Ampicillin And Sulbactam
(Ampicillin Sodium And Sulbactam Sodium)Ampicillin And Sulbactam Prescribing Information
Ampicillin and sulbactam for injection, USP is indicated for the treatment of infections due to susceptible strains of the designated microorganisms in the conditions listed below.
NOTE: For information on use in pediatric patients (see
The safety and effectiveness of ampicillin and sulbactam for injection, USP have been established for pediatric patients one year of age and older for skin and skin structure infections as approved in adults. Use of ampicillin and sulbactam injection, USP in pediatric patients is supported by evidence from adequate and well-controlled studies in adults with additional data from pediatric pharmacokinetic studies, a controlled clinical trial conducted in pediatric patients and post-marketing adverse events surveillance (see
The safety and effectiveness of ampicillin and sulbactam for injection, USP have not been established for pediatric patients for intra-abdominal infections.
Data from a controlled clinical trial conducted in pediatric patients provided evidence supporting the safety and efficacy of ampicillin and sulbactam for injection, USP for the treatment of skin and skin structure infections. Of 99 pediatric patients evaluable for clinical efficacy, 60 patients received a regimen containing intravenous ampicillin and sulbactam for injection, USP, and 39 patients received a regimen containing intravenous cefuroxime. This trial demonstrated similar outcomes (assessed at an appropriate interval after discontinuation of all antimicrobial therapy) for ampicillin and sulbactam for injection, USP - and cefuroxime-treated patients:
| Therapeutic Regimen | Clinical Success | Clinical Failure |
|---|---|---|
| Ampicillin and Sulbactam for Injection, USP | 51/60 (85%) | 9/60 (15%) |
| Cefuroxime | 34/39 (87%) | 5/39 (13%) |
Most patients received a course of oral antimicrobials following initial treatment with intravenous administration of parenteral antimicrobials. The study protocol required that the following three criteria be met prior to transition from intravenous to oral antimicrobial therapy:
(1) receipt of a minimum of 72 hours of intravenous therapy; (2) no documented fever for prior 24 hours; and (3) improvement or resolution of the signs and symptoms of infection.
The choice of oral antimicrobial agent used in this trial was determined by susceptibility testing of the original pathogen, if isolated, to oral agents available. The course of oral antimicrobial therapy should not routinely exceed 14 days.
While ampicillin and sulbactam for injection, USP is indicated only for the conditions listed above, infections caused by ampicillin-susceptible organisms are also amenable to treatment with ampicillin and sulbactam for injection, USP due to its ampicillin content. Therefore, mixed infections caused by ampicillin-susceptible organisms and beta-lactamase producing organisms susceptible to ampicillin and sulbactam for injection, USP should not require the addition of another antibacterial.
Appropriate culture and susceptibility tests should be performed before treatment in order to isolate and identify the organisms causing infection and to determine their susceptibility to ampicillin and sulbactam for injection, USP.
Therapy may be instituted prior to obtaining the results from bacteriological and susceptibility studies when there is reason to believe the infection may involve any of the beta-lactamase producing organisms listed above in the indicated organ systems. Once the results are known, therapy should be adjusted if appropriate.
To reduce the development of drug-resistant bacteria and maintain effectiveness of ampicillin and sulbactam for injection, USP and other antibacterial drugs, ampicillin and sulbactam for injection, USP should be used only to treat infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
Ampicillin and sulbactam for injection may be administered by either the IV or the IM routes.
For IV administration, the dose can be given by slow intravenous injection over at least 10–15 minutes or can also be delivered in greater dilutions with 50–100 mL of a compatible diluent as an intravenous infusion over 15–30 minutes.
Ampicillin and sulbactam for injection may be administered by deep intramuscular injection. (see
Vials for intramuscular use may be reconstituted with Sterile Water for Injection, 0.5% Lidocaine Hydrochloride Injection USP or 2% Lidocaine Hydrochloride Injection. Consult the following table for recommended volumes to be added to obtain solutions containing 375 mg ampicillin and sulbactam for injection, USP per mL (250 mg ampicillin/125 mg sulbactam per mL). Note:
| Ampicillin and sulbactam for Injection, USP Vial Size | Volume of Diluent to be Added | Withdrawal VolumeThere is sufficient excess present to allow withdrawal and administration of the stated volumes |
|---|---|---|
| 1.5 g | 3.2 mL | 4.0 mL |
| 3.0 g | 6.4 mL | 8.0 mL |
The recommended adult dosage of ampicillin and sulbactam for injection is 1.5 g (1 g ampicillin as the sodium salt plus 0.5 g sulbactam as the sodium salt) to 3 g (2 g ampicillin as the sodium salt plus 1 g sulbactam as the sodium salt) every six hours. This 1.5 to 3 g range represents the total of ampicillin content plus the sulbactam content of ampicillin and sulbactam for injection, and corresponds to a range of 1 g ampicillin/0.5 g sulbactam to 2 g ampicillin/1 g sulbactam. The total dose of sulbactam should not exceed 4 grams per day.
The use of ampicillin and sulbactam for injection, USP is contraindicated in individuals with a history of serious hypersensitivity reactions (e.g., anaphylaxis or Stevens-Johnson syndrome) to ampicillin, sulbactam or to other beta-lactam antibacterial drugs (e.g., penicillins and cephalosporins).
Ampicillin and sulbactam for injection, USP is contraindicated in patients with a previous history of cholestatic jaundice/hepatic dysfunction associated with ampicillin and sulbactam for injection, USP.
Ampicillin and sulbactam for injection, USP is generally well tolerated. The following adverse reactions have been reported in clinical trials.
Probenecid decreases the renal tubular secretion of ampicillin and sulbactam. Concurrent use of probenecid with ampicillin and sulbactam for injection, USP may result in increased and prolonged blood levels of ampicillin and sulbactam. The concurrent administration of allopurinol and ampicillin increases substantially the incidence of rashes in patients receiving both drugs as compared to patients receiving ampicillin alone. It is not known whether this potentiation of ampicillin rashes is due to allopurinol or the hyperuricemia present in these patients. There are no data with ampicillin and sulbactam for injection, USP and allopurinol administered concurrently. Ampicillin and sulbactam for injection, USP and aminoglycosides should not be reconstituted together due to the
Ampicillin and Sulbactam for Injection is an injectable antibacterial combination consisting of the semisynthetic antibacterial ampicillin sodium and the beta-lactamase inhibitor sulbactam sodium for intravenous and intramuscular administration.
Ampicillin sodium is derived from the penicillin nucleus, 6-aminopenicillanic acid. Chemically, it is monosodium (2S, 5R, 6R)-6-[(R)-2-amino-2-phenylacetamido]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0] heptane-2-carboxylate and has a molecular weight of 371.39. Its chemical formula is C16H18N3NaO4S. The structural formula is:
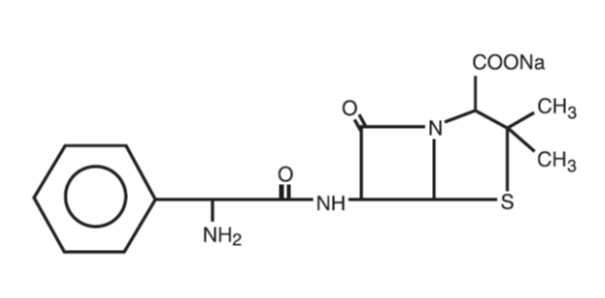
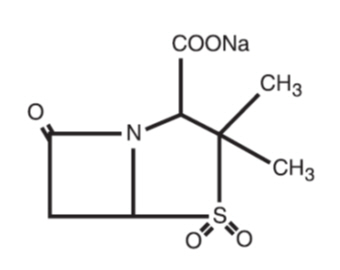
Sulbactam sodium is a derivative of the basic penicillin nucleus. Chemically, sulbactam sodium is sodium penicillinate sulfone; sodium (2S, 5R)-3,3-dimethyl-7-oxo-4-thia- 1-azabicyclo [3.2.0] heptane-2-carboxylate 4,4-dioxide. Its chemical formula is C8H10NNaO5S with a molecular weight of 255.22. The structural formula is:
Ampicillin and Sulbactam for Injection, ampicillin sodium/sulbactam sodium parenteral combination, is available as a white to off-white dry powder for reconstitution. Ampicillin and Sulbactam for Injection dry powder is freely soluble in aqueous diluents to yield pale yellow to yellow solutions containing ampicillin sodium and sulbactam sodium equivalent to 250 mg ampicillin per mL and 125 mg sulbactam per mL. The pH of the solutions is between 8.0 and 10.0.
Dilute solutions (up to 30 mg ampicillin and 15 mg sulbactam per mL) are essentially colorless to pale yellow. The pH of dilute solutions remains the same.
Each 1.5 gram of Ampicillin and Sulbactam for Injection contains 1 gram ampicillin as the sodium salt plus 0.5 gram sulbactam as the sodium salt. The sodium content is approximately 115 mg (5 mEq) of sodium per vial.
Each 3 gram of Ampicillin and Sulbactam for Injection contains 2 gram ampicillin as the sodium salt plus 1 gram sulbactam as the sodium salt. The sodium content is approximately 230 mg (10 mEq) of sodium per vial.