Ampicillin
Ampicillin Prescribing Information
To reduce the development of drug-resistant bacteria and maintain the effectiveness of ampicillin capsules and other antibacterial drugs, ampicillin capsules should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria. When culture and susceptibility information are available, they should be considered in selecting of modifying antimicrobial therapy, in the absence of such data, local epidemiology and susceptibility patterns contribute to the empiric selection of therapy.
Ampicillin capsules are indicated in the treatment of infections caused by susceptible strains of the designated organisms listed below:
Bacteriology studies to determine the causative organisms and their susceptibility to ampicillin capsules should be performed. Therapy may be instituted prior to the results of susceptibility testing.
For
Larger doses may be required for severe or chronic infections. Although ampicillin is resistant to degradation by gastric acid, it should be administered at least one-half hour before or two hours after meals for maximal absorption. Except for the single dose regimen for gonorrhea referred to above, therapy should be continued for a minimum of 48 to 72 hours after the patient becomes asymptomatic or evidence of bacterial eradication has been obtained. In infections caused by hemolytic strains of streptococci, a minimum of 10 days’ treatment is recommended to guard against the risk of rheumatic fever or glomerulonephritis (see
In prolonged therapy, and particularly with high dosage regimens, periodic evaluation of the renal, hepatic, and hematopoietic systems is recommended.
In streptococcal infections, therapy, must be sufficient to eliminate the organism (10 days minimum); otherwise the sequelae of streptococcal disease may occur. Cultures should be taken following completion of treatment to determine whether streptococci have been eradicated.
Cases of gonococcal infection with a suspected lesion of syphilis should have darkfield examinations ruling out syphilis before receiving ampicillin. Patients who do not have suspected lesions of syphilis and are treated with ampicillin should have a follow-up serologic test for syphilis each month for four months to detect syphilis that may have been masked from treatment for gonorrhea.
The use of ampicillin is contraindicated in individuals with a history of serious hypersensitivity reactions (e.g., anaphylaxis or Stevens-Johnson syndrome) to ampicillin or to other beta-lactam antibacterial drugs. Ampicillin is also contraindicated in infections caused by penicillinase-producing organisms.
Ampicillin is contraindicated in patients with a previous history of cholestatic jaundice/hepatic dysfunction associated with treatment with ampicillin.
As with other penicillins, it may be expected that untoward reactions will be essentially limited to sensitivity phenomena. They are more likely to occur in individuals who have previously demonstrated hypersensitivity to penicillin and in those with a history of allergy, asthma, hay fever, or urticaria.
The following adverse reactions have been reported as associated with the use of ampicillin:
SERIOUS AND OCCASIONALLY FATAL HYPERSENSITIVITY (ANAPHYLACTIC) REACTIONS HAVE BEEN REPORTED IN PATIENTS ON PENICILLIN THERAPY. ALTHOUGH ANAPHYLAXIS IS MORE FREQUENT FOLLOWING PARENTERAL THERAPY, IT HAS OCCURRED IN PATIENTS ON ORAL PENICILLINS. THESE REACTIONS ARE MORE LIKELY TO OCCUR IN INDIVIDUALS WITH A HISTORY OF SENSITIVITY TO MULTIPLE ALLERGENS.
THERE HAVE BEEN REPORTS OF INDIVIDUALS WITH A HISTORY OF PENICILLIN HYPERSENSITIVITY WHO HAVE EXPERIENCED SEVERE REACTIONS WHEN TREATED WITH CEPHALOSPORINS. BEFORE THERAPY WITH ANY PENICILLIN, CAREFUL INQUIRY SHOULD BE MADE CONCERNING PREVIOUS HYPERSENSITIVITY REACTIONS TO PENICILLINS, CEPHALOSPORINS OR OTHER ALLERGENS. IF AN ALLERGIC REACTION OCCURS, APPROPRIATE THERAPY SHOULD BE CONSIDERED.
Ampicillin may cause severe cutaneous adverse reactions (SCARs), such as toxic epidermal necrolysis (TEN), Stevens-Johnson syndrome (SJS), drug reaction with eosinophilia and systemic symptoms (DRESS), exfoliative dermatitis, erythema multiforme, and acute generalized exanthematous pustulosis (AGEP). If patients develop a skin rash, they should be monitored closely, and ampicillin discontinued if lesions progress.
Hepatic dysfunction, including hepatitis and cholestatic jaundice has been associated with the use of ampicillin. Hepatic toxicity is usually reversible; however, deaths have been reported. Hepatic function should be monitored at regular intervals in patients with hepatic impairment.
Clostridioides difficile-associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including ampicillin, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of
If CDAD is suspected or confirmed, ongoing antibacterial drug use not directed against
SERIOUS AND OCCASIONALLY FATAL HYPERSENSITIVITY (ANAPHYLACTIC) REACTIONS HAVE BEEN REPORTED IN PATIENTS ON PENICILLIN THERAPY. ALTHOUGH ANAPHYLAXIS IS MORE FREQUENT FOLLOWING PARENTERAL THERAPY, IT HAS OCCURRED IN PATIENTS ON ORAL PENICILLINS. THESE REACTIONS ARE MORE LIKELY TO OCCUR IN INDIVIDUALS WITH A HISTORY OF SENSITIVITY TO MULTIPLE ALLERGENS.
THERE HAVE BEEN REPORTS OF INDIVIDUALS WITH A HISTORY OF PENICILLIN HYPERSENSITIVITY WHO HAVE EXPERIENCED SEVERE REACTIONS WHEN TREATED WITH CEPHALOSPORINS. BEFORE THERAPY WITH ANY PENICILLIN, CAREFUL INQUIRY SHOULD BE MADE CONCERNING PREVIOUS HYPERSENSITIVITY REACTIONS TO PENICILLINS, CEPHALOSPORINS OR OTHER ALLERGENS. IF AN ALLERGIC REACTION OCCURS, APPROPRIATE THERAPY SHOULD BE CONSIDERED.
Ampicillin may cause severe cutaneous adverse reactions (SCARs), such as toxic epidermal necrolysis (TEN), Stevens-Johnson syndrome (SJS), drug reaction with eosinophilia and systemic symptoms (DRESS), exfoliative dermatitis, erythema multiforme, and acute generalized exanthematous pustulosis (AGEP). If patients develop a skin rash, they should be monitored closely, and ampicillin discontinued if lesions progress.
Hepatic dysfunction, including hepatitis and cholestatic jaundice has been associated with the use of ampicillin. Hepatic toxicity is usually reversible; however, deaths have been reported. Hepatic function should be monitored at regular intervals in patients with hepatic impairment.
Clostridioides difficile-associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including ampicillin, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of
If CDAD is suspected or confirmed, ongoing antibacterial drug use not directed against
SERIOUS AND OCCASIONALLY FATAL HYPERSENSITIVITY (ANAPHYLACTIC) REACTIONS HAVE BEEN REPORTED IN PATIENTS ON PENICILLIN THERAPY. ALTHOUGH ANAPHYLAXIS IS MORE FREQUENT FOLLOWING PARENTERAL THERAPY, IT HAS OCCURRED IN PATIENTS ON ORAL PENICILLINS. THESE REACTIONS ARE MORE LIKELY TO OCCUR IN INDIVIDUALS WITH A HISTORY OF SENSITIVITY TO MULTIPLE ALLERGENS.
THERE HAVE BEEN REPORTS OF INDIVIDUALS WITH A HISTORY OF PENICILLIN HYPERSENSITIVITY WHO HAVE EXPERIENCED SEVERE REACTIONS WHEN TREATED WITH CEPHALOSPORINS. BEFORE THERAPY WITH ANY PENICILLIN, CAREFUL INQUIRY SHOULD BE MADE CONCERNING PREVIOUS HYPERSENSITIVITY REACTIONS TO PENICILLINS, CEPHALOSPORINS OR OTHER ALLERGENS. IF AN ALLERGIC REACTION OCCURS, APPROPRIATE THERAPY SHOULD BE CONSIDERED.
Ampicillin may cause severe cutaneous adverse reactions (SCARs), such as toxic epidermal necrolysis (TEN), Stevens-Johnson syndrome (SJS), drug reaction with eosinophilia and systemic symptoms (DRESS), exfoliative dermatitis, erythema multiforme, and acute generalized exanthematous pustulosis (AGEP). If patients develop a skin rash, they should be monitored closely, and ampicillin discontinued if lesions progress.
Hepatic dysfunction, including hepatitis and cholestatic jaundice has been associated with the use of ampicillin. Hepatic toxicity is usually reversible; however, deaths have been reported. Hepatic function should be monitored at regular intervals in patients with hepatic impairment.
Clostridioides difficile-associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including ampicillin, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of
If CDAD is suspected or confirmed, ongoing antibacterial drug use not directed against
Other adverse reactions that have been reported with the use of ampicillin are laryngeal stridor and high fever. An occasional patient may complain of sore mouth or tongue as with any oral penicillin preparation.
When administered concurrently, the following drugs may interact with ampicillin.
To reduce the development of drug-resistant bacteria and maintain the effectiveness of ampicillin and other antibacterial drugs, ampicillin should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.
Ampicillin trihydrate is a semisynthetic penicillin derived from the basic penicillin nucleus, 6-aminopenicillanic acid. Ampicillin is designated chemically as (2S, 5
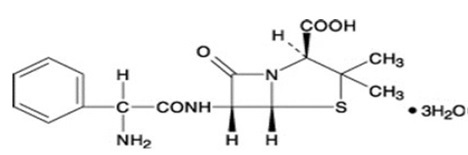
Molecular formula: C16H19N3O4S
Molecular weight: 403.5
Ampicillin capsules, USP for oral administration provide ampicillin trihydrate equivalent to 250 mg and 500 mg ampicillin USP. Inactive ingredients: ammonium hydroxide, black iron oxide, gelatin, magnesium stearate, potassium hydroxide, propylene glycol, shellac, sodium lauryl sulfate and titanium dioxide.
Meets USP dissolution test 2.