of the prospectively followed pregnancies, drug treatment was initiated prior to pregnancy and was discontinued at some point in the first trimester when pregnancy was identified.
Atorvastatin crosses the rat placenta and reaches a level in fetal liver equivalent to that of maternal plasma. When administered to pregnant rats and rabbits during organogenesis at oral doses up to 300 mg/kg/day and 100 mg/kg/day, respectively, atorvastatin was not teratogenic in rats at doses up to 300 mg/kg/day or in rabbits at doses up to 100 mg/kg/day. These doses resulted in multiples of about 30 times (rat) or 20 times (rabbit) the human exposure at the MRHD based on surface area (mg/m2). In rats, the maternally toxic dose of 300 mg/kg resulted in increased post-implantation loss and decreased fetal body weight. At the maternally toxic doses of 50 and 100 mg/kg/day in rabbits, there was increased post-implantation loss, and at 100 mg/kg/day fetal body weights were decreased.
In a study in pregnant rats administered atorvastatin calcium at doses equivalent to 20, 100, or 225 mg/kg/day, from gestation day 7 through to lactation day 20 (weaning), there was decreased survival at birth, postnatal day 4, weaning, and post-weaning in pups of mothers dosed with 225 mg/kg/day, a dose at which maternal toxicity was observed. Pup body weight was decreased through postnatal day 21 at 100 mg/kg/day, and through postnatal day 91 at 225 mg/kg/day. Pup development was delayed (rotorod performance at 100 mg/kg/day and acoustic startle at 225 mg/kg/day; pinnae detachment and eye-opening at 225 mg/kg/day). These doses of atorvastatin correspond to 6 times (100 mg/kg) and 22 times (225 mg/kg) the human exposure at the MRHD, based on AUC.
No evidence of teratogenicity or other embryo/fetal toxicity was found when pregnant rats and rabbits were treated orally with amlodipine maleate at doses up to 10 mg amlodipine/kg/day (approximately 10 and 20 times the MRHD based on body surface area, respectively) during their respective periods of major organogenesis. However, for rats, litter size was significantly decreased (by about 50%) and the number of intrauterine deaths was significantly increased (about 5-fold) in rats receiving amlodipine maleate at a dose equivalent to 10 mg amlodipine/kg/day for 14 days before mating and throughout mating and gestation. Amlodipine maleate has been shown to prolong both the gestation period and the duration of labor in rats at this dose.
•
Amlodipine besylate and atorvastatin calcium tablets are contraindicated during breastfeeding.
Atorvastatin use is contraindicated during breastfeeding [see
Limited available data from a published clinical lactation study reports that amlodipine is present in human milk at an estimated median relative infant dose of 4.2%. No adverse effects of amlodipine on the breastfed infant have been observed. There is no available information on the effects of amlodipine on milk production.
The following serious adverse reactions are discussed in greater detail in other sections of the label:
- Myopathy and Rhabdomyolysis [see Warnings and Precautions (
5.1 Myopathy and RhabdomyolysisAmlodipine and atorvastatin may cause myopathy (muscle pain, tenderness, or weakness with creatine kinase (CK) above ten times the upper limit of normal) and rhabdomyolysis (with or without acute renal failure secondary to myoglobinuria). Rare fatalities have occurred as a result of rhabdomyolysis with statin use, including amlodipine and atorvastatin.Risk Factors for MyopathyRisk factors for myopathy include age 65 years or greater, uncontrolled hypothyroidism, renal impairment, concomitant use with certain other drugs, and higher amlodipine and atorvastatin dosage [see Drug Interactions ].Steps to Prevent or Reduce the Risk of Myopathy and RhabdomyolysisAmlodipine and atorvastatin exposure may be increased by drug interactions due to inhibition of cytochrome P450 enzyme 3A4 (CYP3A4) and/or transporters (e.g., breast cancer resistant protein [BCRP], organic anion-transporting polypeptide [OATP1B1/OATP1B3] and P-glycoprotein [P-gp]), resulting in an increased risk of myopathy and rhabdomyolysis. Concomitant use of cyclosporine, gemfibrozil, tipranavir plus ritonavir or glecaprevir plus pibrentasvir with amlodipine and atorvastatin is not recommended. Amlodipine and atorvastatin dosage modifications are recommended for patients taking certain anti-viral, azole antifungals, or macrolide antibiotic medications [see Dosage and Administration ]. Cases of myopathy/rhabdomyolysis have been reported with atorvastatin co-administered with lipid modifying doses (>1 gram/day) of niacin, fibrates, colchicine, and ledipasvir plus sofosbuvir. Consider if the benefit of use of these products outweighs the increased risk of myopathy and rhabdomyolysis [see Drug Interactions (7.3)].Concomitant intake of large quantities, more than 1.2 liters daily, of grapefruit juice is not recommended in patients taking amlodipine and atorvastatin tablets[see Drug Interactions ].Discontinue amlodipine and atorvastatin tablets if markedly elevated CK levels occur or myopathy is diagnosed or suspected. Muscle symptoms and CK increases may resolve if amlodipine and atorvastatin tablets are discontinued. Temporarily discontinue amlodipine and atorvastatin tablets in patients experiencing an acute or serious condition at high risk of developing renal failure secondary to rhabdomyolysis (e.g., sepsis; shock; severe hypovolemia; major surgery; trauma; severe metabolic, endocrine, or electrolyte disorders; or uncontrolled epilepsy).Inform patients of the risk of myopathy and rhabdomyolysis when starting or increasing the amlodipine and atorvastatin dosage. Instruct patients to promptly report any unexplained muscle pain, tenderness or weakness, particularly if accompanied by malaise or fever.)] Liver enzyme abnormalities [see
Warnings and Precautions (])5.3 Liver DysfunctionStatins, like atorvastatin, and some other lipid-lowering therapies, have been associated with biochemical abnormalities of liver function. Persistent elevations (>3 times the upper limit of normal [ULN] occurring on 2 or more occasions) in serum transaminases occurred in 0.7% of patients who received atorvastatin in clinical trials. The incidence of these abnormalities was 0.2%, 0.2%, 0.6%, and 2.3% for 10, 20, 40, and 80 mg, respectively.
One patient in clinical trials with atorvastatin developed jaundice. Increases in liver function tests (LFT) in other patients were not associated with jaundice or other clinical signs or symptoms. Upon dose reduction, drug interruption, or discontinuation, transaminase levels returned to or near pretreatment levels without sequelae. Eighteen of 30 patients with persistent LFT elevations continued treatment with a reduced dose of atorvastatin.
It is recommended that liver enzyme tests be obtained prior to initiating therapy with atorvastatin and repeated as clinically indicated. There have been rare postmarketing reports of fatal and non-fatal hepatic failure in patients taking statins, including atorvastatin. If serious liver injury with clinical symptoms and/or hyperbilirubinemia or jaundice occurs during treatment with amlodipine besylate and atorvastatin calcium, promptly interrupt therapy. If an alternate etiology is not found, do not restart amlodipine besylate and atorvastatin calcium.
Active liver disease or unexplained persistent transaminase elevations are contraindications to the use of amlodipine besylate and atorvastatin calcium [see
Contraindications].