Amoxicillin And Clavulanate Potassium
Amoxicillin And Clavulanate Potassium Prescribing Information
Amoxicillin and Clavulanate Potassium for Oral Suspension is indicated for the treatment of infections in adults and pediatric patients, due to susceptible isolates of the designated bacteria in the conditions listed below:
- Lower Respiratory Tract Infections-caused by beta‑lactamase‑producing isolates ofHaemophilus influenzaeandMoraxella catarrhalis.
- Acute Bacterial Otitis Media-caused by beta‑lactamase‑producing isolates ofH. influenzaeandM. catarrhalis.
- Sinusitis-caused by beta‑lactamase‑producing isolates ofH. influenzaeandM. catarrhalis.
- Skin and Skin Structure Infections-caused by beta‑lactamase‑producing isolates ofStaphylococcus aureus,Escherichia coli, andKlebsiellaspecies.
- Urinary Tract Infections-caused by beta‑lactamase‑producing isolates ofE. coli,Klebsiellaspecies, andEnterobacterspecies.
When susceptibility test results show susceptibility to amoxicillin, indicating no beta-lactamase production, Amoxicillin and Clavulanate Potassium for Oral Suspension should not be used.
To reduce the development of drug‑resistant bacteria and maintain the effectiveness of Amoxicillin and Clavulanate Potassium for Oral Suspension and other antibacterial drugs, Amoxicillin and Clavulanate Potassium for Oral Suspension should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
- Amoxicillin and Clavulanate Potassium for Oral Suspension, USP 200 mg/28.5 mg per 5 mL:The dry powder is white to off white with fruity flavor. Each 5 mL of reconstituted creamy suspension contains 200 mg amoxicillin and 28.5 mg clavulanic acid as the potassium salt.
- Amoxicillin and Clavulanate Potassium for Oral Suspension, USP 400 mg/57 mg per 5 mL:The dry powder is white to off white with fruity flavor. Each 5 ml of reconstituted creamy suspension contains 400 mg amoxicillin and 57 mg clavulanic acid as the potassium salt.
The following are discussed in more detail in other sections of the labeling:
- Anaphylactic reactions [see Warnings and Precautions ]
- Severe Cutaneous Adverse Reactions [see Warnings and Precautions ]
- Drug-Induced Enterocolitis Syndrome (DIES) [see Warnings and Precautions ()]
5.3 Drug-Induced Enterocolitis Syndrome (DIES)Drug-induced enterocolitis syndrome (DIES) has been reported with the use of amoxicillin, a component of amoxicillin and clavulanate potassium for oral suspension,[see Adverse Reactions ], with most cases occurring in pediatric patients ≤18 years of age. DIES is a non-IgE mediated hypersensitivity reaction characterized by protracted vomiting occurring 1 to 4 hours after drug ingestion in the absence of skin or respiratory symptoms. DIES may be associated with pallor, lethargy, hypotension, shock, diarrhea within 24 hours after ingesting amoxicillin, and leukocytosis with neutrophilia. If DIES occurs, discontinue Amoxicillin and clavulanate potassium suspension and institute appropriate therapy.
- Hepatic Dysfunction [see Warnings and Precautions ()]
5.4 Hepatic DysfunctionHepatic dysfunction, including hepatitis and cholestatic jaundice has been associated with the use of amoxicillin and clavulanate potassium suspension. Hepatic toxicity is usually reversible; however, deaths have been reported. Hepatic function should be monitored at regular intervals in patients with hepatic impairment.
- Clostridioides difficileAssociated Diarrhea (CDAD)[see Warnings and Precautions ()]
5.5ClostridioidesdifficileAssociated Diarrhea (CDAD)Clostridioides difficileassociated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including amoxicillin and clavulanate potassium suspension, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth ofC. difficile.C. difficileproduces toxins A and B which contribute to the development of CDAD. Hypertoxin-producing strains ofC. difficilecause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibacterial use. Careful medical history is necessary since CDAD has been reported to occur over 2 months after the administration of antibacterial agents.If CDAD is suspected or confirmed, ongoing antibacterial use not directed against
C. difficilemay need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibacterial treatment ofC. difficile, and surgical evaluation should be instituted as clinically indicated.
Amoxicillin and Clavulanate Potassium for Oral Suspension, USP is an oral antibacterial combination consisting of amoxicillin and the beta-lactamase inhibitor, clavulanate potassium (the potassium salt of clavulanic acid).
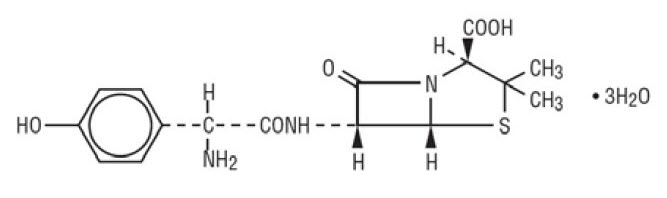
Amoxicillin is an analog of ampicillin, derived from the basic penicillin nucleus, 6-aminopenicillanic acid. The amoxicillin molecular formula is C16H19N3O5S•3H2O, and the molecular weight is 419.46. Chemically, amoxicillin is (
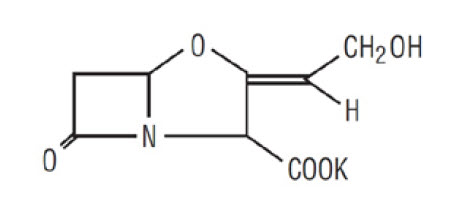
Clavulanic acid is produced by the fermentation of
Each 5 mL of reconstituted amoxicillin and clavulanate potassium 400 mg/57 mg per 5 mL suspension contains 0.268 mEq of potassium.
Each 5 mL of reconstituted amoxicillin and clavulanate potassium 200 mg/28.5 mg per 5 mL suspension contains 0.143 mEq of potassium
NDC 0143-9981-50 .................. 50 mL bottle
NDC 0143-9981-75 .................. 75 mL bottle
NDC 0143-9981-01 .................. 100 mL bottle
NDC 0143-9982-50 .................. 50 mL bottle
NDC 0143-9982-75 .................. 75 mL bottle
NDC 0143-9982-01 .................. 100 mL bottle
Dispense in original container.
Store dry powder at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature].
Store reconstituted suspension under refrigeration. Discard unused suspension after 10 days. Keep out of the reach of children.
Amoxicillin and clavulanate potassium for oral suspension is an antibacterial drug.