Apixaban Prescribing Information
(B) SPINAL/EPIDURAL HEMATOMA
Premature discontinuation of any oral anticoagulant, including apixaban tablets, increases the risk of thrombotic events. If anticoagulation with apixaban tablets is discontinued for a reason other than pathological bleeding or completion of a course of therapy, consider coverage with another anticoagulant
Discontinue apixaban tablets and begin taking the new anticoagulant other than warfarin at the usual time of the next dose of apixaban tablets.
Discontinue the anticoagulant other than warfarin and begin taking apixaban tablets at the usual time of the next dose of the anticoagulant other than warfarin.
Premature discontinuation of any oral anticoagulant, including apixaban tablets, in the absence of adequate alternative anticoagulation increases the risk of thrombotic events. An increased rate of stroke was observed during the transition from apixaban tablets to warfarin in clinical trials in atrial fibrillation patients. If apixaban tablets is discontinued for a reason other than pathological bleeding or completion of a course of therapy, consider coverage with another anticoagulant
Evidence for the efficacy and safety of apixaban was derived from ARISTOTLE, a multinational, double-blind study in patients with nonvalvular AF comparing the effects of apixaban and warfarin on the risk of stroke and non-central nervous system (CNS) systemic embolism. In ARISTOTLE, patients were randomized to apixaban tablets 5 mg orally twice daily (or 2.5 mg twice daily in subjects with at least 2 of the following characteristics: age greater than or equal to 80 years, body weight less than or equal to 60 kg, or serum creatinine greater than or equal to 1.5 mg/dL) or to warfarin (targeted to an INR range of 2.0-3.0). Patients had to have one or more of the following additional risk factors for stroke:
• prior stroke or transient ischemic attack (TIA)
• prior systemic embolism
• age greater than or equal to 75 years
• arterial hypertension requiring treatment
• diabetes mellitus
• heart failure ≥New York Heart Association Class 2
• left ventricular ejection fraction ≤40%
The primary objective of ARISTOTLE was to determine whether apixaban tablets 5 mg twice daily (or 2.5 mg twice daily) was effective (noninferior to warfarin) in reducing the risk of stroke (ischemic or hemorrhagic) and systemic embolism. Superiority of apixaban to warfarin was also examined for the primary endpoint (rate of stroke and systemic embolism), major bleeding, and death from any cause.
A total of 18,201 patients were randomized and followed on study treatment for a median of 89 weeks. Forty-three percent of patients were vitamin K antagonist (VKA) “naive,” defined as having received ≤30 consecutive days of treatment with warfarin or another VKA before entering the study. The mean age was 69 years and the mean CHADS2 score (a scale from 0 to 6 used to estimate risk of stroke, with higher scores predicting greater risk) was 2.1. The population was 65% male, 83% Caucasian, 14% Asian, and 1% Black. There was a history of stroke, TIA, or non-CNS systemic embolism in 19% of patients. Concomitant diseases of patients in this study included hypertension 88%, diabetes 25%, congestive heart failure (or left ventricular ejection fraction ≤40%) 35%, and prior myocardial infarction 14%. Patients treated with warfarin in ARISTOTLE had a mean percentage of time in therapeutic range (INR 2.0–3.0) of 62%.
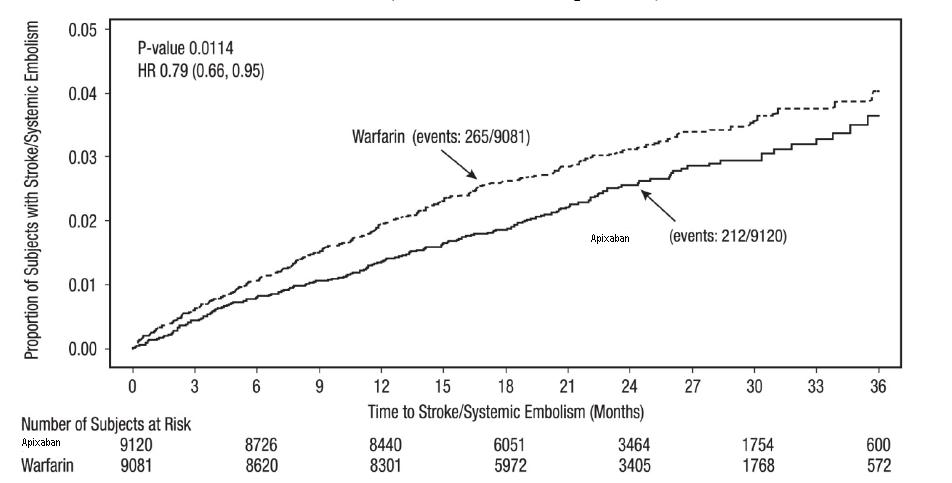
Apixaban was superior to warfarin for the primary endpoint of reducing the risk of stroke and systemic embolism (Table 9 and Figure 4). Superiority to warfarin was primarily attributable to a reduction in hemorrhagic stroke and ischemic strokes with hemorrhagic conversion compared to warfarin. Purely ischemic strokes occurred with similar rates on both drugs.
Apixaban also showed significantly fewer major bleeds than warfarin [
Apixaban N=9120 n (%/year) | Warfarin N=9081 n (%/year) | Hazard Ratio (95% CI) | P-value | |
| Stroke or systemic embolism | 212 (1.27) | 265 (1.60) | 0.79 (0.66, 0.95) | 0.01 |
| Stroke | 199 (1.19) | 250 (1.51) | 0.79 (0.65, 0.95) | |
| Ischemic without hemorrhage | 140 (0.83) | 136 (0.82) | 1.02 (0.81, 1.29) | |
| Ischemic with hemorrhagic conversion | 12 (0.07) | 20 (0.12) | 0.60 (0.29, 1.23) | |
| Hemorrhagic | 40 (0.24) | 78 (0.47) | 0.51 (0.35, 0.75) | |
| Unknown | 14 (0.08) | 21 (0.13) | 0.65 (0.33, 1.29) | |
| Systemic embolism | 15 (0.09) | 17 (0.10) | 0.87 (0.44, 1.75) |
The primary endpoint was based on the time to first event (one per subject). Component counts are for subjects with any event, not necessarily the first.
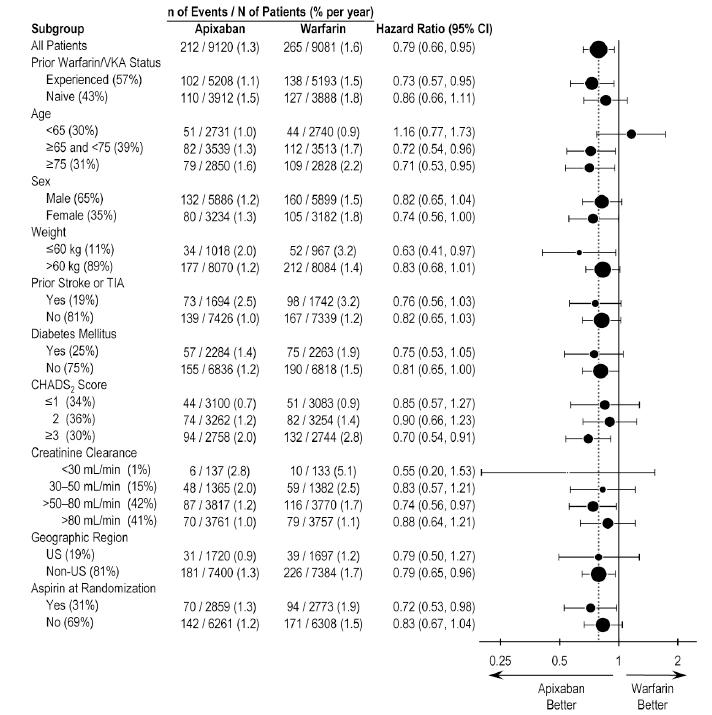
In ARISTOTLE, the results for the primary efficacy endpoint were generally consistent across most major subgroups including weight, CHADS2 score (a scale from 0 to 6 used to predict risk of stroke in patients with AF, with higher scores predicting greater risk), prior warfarin use, level of renal impairment, geographic region, and aspirin use at randomization (Figure 5).
At the end of the ARISTOTLE study, warfarin patients who completed the study were generally maintained on a VKA with no interruption of anticoagulation. Apixaban patients who completed the study were generally switched to a VKA with a 2-day period of coadministration of apixaban and VKA, so that some patients may not have been adequately anticoagulated after stopping apixaban until attaining a stable and therapeutic INR. During the 30 days following the end of the study, there were 21 stroke or systemic embolism events in the 6791 patients (0.3%) in the apixaban arm compared to 5 in the 6569 patients (0.1%) in the warfarin arm
Apixaban N=2807 n (%/year) | Aspirin N=2791 n (%/year) | Hazard Ratio (95% CI) | P-value | |
| Stroke or systemic embolism | 51 (1.62) | 113 (3.63) | 0.45 (0.32, 0.62) | <0.0001 |
| Stroke | ||||
| Ischemic or undetermined | 43 (1.37) | 97 (3.11) | 0.44 (0.31, 0.63) | - |
| Hemorrhagic | 6 (0.19) | 9 (0.28) | 0.67 (0.24, 1.88) | - |
| Systemic embolism | 2 (0.06) | 13 (0.41) | 0.15 (0.03, 0.68) | - |
| MI | 24 (0.76) | 28 (0.89) | 0.86 (0.50, 1.48) | - |
| All-cause death | 111 (3.51) | 140 (4.42) | 0.79 (0.62, 1.02) | 0.068 |
| Vascular death | 84 (2.65) | 96 (3.03) | 0.87 (0.65, 1.17) | - |


(B) SPINAL/EPIDURAL HEMATOMA
Epidural or spinal hematomas may occur in patients treated with apixaban tablets who are receiving neuraxial anesthesia or undergoing spinal puncture. These hematomas may result in long-term or permanent paralysis. Consider these risks when scheduling patients for spinal procedures. Factors that can increase the risk of developing epidural or spinal hematomas in these patients include:
• use of indwelling epidural catheters
• concomitant use of other drugs that affect hemostasis, such as nonsteroidal anti-inflammatory drugs (NSAIDs), platelet inhibitors, other anticoagulants
• a history of traumatic or repeated epidural or spinal punctures
• a history of spinal deformity or spinal surgery
• optimal timing between the administration of apixaban tablets and neuraxial procedures is not known
When neuraxial anesthesia (spinal/epidural anesthesia) or spinal/epidural puncture is employed, patients treated with antithrombotic agents for prevention of thromboembolic complications are at risk of developing an epidural or spinal hematoma which can result in long-term or permanent paralysis.
The risk of these events may be increased by the postoperative use of indwelling epidural catheters or the concomitant use of medicinal products affecting hemostasis. Indwelling epidural or intrathecal catheters should not be removed earlier than 24 hours after the last administration of apixaban tablets. The next dose of apixaban tablets should not be administered earlier than 5 hours after the removal of the catheter. The risk may also be increased by traumatic or repeated epidural or spinal puncture. If traumatic puncture occurs, delay the administration of apixaban tablets for 48 hours.
Monitor patients frequently for signs and symptoms of neurological impairment (e.g., numbness or weakness of the legs, or bowel or bladder dysfunction). If neurological compromise is noted, urgent diagnosis and treatment is necessary. Prior to neuraxial intervention the physician should consider the potential benefit versus the risk in anticoagulated patients or in patients to be anticoagulated for thromboprophylaxis.
Monitor patients frequently for signs and symptoms of neurological impairment. If neurological compromise is noted, urgent treatment is necessary
When neuraxial anesthesia (spinal/epidural anesthesia) or spinal/epidural puncture is employed, patients treated with antithrombotic agents for prevention of thromboembolic complications are at risk of developing an epidural or spinal hematoma which can result in long-term or permanent paralysis.
The risk of these events may be increased by the postoperative use of indwelling epidural catheters or the concomitant use of medicinal products affecting hemostasis. Indwelling epidural or intrathecal catheters should not be removed earlier than 24 hours after the last administration of apixaban tablets. The next dose of apixaban tablets should not be administered earlier than 5 hours after the removal of the catheter. The risk may also be increased by traumatic or repeated epidural or spinal puncture. If traumatic puncture occurs, delay the administration of apixaban tablets for 48 hours.
Monitor patients frequently for signs and symptoms of neurological impairment (e.g., numbness or weakness of the legs, or bowel or bladder dysfunction). If neurological compromise is noted, urgent diagnosis and treatment is necessary. Prior to neuraxial intervention the physician should consider the potential benefit versus the risk in anticoagulated patients or in patients to be anticoagulated for thromboprophylaxis.
When neuraxial anesthesia (spinal/epidural anesthesia) or spinal/epidural puncture is employed, patients treated with antithrombotic agents for prevention of thromboembolic complications are at risk of developing an epidural or spinal hematoma which can result in long-term or permanent paralysis.
The risk of these events may be increased by the postoperative use of indwelling epidural catheters or the concomitant use of medicinal products affecting hemostasis. Indwelling epidural or intrathecal catheters should not be removed earlier than 24 hours after the last administration of apixaban tablets. The next dose of apixaban tablets should not be administered earlier than 5 hours after the removal of the catheter. The risk may also be increased by traumatic or repeated epidural or spinal puncture. If traumatic puncture occurs, delay the administration of apixaban tablets for 48 hours.
Monitor patients frequently for signs and symptoms of neurological impairment (e.g., numbness or weakness of the legs, or bowel or bladder dysfunction). If neurological compromise is noted, urgent diagnosis and treatment is necessary. Prior to neuraxial intervention the physician should consider the potential benefit versus the risk in anticoagulated patients or in patients to be anticoagulated for thromboprophylaxis.
Apixaban tablet is a factor Xa inhibitor indicated:
• to reduce the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation. (
Apixaban tablets are indicated to reduce the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation.
• for the prophylaxis of deep vein thrombosis (DVT), which may lead to pulmonary embolism (PE), in patients who have undergone hip or knee replacement surgery. (
Apixaban tablets are indicated for the prophylaxis of deep vein thrombosis (DVT), which may lead to pulmonary embolism (PE), in patients who have undergone hip or knee replacement surgery.
• for the treatment of DVT and PE, and for the reduction in the risk of recurrent DVT and PE following initial therapy. (
Apixaban tablets are indicated for the treatment of DVT.
Apixaban tablets are indicated for the treatment of PE.
Apixaban tablets are indicated to reduce the risk of recurrent DVT and PE following initial therapy.
• Reduction of risk of stroke and systemic embolism in nonvalvular atrial fibrillation:
• The recommended dose is 5 mg orally twice daily. (
The recommended dose of apixaban tablets is 2.5 mg twice daily in patients with at least two of the following characteristics:
• age greater than or equal to 80 years
• body weight less than or equal to 60 kg
• serum creatinine greater than or equal to 1.5 mg/dL
The recommended dose of apixaban tablets is 2.5 mg taken orally twice daily. The initial dose should be taken 12 to 24 hours after surgery.
• In patients undergoing hip replacement surgery, the recommended duration of treatment is 35 days.
• In patients undergoing knee replacement surgery, the recommended duration of treatment is 12 days.
The recommended dose of apixaban tablets is 10 mg taken orally twice daily for the first 7 days of therapy. After 7 days, the recommended dose is 5 mg taken orally twice daily.
The recommended dose of apixaban tablets is 2.5 mg taken orally twice daily after at least 6 months of treatment for DVT or PE
• In patients with at least 2 of the following characteristics: age greater than or equal to 80 years, body weight less than or equal to 60 kg, or serum creatinine greater than or equal to 1.5 mg/dL, the recommended dose is 2.5 mg orally twice daily. (
The recommended dose of apixaban tablets is 2.5 mg twice daily in patients with at least two of the following characteristics:
• age greater than or equal to 80 years
• body weight less than or equal to 60 kg
• serum creatinine greater than or equal to 1.5 mg/dL
The recommended dose of apixaban tablets is 2.5 mg taken orally twice daily. The initial dose should be taken 12 to 24 hours after surgery.
• In patients undergoing hip replacement surgery, the recommended duration of treatment is 35 days.
• In patients undergoing knee replacement surgery, the recommended duration of treatment is 12 days.
The recommended dose of apixaban tablets is 10 mg taken orally twice daily for the first 7 days of therapy. After 7 days, the recommended dose is 5 mg taken orally twice daily.
The recommended dose of apixaban tablets is 2.5 mg taken orally twice daily after at least 6 months of treatment for DVT or PE
• Prophylaxis of DVT following hip or knee replacement surgery:
• The recommended dose is 2.5 mg orally twice daily. (
The recommended dose of apixaban tablets is 2.5 mg twice daily in patients with at least two of the following characteristics:
• age greater than or equal to 80 years
• body weight less than or equal to 60 kg
• serum creatinine greater than or equal to 1.5 mg/dL
The recommended dose of apixaban tablets is 2.5 mg taken orally twice daily. The initial dose should be taken 12 to 24 hours after surgery.
• In patients undergoing hip replacement surgery, the recommended duration of treatment is 35 days.
• In patients undergoing knee replacement surgery, the recommended duration of treatment is 12 days.
The recommended dose of apixaban tablets is 10 mg taken orally twice daily for the first 7 days of therapy. After 7 days, the recommended dose is 5 mg taken orally twice daily.
The recommended dose of apixaban tablets is 2.5 mg taken orally twice daily after at least 6 months of treatment for DVT or PE
• Treatment of DVT and PE:
• The recommended dose is 10 mg taken orally twice daily for 7 days, followed by 5 mg taken orally twice daily. (
The recommended dose of apixaban tablets is 2.5 mg twice daily in patients with at least two of the following characteristics:
• age greater than or equal to 80 years
• body weight less than or equal to 60 kg
• serum creatinine greater than or equal to 1.5 mg/dL
The recommended dose of apixaban tablets is 2.5 mg taken orally twice daily. The initial dose should be taken 12 to 24 hours after surgery.
• In patients undergoing hip replacement surgery, the recommended duration of treatment is 35 days.
• In patients undergoing knee replacement surgery, the recommended duration of treatment is 12 days.
The recommended dose of apixaban tablets is 10 mg taken orally twice daily for the first 7 days of therapy. After 7 days, the recommended dose is 5 mg taken orally twice daily.
The recommended dose of apixaban tablets is 2.5 mg taken orally twice daily after at least 6 months of treatment for DVT or PE
• Reduction in the risk of recurrent DVT and PE following initial therapy:
• The recommended dose is 2.5 mg taken orally twice daily. (
The recommended dose of apixaban tablets is 2.5 mg twice daily in patients with at least two of the following characteristics:
• age greater than or equal to 80 years
• body weight less than or equal to 60 kg
• serum creatinine greater than or equal to 1.5 mg/dL
The recommended dose of apixaban tablets is 2.5 mg taken orally twice daily. The initial dose should be taken 12 to 24 hours after surgery.
• In patients undergoing hip replacement surgery, the recommended duration of treatment is 35 days.
• In patients undergoing knee replacement surgery, the recommended duration of treatment is 12 days.
The recommended dose of apixaban tablets is 10 mg taken orally twice daily for the first 7 days of therapy. After 7 days, the recommended dose is 5 mg taken orally twice daily.
The recommended dose of apixaban tablets is 2.5 mg taken orally twice daily after at least 6 months of treatment for DVT or PE
• 2.5 mg, yellow, round, biconvex, film-coated tablets with “F51” debossed on one side and plain on the other side.
• 5 mg, pink, capsule shaped, film-coated tablets with “F52” debossed on one side and plain on the other side.
•
The limited available data on apixaban tablets use in pregnant women are insufficient to inform drug-associated risks of major birth defects, miscarriage, or adverse developmental outcomes. Treatment may increase the risk of bleeding during pregnancy and delivery. In animal reproduction studies, no adverse developmental effects were seen when apixaban was administered to rats (orally), rabbits (intravenously) and mice (orally) during organogenesis at unbound apixaban exposure levels up to 4, 1 and 19 times, respectively, the human exposure based on area under plasma-concentration time curve (AUC) at the Maximum Recommended Human Dose (MRHD) of 5 mg twice daily.
The estimated background risk of major birth defects and miscarriage for the indicated populations is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Pregnancy confers an increased risk of thromboembolism that is higher for women with underlying thromboembolic disease and certain high-risk pregnancy conditions. Published data describe that women with a previous history of venous thrombosis are at high risk for recurrence during pregnancy.
Use of anticoagulants, including apixaban, may increase the risk of bleeding in the fetus and neonate.
All patients receiving anticoagulants, including pregnant women, are at risk for bleeding. apixaban tablets use during labor or delivery in women who are receiving neuraxial anesthesia may result in epidural or spinal hematomas. Consider use of a shorter acting anticoagulant as delivery approaches
No developmental toxicities were observed when apixaban was administered during organogenesis to rats (orally), rabbits (intravenously) and mice (orally) at unbound apixaban exposure levels 4, 1, and 19 times, respectively, the human exposures at the MRHD. There was no evidence of fetal bleeding, although conceptus exposure was confirmed in rats and rabbits. Oral administration of apixaban to rat dams from gestation day 6 through lactation day 21 at maternal unbound apixaban exposures ranging from 1.4 to 5 times the human exposures at the MRHD was not associated with reduced maternal mortality or reduced conceptus/neonatal viability, although increased incidences of peri-vaginal bleeding were observed in dams at all doses. There was no evidence of neonatal bleeding.
•
There are no data on the presence of apixaban or its metabolites in human milk, the effects on the breastfed child, or the effects on milk production. Apixaban and/or its metabolites were present in the milk of rats (see Data). Because human exposure through milk is unknown, breastfeeding is not recommended during treatment with apixaban tablets.
Maximal plasma concentrations were observed after 30 minutes following a single oral administration of a 5 mg dose to lactating rats. Maximal milk concentrations were observed 6 hours after dosing. The milk to plasma AUC (0-24) ratio is 30:1 indicating that apixaban can accumulate in milk. The concentrations of apixaban in animal milk does not necessarily predict the concentration of drug in human milk.
•
No dose adjustment is required in patients with mild hepatic impairment (Child-Pugh class A).
Because patients with moderate hepatic impairment (Child-Pugh class B) may have intrinsic coagulation abnormalities and there is limited clinical experience with apixaban tablets in these patients, dosing recommendations cannot be provided
As a result of FXa inhibition, apixaban prolongs clotting tests such as prothrombin time (PT), INR, and activated partial thromboplastin time (aPTT). Changes observed in these clotting tests at the expected therapeutic dose, however, are small, subject to a high degree of variability, and not useful in monitoring the anticoagulation effect of apixaban.
The Rotachrom®Heparin chromogenic assay was used to measure the effect of apixaban on FXa activity in humans during the apixaban development program. A concentration-dependent increase in anti-FXa activity was observed in the dose range tested and was similar in healthy subjects and patients with AF.
This test is not recommended for assessing the anticoagulant effect of apixaban.
There is no clinical experience to reverse bleeding with the use of 4-factor PCC products in individuals who have received apixaban tablets.
Effects of 4-factor PCCs on the pharmacodynamics of apixaban were studied in healthy subjects. Following administration of apixaban dosed to steady state, endogenous thrombin potential (ETP) returned to pre-apixaban levels 4 hours after the initiation of a 30-minute PCC infusion, compared to 45 hours with placebo. Mean ETP levels continued to increase and exceeded pre-apixaban levels reaching a maximum (34%-51% increase over pre-apixaban levels) at 21 hours after initiating PCC and remained elevated (21%-27% increase) at the end of the study (69 hours after initiation of PCC). The clinical relevance of this increase in ETP is unknown.
Pharmacodynamic drug interaction studies with aspirin, clopidogrel, aspirin and clopidogrel, prasugrel, enoxaparin, and naproxen were conducted. No pharmacodynamic interactions were observed with aspirin, clopidogrel, or prasugrel
Apixaban has no effect on the QTc interval in humans at doses up to 50 mg.
Apixaban tablets are contraindicated in patients with the following conditions:
• Active pathological bleeding
Apixaban tablets increases the risk of bleeding and can cause serious, potentially fatal, bleeding
Concomitant use of drugs affecting hemostasis increases the risk of bleeding. These include aspirin and other antiplatelet agents, other anticoagulants, heparin, thrombolytic agents, selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors, and nonsteroidal anti-inflammatory drugs (NSAIDs)
Hemodialysis does not appear to have a substantial impact on apixaban exposure
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of apixaban was evaluated in the ARISTOTLE and AVERROES studies
The most common reason for treatment discontinuation in both studies was for bleeding-related adverse reactions; in ARISTOTLE this occurred in 1.7% and 2.5% of patients treated with apixaban and warfarin, respectively, and in AVERROES, in 1.5% and 1.3% on apixaban and aspirin, respectively.
Bleeding in Patients with Nonvalvular Atrial Fibrillation in ARISTOTLE and AVERROES
Tables 1 and 2 show the number of patients experiencing major bleeding during the treatment period and the bleeding rate (percentage of subjects with at least one bleeding event per 100 patient-years) in ARISTOTLE and AVERROES.
Apixaban N=9088 n (per 100 pt-year) | Warfarin N=9052 n (per 100 pt-year) | Hazard Ratio (95% CI) | P-value | |
| Major† | 327 (2.13) | 462 (3.09) | 0.69 (0.60, 0.80) | <0.0001 |
| Intracranial (ICH)‡ | 52 (0.33) | 125 (0.82) | 0.41 (0.30, 0.57) | - |
| Hemorrhagic stroke§ | 38 (0.24) | 74 (0.49) | 0.51 (0.34, 0.75) | - |
| Other ICH | 15 (0.10) | 51 (0.34) | 0.29 (0.16, 0.51) | - |
| Gastrointestinal (GI)¶ | 128 (0.83) | 141 (0.93) | 0.89 (0.70, 1.14) | - |
| Fatal** | 10 (0.06) | 37 (0.24) | 0.27 (0.13, 0.53) | - |
| Intracranial | 4 (0.03) | 30 (0.20) | 0.13 (0.05, 0.37) | - |
| Non-intracranial | 6 (0.04) | 7 (0.05) | 0.84 (0.28, 2.15) | - |
* Bleeding events within each subcategory were counted once per subject, but subjects may have contributed events to multiple endpoints. Bleeding events were counted during treatment or within 2 days of stopping study treatment (on-treatment period).
† Defined as clinically overt bleeding accompanied by one or more of the following: a decrease in hemoglobin of ≥2 g/dL, a transfusion of 2 or more units of packed red blood cells, bleeding at a critical site: intracranial, intraspinal, intraocular, pericardial, intra-articular, intramuscular with compartment syndrome, retroperitoneal or with fatal outcome.
‡ Intracranial bleed includes intracerebral, intraventricular, subdural, and subarachnoid bleeding. Any type of hemorrhagic stroke was adjudicated and counted as an intracranial major bleed.
§On-treatment analysis based on the safety population, compared to ITT analysis presented in Section 14.
¶GI bleed includes upper GI, lower GI, and rectal bleeding.
** Fatal bleeding is an adjudicated death with the primary cause of death as intracranial bleeding or non-intracranial bleeding during the on-treatment period.
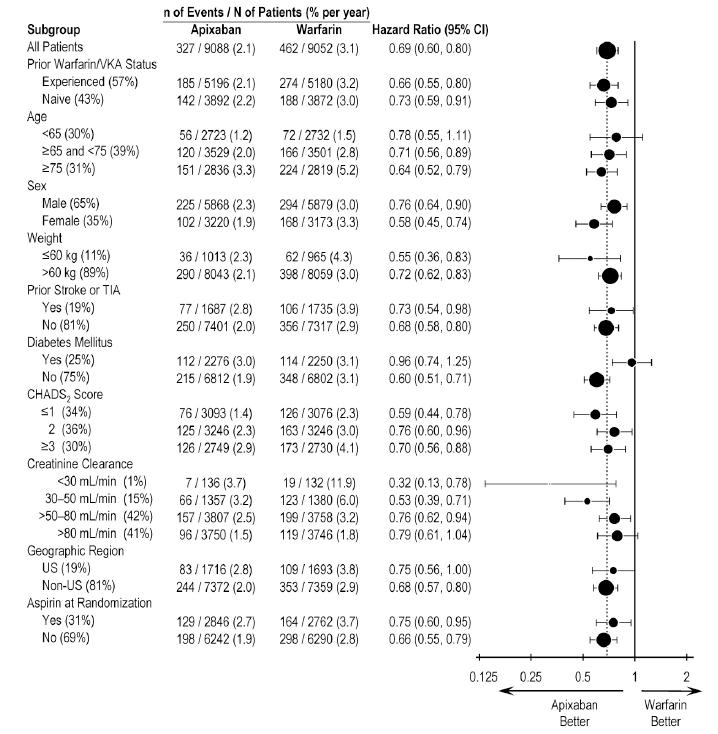
In ARISTOTLE, the results for major bleeding were generally consistent across most major subgroups including age, weight, CHADS2score (a scale from 0 to 6 used to estimate risk of stroke, with higher scores predicting greater risk), prior warfarin use, geographic region, and aspirin use at randomization (Figure 1). Subjects treated with apixaban with diabetes bled more (3% per year) than did subjects without diabetes (1.9% per year).
Apixaban N=2798 n (%year) | Aspirin N=2780 n (%/year) | Hazard Ratio (95% CI) | P-value | |
| Major | 45 (1.41) | 29 (0.92) | 1.54 (0.96, 2.45) | 0.07 |
| Fatal | 5 (0.16) | 5 (0.16) | 0.99 (0.23, 4.29) | - |
| Intracranial | 11 (0.34) | 11 (0.35) | 0.99 (0.39, 2.51) | - |
Events associated with each endpoint were counted once per subject, but subjects may have contributed events to multiple endpoints
Other Adverse Reactions
Hypersensitivity reactions (including drug hypersensitivity, such as skin rash, and anaphylactic reactions, such as allergic edema) and syncope were reported in <1% of patients receiving apixaban.
The safety of apixaban has been evaluated in 1 Phase II and 3 Phase III studies including 5924 patients exposed to apixaban tablets 2.5 mg twice daily undergoing major orthopedic surgery of the lower limbs (elective hip replacement or elective knee replacement) treated for up to 38 days.
In total, 11% of the patients treated with apixaban tablets 2.5 mg twice daily experienced adverse reactions.
Bleeding results during the treatment period in the Phase III studies are shown in Table 3. Bleeding was assessed in each study beginning with the first dose of double-blind study drug.
Bleeding Endpoint* | ADVANCE-3 Hip Replacement Surgery | ADVANCE-2 Knee Replacement Surgery | ADVANCE-1 Knee Replacement Surgery | |||
| Apixaban tablets 2.5 mg po bid 35±3 days | Enoxaparin 40 mg sc qd 35±3 days | Apixaban tablets 2.5 mg po bid 12±2 days | Enoxaparin 40 mg sc qd 12±2 days | Apixaban tablets 2.5 mg po bid 12±2 days | Enoxaparin 30 mg sc q12h 12±2 days | |
| First dose 12 to 24 hours post surgery | First dose 9 to 15 hours prior to surgery | First dose 12 to 24 hours post surgery | First dose 9 to 15 hours prior to surgery | First dose 12 to 24 hours post surgery | First dose 12 to 24 hours post surgery | |
| All treated | N=2673 | N=2659 | N=1501 | N=1508 | N=1596 | N=1588 |
| Major (including surgical site) | 22 (0.82%)† | 18 (0.68%) | 9 (0.60%)‡ | 14 (0.93%) | 11 (0.69%) | 22 (1.39%) |
| Fatal | 0 | 0 | 0 | 0 | 0 | 1 (0.06%) |
| Hgb decrease ≥2 g/dL | 13 (0.49%) | 10 (0.38%) | 8 (0.53%) | 9 (0.60%) | 10 (0.63%) | 16 (1.01%) |
| Transfusion of ≥2 units RBC | 16 (0.60%) | 14 (0.53%) | 5 (0.33%) | 9 (0.60%) | 9 (0.56%) | 18 (1.13%) |
| Bleed at critical site§ | 1 (0.04%) | 1 (0.04%) | 1 (0.07%) | 2 (0.13%) | 1 (0.06%) | 4 (0.25%) |
| Major + CRNM¶ | 129 (4.83%) | 134 (5.04%) | 53 (3.53%) | 72 (4.77%) | 46 (2.88%) | 68 (4.28%) |
| All | 313 (11.71%) | 334 (12.56%) | 104 (6.93%) | 126 (8.36%) | 85 (5.33%) | 108 (6.80%) |
* All bleeding criteria included surgical site bleeding.
† Includes 13 subjects with major bleeding events that occurred before the first dose of apixaban (administered 12 to 24 hours post-surgery).
‡Includes 5 subjects with major bleeding events that occurred before the first dose of apixaban (administered 12 to 24 hours post-surgery).
§Intracranial, intraspinal, intraocular, pericardial, an operated joint requiring re-operation or intervention, intramuscular with compartment syndrome, or retroperitoneal. Bleeding into an operated joint requiring re-operation or intervention was present in all patients with this category of bleeding. Events and event rates include one enoxaparin-treated patient in ADVANCE-1 who also had intracranial hemorrhage.
¶CRNM = clinically relevant nonmajor.
Adverse reactions occurring in ≥1% of patients undergoing hip or knee replacement surgery in the 1 Phase II study and the 3 Phase III studies are listed in Table 4.
Surgery
Apixaban tablets, n (%) 2.5 mg po bid N=5924 | Enoxaparin, n (%) 40 mg sc qd or 30 mg sc q12h N=5904 | |
| Nausea | 153 (2.6) | 159 (2.7) |
| Anemia (including postoperative and hemorrhagic anemia, and respective laboratory parameters) | 153 (2.6) | 178 (3.0) |
| Contusion | 83 (1.4) | 115 (1.9) |
| Hemorrhage (including hematoma, and vaginal and urethral hemorrhage) | 67 (1.1) | 81 (1.4) |
| Postprocedural hemorrhage (including postprocedural hematoma, wound hemorrhage, vessel puncture-site hematoma, and catheter-site hemorrhage) | 54 (0.9) | 60 (1.0) |
| Transaminases increased (including alanine aminotransferase increased and alanine aminotransferase abnormal) | 50 (0.8) | 71 (1.2) |
| Aspartate aminotransferase increased | 47 (0.8) | 69 (1.2) |
| Gamma-glutamyltransferase increased | 38 (0.6) | 65 (1.1) |
Less common adverse reactions in apixaban-treated patients undergoing hip or knee replacement surgery occurring at a frequency of ≥0.1% to <1%:
Blood and lymphatic system disorders: thrombocytopenia (including platelet count decreases)
Less common adverse reactions in apixaban-treated patients undergoing hip or knee replacement surgery occurring at a frequency of <0.1%:
Gingival bleeding, hemoptysis, hypersensitivity, muscle hemorrhage, ocular hemorrhage (including conjunctival hemorrhage), rectal hemorrhage
The safety of apixaban has been evaluated in the AMPLIFY and AMPLIFY-EXT studies, including 2676 patients exposed to apixaban tablets 10 mg twice daily, 3359 patients exposed to apixaban tablets 5 mg twice daily, and 840 patients exposed to apixaban tablets 2.5 mg twice daily.
Common adverse reactions (≥1%) were gingival bleeding, epistaxis, contusion, hematuria, rectal hemorrhage, hematoma, menorrhagia, and hemoptysis.
AMPLIFY Study
The mean duration of exposure to apixaban was 154 days and to enoxaparin/warfarin was 152 days in the AMPLIFY study. Adverse reactions related to bleeding occurred in 417 (15.6%) apixaban-treated patients compared to 661 (24.6%) enoxaparin/warfarin-treated patients. The discontinuation rate due to bleeding events was 0.7% in the apixaban-treated patients compared to 1.7% in enoxaparin/warfarin-treated patients in the AMPLIFY study.
In the AMPLIFY study, apixaban was statistically superior to enoxaparin/warfarin in the primary safety endpoint of major bleeding (relative risk 0.31, 95% CI [0.17, 0.55], P-value <0.0001).
Bleeding results from the AMPLIFY study are summarized in Table 5.
Apixaban N=2676 n (%) | Enoxaparin/Warfarin N=2689 n (%) | Relative Risk (95% CI) | |
| Major | 15 (0.6) | 49 (1.8) | 0.31 (0.17, 0.55) p<0.0001 |
| CRNM* | 103 (3.9) | 215 (8.0) | |
| Major + CRNM | 115 (4.3) | 261 (9.7) | |
| Minor | 313 (11.7) | 505 (18.8) | |
| All | 402 (15.0) | 676 (25.1) |
* CRNM = clinically relevant nonmajor bleeding
Events associated with each endpoint were counted once per subject, but subjects may have contributed events to multiple endpoints
Adverse reactions occurring in ≥1% of patients in the AMPLIFY study are listed in Table 6.
Apixaban N=2676 n (%) | Enoxaparin/Warfarin N=2689 n (%) | |
| Epistaxis | 77 (2.9) | 146 (5.4) |
| Contusion | 49 (1.8) | 97 (3.6) |
| Hematuria | 46 (1.7) | 102 (3.8) |
| Menorrhagia | 38 (1.4) | 30 (1.1) |
| Hematoma | 35 (1.3) | 76 (2.8) |
| Hemoptysis | 32 (1.2) | 31 (1.2) |
| Rectal hemorrhage | 26 (1.0) | 39 (1.5) |
| Gingival bleeding | 26 (1.0) | 50 (1.9) |
AMPLIFY-EXT Study
The mean duration of exposure to apixaban was approximately 330 days and to placebo was 312 days in the AMPLIFY-EXT study. Adverse reactions related to bleeding occurred in 219 (13.3%) apixaban-treated patients compared to 72 (8.7%) placebo-treated patients. The discontinuation rate due to bleeding events was approximately 1% in the apixaban-treated patients compared to 0.4% in those patients in the placebo group in the AMPLIFY-EXT study.
Bleeding results from the AMPLIFY-EXT study are summarized in Table 7.
Apixaban tablets 2.5 mg bid N=840 n (%) | Apixaban tablets 5 mg bid N=811 n (%) | Placebo N=826 n (%) | |
| Major | 2 (0.2) | 1 (0.1) | 4 (0.5) |
| CRNM* | 25 (3.0) | 34 (4.2) | 19 (2.3) |
| Major + CRNM | 27 (3.2) | 35 (4.3) | 22 (2.7) |
| Minor | 75 (8.9) | 98 (12.1) | 58 (7.0) |
| All | 94 (11.2) | 121 (14.9) | 74 (9.0) |
* CRNM = clinically relevant nonmajor bleeding.
Events associated with each endpoint were counted once per subject, but subjects may have contributed events to multiple endpoints.
Adverse reactions occurring in ≥1% of patients in the AMPLIFY-EXT study are listed in Table 8.
Apixaban tablets 2.5 mg bid N=840 n (%) | Apixaban tablets 5 mg bid N=811 n (%) | Placebo N=826 n (%) | |
| Epistaxis | 13 (1.5) | 29 (3.6) | 9 (1.1) |
| Hematuria | 12 (1.4) | 17 (2.1) | 9 (1.1) |
| Hematoma | 13 (1.5) | 16 (2.0) | 10 (1.2) |
| Contusion | 18 (2.1) | 18 (2.2) | 18 (2.2) |
| Gingival bleeding | 12 (1.4) | 9 (1.1) | 3 (0.4) |
Other Adverse Reactions
Less common adverse reactions in apixaban-treated patients in the AMPLIFY or AMPLIFY-EXT studies occurring at a frequency of ≥0.1% to <1%:

• Severe hypersensitivity reaction to apixaban tablets (e.g., anaphylactic reactions)
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of apixaban was evaluated in the ARISTOTLE and AVERROES studies
The most common reason for treatment discontinuation in both studies was for bleeding-related adverse reactions; in ARISTOTLE this occurred in 1.7% and 2.5% of patients treated with apixaban and warfarin, respectively, and in AVERROES, in 1.5% and 1.3% on apixaban and aspirin, respectively.
Bleeding in Patients with Nonvalvular Atrial Fibrillation in ARISTOTLE and AVERROES
Tables 1 and 2 show the number of patients experiencing major bleeding during the treatment period and the bleeding rate (percentage of subjects with at least one bleeding event per 100 patient-years) in ARISTOTLE and AVERROES.
Apixaban N=9088 n (per 100 pt-year) | Warfarin N=9052 n (per 100 pt-year) | Hazard Ratio (95% CI) | P-value | |
| Major† | 327 (2.13) | 462 (3.09) | 0.69 (0.60, 0.80) | <0.0001 |
| Intracranial (ICH)‡ | 52 (0.33) | 125 (0.82) | 0.41 (0.30, 0.57) | - |
| Hemorrhagic stroke§ | 38 (0.24) | 74 (0.49) | 0.51 (0.34, 0.75) | - |
| Other ICH | 15 (0.10) | 51 (0.34) | 0.29 (0.16, 0.51) | - |
| Gastrointestinal (GI)¶ | 128 (0.83) | 141 (0.93) | 0.89 (0.70, 1.14) | - |
| Fatal** | 10 (0.06) | 37 (0.24) | 0.27 (0.13, 0.53) | - |
| Intracranial | 4 (0.03) | 30 (0.20) | 0.13 (0.05, 0.37) | - |
| Non-intracranial | 6 (0.04) | 7 (0.05) | 0.84 (0.28, 2.15) | - |
* Bleeding events within each subcategory were counted once per subject, but subjects may have contributed events to multiple endpoints. Bleeding events were counted during treatment or within 2 days of stopping study treatment (on-treatment period).
† Defined as clinically overt bleeding accompanied by one or more of the following: a decrease in hemoglobin of ≥2 g/dL, a transfusion of 2 or more units of packed red blood cells, bleeding at a critical site: intracranial, intraspinal, intraocular, pericardial, intra-articular, intramuscular with compartment syndrome, retroperitoneal or with fatal outcome.
‡ Intracranial bleed includes intracerebral, intraventricular, subdural, and subarachnoid bleeding. Any type of hemorrhagic stroke was adjudicated and counted as an intracranial major bleed.
§On-treatment analysis based on the safety population, compared to ITT analysis presented in Section 14.
¶GI bleed includes upper GI, lower GI, and rectal bleeding.
** Fatal bleeding is an adjudicated death with the primary cause of death as intracranial bleeding or non-intracranial bleeding during the on-treatment period.
In ARISTOTLE, the results for major bleeding were generally consistent across most major subgroups including age, weight, CHADS2score (a scale from 0 to 6 used to estimate risk of stroke, with higher scores predicting greater risk), prior warfarin use, geographic region, and aspirin use at randomization (Figure 1). Subjects treated with apixaban with diabetes bled more (3% per year) than did subjects without diabetes (1.9% per year).

Apixaban N=2798 n (%year) | Aspirin N=2780 n (%/year) | Hazard Ratio (95% CI) | P-value | |
| Major | 45 (1.41) | 29 (0.92) | 1.54 (0.96, 2.45) | 0.07 |
| Fatal | 5 (0.16) | 5 (0.16) | 0.99 (0.23, 4.29) | - |
| Intracranial | 11 (0.34) | 11 (0.35) | 0.99 (0.39, 2.51) | - |
Events associated with each endpoint were counted once per subject, but subjects may have contributed events to multiple endpoints
Other Adverse Reactions
Hypersensitivity reactions (including drug hypersensitivity, such as skin rash, and anaphylactic reactions, such as allergic edema) and syncope were reported in <1% of patients receiving apixaban.
The safety of apixaban has been evaluated in 1 Phase II and 3 Phase III studies including 5924 patients exposed to apixaban tablets 2.5 mg twice daily undergoing major orthopedic surgery of the lower limbs (elective hip replacement or elective knee replacement) treated for up to 38 days.
In total, 11% of the patients treated with apixaban tablets 2.5 mg twice daily experienced adverse reactions.
Bleeding results during the treatment period in the Phase III studies are shown in Table 3. Bleeding was assessed in each study beginning with the first dose of double-blind study drug.
Bleeding Endpoint* | ADVANCE-3 Hip Replacement Surgery | ADVANCE-2 Knee Replacement Surgery | ADVANCE-1 Knee Replacement Surgery | |||
| Apixaban tablets 2.5 mg po bid 35±3 days | Enoxaparin 40 mg sc qd 35±3 days | Apixaban tablets 2.5 mg po bid 12±2 days | Enoxaparin 40 mg sc qd 12±2 days | Apixaban tablets 2.5 mg po bid 12±2 days | Enoxaparin 30 mg sc q12h 12±2 days | |
| First dose 12 to 24 hours post surgery | First dose 9 to 15 hours prior to surgery | First dose 12 to 24 hours post surgery | First dose 9 to 15 hours prior to surgery | First dose 12 to 24 hours post surgery | First dose 12 to 24 hours post surgery | |
| All treated | N=2673 | N=2659 | N=1501 | N=1508 | N=1596 | N=1588 |
| Major (including surgical site) | 22 (0.82%)† | 18 (0.68%) | 9 (0.60%)‡ | 14 (0.93%) | 11 (0.69%) | 22 (1.39%) |
| Fatal | 0 | 0 | 0 | 0 | 0 | 1 (0.06%) |
| Hgb decrease ≥2 g/dL | 13 (0.49%) | 10 (0.38%) | 8 (0.53%) | 9 (0.60%) | 10 (0.63%) | 16 (1.01%) |
| Transfusion of ≥2 units RBC | 16 (0.60%) | 14 (0.53%) | 5 (0.33%) | 9 (0.60%) | 9 (0.56%) | 18 (1.13%) |
| Bleed at critical site§ | 1 (0.04%) | 1 (0.04%) | 1 (0.07%) | 2 (0.13%) | 1 (0.06%) | 4 (0.25%) |
| Major + CRNM¶ | 129 (4.83%) | 134 (5.04%) | 53 (3.53%) | 72 (4.77%) | 46 (2.88%) | 68 (4.28%) |
| All | 313 (11.71%) | 334 (12.56%) | 104 (6.93%) | 126 (8.36%) | 85 (5.33%) | 108 (6.80%) |
* All bleeding criteria included surgical site bleeding.
† Includes 13 subjects with major bleeding events that occurred before the first dose of apixaban (administered 12 to 24 hours post-surgery).
‡Includes 5 subjects with major bleeding events that occurred before the first dose of apixaban (administered 12 to 24 hours post-surgery).
§Intracranial, intraspinal, intraocular, pericardial, an operated joint requiring re-operation or intervention, intramuscular with compartment syndrome, or retroperitoneal. Bleeding into an operated joint requiring re-operation or intervention was present in all patients with this category of bleeding. Events and event rates include one enoxaparin-treated patient in ADVANCE-1 who also had intracranial hemorrhage.
¶CRNM = clinically relevant nonmajor.
Adverse reactions occurring in ≥1% of patients undergoing hip or knee replacement surgery in the 1 Phase II study and the 3 Phase III studies are listed in Table 4.
Surgery
Apixaban tablets, n (%) 2.5 mg po bid N=5924 | Enoxaparin, n (%) 40 mg sc qd or 30 mg sc q12h N=5904 | |
| Nausea | 153 (2.6) | 159 (2.7) |
| Anemia (including postoperative and hemorrhagic anemia, and respective laboratory parameters) | 153 (2.6) | 178 (3.0) |
| Contusion | 83 (1.4) | 115 (1.9) |
| Hemorrhage (including hematoma, and vaginal and urethral hemorrhage) | 67 (1.1) | 81 (1.4) |
| Postprocedural hemorrhage (including postprocedural hematoma, wound hemorrhage, vessel puncture-site hematoma, and catheter-site hemorrhage) | 54 (0.9) | 60 (1.0) |
| Transaminases increased (including alanine aminotransferase increased and alanine aminotransferase abnormal) | 50 (0.8) | 71 (1.2) |
| Aspartate aminotransferase increased | 47 (0.8) | 69 (1.2) |
| Gamma-glutamyltransferase increased | 38 (0.6) | 65 (1.1) |
Less common adverse reactions in apixaban-treated patients undergoing hip or knee replacement surgery occurring at a frequency of ≥0.1% to <1%:
Blood and lymphatic system disorders: thrombocytopenia (including platelet count decreases)
Less common adverse reactions in apixaban-treated patients undergoing hip or knee replacement surgery occurring at a frequency of <0.1%:
Gingival bleeding, hemoptysis, hypersensitivity, muscle hemorrhage, ocular hemorrhage (including conjunctival hemorrhage), rectal hemorrhage
The safety of apixaban has been evaluated in the AMPLIFY and AMPLIFY-EXT studies, including 2676 patients exposed to apixaban tablets 10 mg twice daily, 3359 patients exposed to apixaban tablets 5 mg twice daily, and 840 patients exposed to apixaban tablets 2.5 mg twice daily.
Common adverse reactions (≥1%) were gingival bleeding, epistaxis, contusion, hematuria, rectal hemorrhage, hematoma, menorrhagia, and hemoptysis.
AMPLIFY Study
The mean duration of exposure to apixaban was 154 days and to enoxaparin/warfarin was 152 days in the AMPLIFY study. Adverse reactions related to bleeding occurred in 417 (15.6%) apixaban-treated patients compared to 661 (24.6%) enoxaparin/warfarin-treated patients. The discontinuation rate due to bleeding events was 0.7% in the apixaban-treated patients compared to 1.7% in enoxaparin/warfarin-treated patients in the AMPLIFY study.
In the AMPLIFY study, apixaban was statistically superior to enoxaparin/warfarin in the primary safety endpoint of major bleeding (relative risk 0.31, 95% CI [0.17, 0.55], P-value <0.0001).
Bleeding results from the AMPLIFY study are summarized in Table 5.
Apixaban N=2676 n (%) | Enoxaparin/Warfarin N=2689 n (%) | Relative Risk (95% CI) | |
| Major | 15 (0.6) | 49 (1.8) | 0.31 (0.17, 0.55) p<0.0001 |
| CRNM* | 103 (3.9) | 215 (8.0) | |
| Major + CRNM | 115 (4.3) | 261 (9.7) | |
| Minor | 313 (11.7) | 505 (18.8) | |
| All | 402 (15.0) | 676 (25.1) |
* CRNM = clinically relevant nonmajor bleeding
Events associated with each endpoint were counted once per subject, but subjects may have contributed events to multiple endpoints
Adverse reactions occurring in ≥1% of patients in the AMPLIFY study are listed in Table 6.
Apixaban N=2676 n (%) | Enoxaparin/Warfarin N=2689 n (%) | |
| Epistaxis | 77 (2.9) | 146 (5.4) |
| Contusion | 49 (1.8) | 97 (3.6) |
| Hematuria | 46 (1.7) | 102 (3.8) |
| Menorrhagia | 38 (1.4) | 30 (1.1) |
| Hematoma | 35 (1.3) | 76 (2.8) |
| Hemoptysis | 32 (1.2) | 31 (1.2) |
| Rectal hemorrhage | 26 (1.0) | 39 (1.5) |
| Gingival bleeding | 26 (1.0) | 50 (1.9) |
AMPLIFY-EXT Study
The mean duration of exposure to apixaban was approximately 330 days and to placebo was 312 days in the AMPLIFY-EXT study. Adverse reactions related to bleeding occurred in 219 (13.3%) apixaban-treated patients compared to 72 (8.7%) placebo-treated patients. The discontinuation rate due to bleeding events was approximately 1% in the apixaban-treated patients compared to 0.4% in those patients in the placebo group in the AMPLIFY-EXT study.
Bleeding results from the AMPLIFY-EXT study are summarized in Table 7.
Apixaban tablets 2.5 mg bid N=840 n (%) | Apixaban tablets 5 mg bid N=811 n (%) | Placebo N=826 n (%) | |
| Major | 2 (0.2) | 1 (0.1) | 4 (0.5) |
| CRNM* | 25 (3.0) | 34 (4.2) | 19 (2.3) |
| Major + CRNM | 27 (3.2) | 35 (4.3) | 22 (2.7) |
| Minor | 75 (8.9) | 98 (12.1) | 58 (7.0) |
| All | 94 (11.2) | 121 (14.9) | 74 (9.0) |
* CRNM = clinically relevant nonmajor bleeding.
Events associated with each endpoint were counted once per subject, but subjects may have contributed events to multiple endpoints.
Adverse reactions occurring in ≥1% of patients in the AMPLIFY-EXT study are listed in Table 8.
Apixaban tablets 2.5 mg bid N=840 n (%) | Apixaban tablets 5 mg bid N=811 n (%) | Placebo N=826 n (%) | |
| Epistaxis | 13 (1.5) | 29 (3.6) | 9 (1.1) |
| Hematuria | 12 (1.4) | 17 (2.1) | 9 (1.1) |
| Hematoma | 13 (1.5) | 16 (2.0) | 10 (1.2) |
| Contusion | 18 (2.1) | 18 (2.2) | 18 (2.2) |
| Gingival bleeding | 12 (1.4) | 9 (1.1) | 3 (0.4) |
Other Adverse Reactions
Less common adverse reactions in apixaban-treated patients in the AMPLIFY or AMPLIFY-EXT studies occurring at a frequency of ≥0.1% to <1%:
