Ampicillin
(Ampicillin Sodium)Ampicillin Prescribing Information
Ampicillin for Injection, USP is indicated in the treatment of infections caused by susceptible strains of the designated organisms in the following conditions:
Respiratory Tract Infections
Bacterial Meningitis
Septicemia and Endocarditis
Urinary Tract Infections
Gastrointestinal Infections
Bacteriology studies to determine the causative organisms and their susceptibility to ampicillin should be performed. Therapy may be instituted prior to obtaining results of susceptibility testing. It is advisable to reserve the parenteral form of this drug for moderately severe and severe infections and for patients who are unable to take the oral forms. A change to oral ampicillin may be made as soon as appropriate.
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Ampicillin for Injection, USP and other antibacterial drugs, Ampicillin for Injection, USP should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
Indicated surgical procedures should be performed.
This insert is for a Pharmacy Bulk Package and is intended for preparing IV admixtures only. Dosage recommendations for intramuscular or direct intravenous injection are for informational purposes only.
In the treatment of chronic urinary tract and intestinal infections, frequent bacteriological and clinical appraisal is necessary. Smaller doses than those recommended above should not be used. Higher doses should be used for stubborn or severe infections. In stubborn infections, therapy may be required for several weeks. It may be necessary to continue clinical and/or bacteriological follow-up for several months after cessation of therapy.
The doses for the preceding infections may be given by either the intramuscular or intravenous route. A change to oral ampicillin may be made when appropriate.
Gestational age (weeks) | Postnatal age (days) | Dosage |
| less than or equal to 34 | less than or equal to 7 | 100 mg/kg/day in equally divided doses every 12 hours |
| less than or equal to 34 | greater than or equal to 8 and less than 28 | 150 mg/kg/day in equally divided doses every 12 hours |
| greater than 34 | less than or equal to 28 | 150 mg/kg/day in equally divided doses every 8 hours |
Treatment of all infections should be continued for a minimum of 48 to 72 hours beyond the time that the patient becomes asymptomatic or evidence of bacterial eradication has been obtained. A minimum of 10 days treatment is recommended for any infection caused by Group A beta-hemolytic streptococci to help prevent the occurrence of acute rheumatic fever or acute glomerulonephritis.
This glass Pharmacy Bulk Package bottle contains ampicillin sodium equivalent to 10 grams of ampicillin and is designed for use in the pharmacy in preparing IV admixtures.
a) Add 94 mL Sterile Water for Injection, USP. The resulting solution will contain 100 milligrams ampicillin activity per mL, and is stable up to
b) Dilute further within
c) Using aseptic technique under a laminar flow hood, the closure should be penetrated only one time after reconstitution using a suitable sterile dispensing set; which allows measured dispensing of the contents. Use of a syringe and needle is not recommended as it may cause leakage.
d) After entry, use entire contents of Pharmacy Bulk Package bottle promptly. The entire contents of the Pharmacy Bulk Package bottle must be dispensed within
e) A plastic ball attached to the pharmacy bulk package provides a suitable hanging device while dispensing contents.
Use of this product is restricted to a suitable work area, such as a laminar flow hood. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Room Temperature (25°C) | ||
Diluent | Concentrations | Stability Periods |
| Sterile Water for Injection | up to 30 mg/mL | 8 hours |
| Sodium Chloride Injection USP, 0.9% | up to 30 mg/mL | 8 hours |
| 5% Dextrose in Water | 10 to 20 mg/mL | 1 hour |
| 5% Dextrose in Water | up to 2 mg/mL | 2 hours |
| 5% Dextrose in 0.45% Sodium Chloride | up to 2 mg/mL | 2 hours |
| Lactated Ringer’s Solution | up to 30 mg/mL | 8 hours |
Refrigerated (4°C) | ||
Diluent | Concentrations | Stability Periods |
| Sterile Water for Injection | 30 mg/mL | 48 hours |
| Sterile Water for Injection | up to 20 mg/mL | 72 hours |
| Sodium Chloride Injection USP, 0.9% | 30 mg/mL | 24 hours |
| Sodium Chloride Injection USP, 0.9% | up to 20 mg/mL | 48 hours |
| Lactated Ringer's Solution | up to 30 mg/mL | 24 hours |
| 5% Dextrose in Water | up to 20 mg/mL | 1 hour |
| 5% Dextrose in 0.45% Sodium Chloride | up to 10 mg/mL | 1 hour |
Only those solutions listed above should be used for the intravenous infusion of Ampicillin for Injection, USP. The concentrations should fall within the range specified. The drug concentration and the rate and volume of the infusion should be adjusted so that the total dose of ampicillin is administered before the drug loses its stability in the solution in use.
A history of a previous hypersensitivity reaction to any of the penicillins is a contraindication.
As with other penicillins, it may be expected that untoward reactions will be essentially limited to sensitivity phenomena. They are more likely to occur in individuals who have previously demonstrated hypersensitivity to penicillins and in those with a history of allergy, asthma, hay fever, or urticaria.
The following adverse reactions have been reported as associated with the use of ampicillin:
Glossitis, stomatitis, black “hairy” tongue, nausea, vomiting, enterocolitis, pseudomembranous colitis, and diarrhea. (These reactions are usually associated with oral dosage forms.)
Skin rashes and urticaria have been reported frequently. A few cases of exfoliative dermatitis and erythema multiforme have been reported.
Note:
Liver
Hemic and Lymphatic Systems
Central Nervous System -
The concurrent administration of allopurinol and ampicillin increases substantially the incidence of skin rashes in patients receiving both drugs as compared to patients receiving ampicillin alone. It is not known whether this potentiation of ampicillin rashes is due to allopurinol or the hyperuricemia present in these patients.
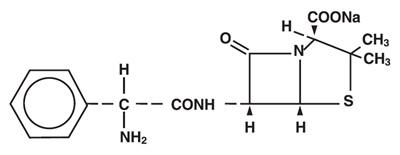
Ampicillin for Injection, USP the monosodium salt of [2S-[2α,5α,6β(S*)]]-6-[(aminophenylacetyl) amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo [3.2.0]heptane-2-carboxylic acid, is a synthetic penicillin for intravenous use.
The pharmacy bulk package contains sterile ampicillin sodium equivalent to 10 grams ampicillin. It is an antibacterial agent with a broad spectrum of bactericidal activity against both penicillin-susceptible Grampositive organisms and many common Gram-negative pathogens.
Ampicillin for Injection, USP is a dry, white to off-white powder. The reconstituted solution is clear, colorless and free from visible particulates.
A Pharmacy Bulk Package is a container of a sterile preparation for parenteral use that contains many single doses. The contents of this pharmacy bulk package are intended for use by a pharmacy admixture service for addition to suitable parenteral fluids in the preparation of admixtures for intravenous infusion (see
This glass Pharmacy Bulk Package bottle contains ampicillin sodium equivalent to 10 grams of ampicillin and is designed for use in the pharmacy in preparing IV admixtures.
a) Add 94 mL Sterile Water for Injection, USP. The resulting solution will contain 100 milligrams ampicillin activity per mL, and is stable up to
b) Dilute further within
c) Using aseptic technique under a laminar flow hood, the closure should be penetrated only one time after reconstitution using a suitable sterile dispensing set; which allows measured dispensing of the contents. Use of a syringe and needle is not recommended as it may cause leakage.
d) After entry, use entire contents of Pharmacy Bulk Package bottle promptly. The entire contents of the Pharmacy Bulk Package bottle must be dispensed within
e) A plastic ball attached to the pharmacy bulk package provides a suitable hanging device while dispensing contents.
Use of this product is restricted to a suitable work area, such as a laminar flow hood. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
It has the following molecular structure: