Apremilast
Apremilast Prescribing Information
Apremilast tablets are available as oval shaped, film coated tablets in the following dosage strengths:
- Apremilast Tablets, 10 mgare supplied as pink colored, oval shaped, film-coated tablets, debossed with “C12” on one side and plain on other side.
- Apremilast Tablets, 20 mgare supplied as brown colored, oval shaped, film-coated tablet, debossed with "C13” on one side and plain on other side.
- Apremilast Tablets, 30 mgare supplied as beige colored, oval shaped, film-coated tablets, debossed with “C14" on one side and plain on other side.
Apremilast tablets are contraindicated in patients with a known hypersensitivity to apremilast or to any of the excipients in the formulation
The following adverse reactions are described elsewhere in the labeling:
- Hypersensitivity[see]
5.1 HypersensitivityHypersensitivity reactions, including cases of angioedema and anaphylaxis, have been reported during post marketing surveillance. Avoid the use of apremilast in patients with known hypersensitivity to apremilast or to any of the excipients in the formulation. If signs or symptoms of serious hypersensitivity reactions develop during treatment, discontinue apremilast and institute appropriate therapy.
- Diarrhea, Nausea, and Vomiting [see]
5.2 Diarrhea, Nausea, and VomitingThere have been reports of severe diarrhea, nausea, and vomiting associated with the use of apremilast. Most events occurred within the first few weeks of treatment. In some cases, patients were hospitalized. Patients 65 years of age or older and patients taking medications that can lead to volume depletion or hypotension may be at a higher risk of complications from severe diarrhea, nausea, or vomiting. Monitor patients who are more susceptible to complications of diarrhea or vomiting. Patients who reduced dosage or discontinued apremilast generally improved quickly. Consider apremilast dose reduction or suspension if patients develop severe diarrhea, nausea, or vomiting.
- Depression [see)]
5.3 DepressionTreatment with apremilast is associated with an increase incidence of depression. Before using apremilast in patients with a history of depression and/or suicidal thoughts or behavior, carefully weigh the risks and benefits of treatment with apremilast. Advise patients, their caregivers, and families of the need to be alert for the emergence or worsening of depression, suicidal thoughts or other mood changes, and if such changes occur to contact their healthcare provider. Carefully evaluate the risks and benefits of continuing treatment with apremilast if such events occur.
Plaque Psoriasis: During the 16-week placebo-controlled period of the 3 controlled clinical trials in adult subjects with moderate to severe plaque psoriasis, 1.3% (12/920) of subjects treated with apremilast reported depression compared to 0.4% (2/506) treated with placebo. During the clinical trials, 0.1% (1/1,308) of subjects treated with apremilast discontinued treatment due to depression compared with none in placebo-treated subjects (0/506). Depression was reported as serious in 0.1% (1/1,308) of subjects exposed to apremilast, compared to none in placebo-treated subjects (0/506). Instances of suicidal behavior have been observed in 0.1% (1/1,308) of subjects while receiving apremilast, compared to 0.2% (1/506) in placebo-treated subjects. In the clinical trials, one subject treated with apremilast attempted suicide while one who received placebo committed suicide.During the 16-week placebo-controlled period of the clinical trial in adults with mild to moderate plaque psoriasis, the incidence of subjects reporting depression was similar to what was observed in the adult moderate to severe plaque psoriasis trials.
- Weight Decrease [see]
5.4 Weight DecreaseWeight loss may occur in adult or pediatric patients treated with apremilast.
Regularly monitor the weight of patients treated with apremilast. If unexplained or clinically significant weight loss occurs, evaluate weight loss and consider discontinuation of apremilast
[see Adverse Reactions (6.1)].Weight Loss in Adult PatientsDuring the placebo-controlled period of the trials in adults with moderate to severe plaque psoriasis, weight decrease between 5% to 10% of body weight occurred in 12% (96/784) of subjects treated with apremilast compared to 5% (19/382) treated with placebo. Weight decrease of ≥ 10% of body weight occurred in 2% (16/784) of subjects treated with apremilast 30 mg twice daily compared to 1% (3/382) subjects treated with placebo.
During the placebo-controlled period of the clinical trial in adults with mild to moderate plaque psoriasis, weight decrease was similar to what was observed in the trials of adults with moderate to severe plaque psoriasis.
Pediatric use information is approved for Amgen Inc.'s Otezla (apremilast) Tablets. However, due to Amgen Inc.'s marketing exclusivity rights, this drug product is not labeled with that information. - Drug Interactions [see]
5.5 Drug InteractionsCo-administration of strong cytochrome P450 enzyme inducer, rifampin, resulted in a reduction of systemic exposure of apremilast, which may result in a loss of efficacy of apremilast. Therefore, the use of cytochrome P450 enzyme inducers (e.g., rifampin, phenobarbital, carbamazepine, phenytoin) with apremilast is not recommended
[seeDrug Interactions (7.1)andClinical Pharmacology (12.3)].
The active ingredient in apremilast tablets is apremilast. Apremilast is a phosphodiesterase 4 (PDE4) inhibitor. Apremilast is known chemically as N-[2-[(1S)-1-(3-ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)ethyl]-2,3-dihydro-1,3-dioxo-1H-isoindol-4-yl]acetamide. Its molecular formula is C22H24N2O7S and the molecular weight is 460.50 g/mol.
The chemical structure is:

Apremilast is a white to pale yellow powder. It is soluble in acetone and practically insoluble in water.
Apremilast tablets are supplied in 10 mg, 20 mg, and 30 mg strengths for oral administration. Each tablet contains apremilast as the active ingredient and the following inactive ingredients: croscarmellose sodium, ferrosoferric oxide (30 mg), iron oxide red, iron oxide yellow (20 mg and 30 mg), lactose monohydrate, magnesium stearate, polyethylene glycol, polyvinyl alcohol, talc and titanium dioxide.
Long-term studies were conducted in mice and rats with apremilast to evaluate its carcinogenic potential. No evidence of apremilast-induced tumors was observed in mice at oral doses up to 8.8-times the Maximum Recommended Human Dose (MRHD) on an AUC basis (1,000 mg/kg/day) or in rats at oral doses up to approximately 0.08- and 1.1-times the MRHD, (20 mg/kg/day in males and 3 mg/kg/day in females, respectively).
Apremilast tested negative in the Ames assay,
In a fertility study of male mice, apremilast at oral doses up to approximately 3-times the MRHD based on AUC (up to 50 mg/kg/day) produced no effects on male fertility. In a fertility study of female mice, apremilast was administered at oral doses of 10, 20, 40, or 80 mg/kg/day. At doses ≥ 1.8-times the MRHD (≥ 20 mg/kg/day), estrous cycles were prolonged, due to lengthening of diestrus which resulted in a longer interval until mating. Mice that became pregnant at doses of 20 mg/kg/day and greater also had increased incidences of early post-implantation losses. There was no effect of apremilast approximately 1.0-times the MRHD (10 mg/kg/day).
Two multicenter, randomized, double-blind, placebo-controlled trials (PSOR-1 [NCT01194219] and PSOR-2 [NCT01232283]) enrolled a total of 1,257 subjects 18 years of age and older with moderate to severe plaque psoriasis [body surface area (BSA) involvement of ≥ 10%, static Physician Global Assessment (sPGA) of ≥ 3 (moderate or severe disease), Psoriasis Area and Severity Index (PASI) score ≥ 12, candidates for phototherapy or systemic therapy]. Subjects were allowed to use low potency topical corticosteroids on the face, axilla and groin. Subjects with plaque psoriasis of the scalp were allowed to use coal tar shampoo and/or salicylic acid scalp preparations on scalp lesions.
Trial PSOR-1 enrolled 844 subjects and trial PSOR-2 enrolled 413 subjects. In both trials, subjects were randomized 2:1 to apremilast 30 mg twice daily (BID) or placebo for 16 weeks. Both trials assessed the proportion of subjects who achieved PASI-75 at Week 16 and the proportion of subjects who achieved an sPGA score of clear (0) or almost clear (1) at Week 16. Across both trials, subjects ranged in age from 18 to 83 years, with an overall median age of 46 years. The mean baseline BSA involvement was 25.2% (median 21.0%), the mean baseline PASI score was 19.1 (median 16.8), and the proportion of subjects with an sPGA score of 3 (moderate) and 4 (severe) at baseline were 70.0% and 29.8%, respectively. Approximately 30% of all subjects had received prior phototherapy and 54% had received prior conventional systemic and/or biologic therapy for the treatment of psoriasis with 37% receiving prior conventional systemic therapy and 30% receiving prior biologic therapy. Approximately one-third of subjects had not received prior phototherapy, conventional systemic nor biologic therapy.
The proportion of subjects who achieved PASI-75 responses, and an sPGA score of clear (0) or almost clear (1), are presented in Table 8.
Trial PSOR-1 | Trial PSOR-2 | |||
Placebo | Apremilast 30 mg BID | Placebo | Apremilast 30 mg BIDd | |
N a | N=282 | N=562 | N=137 | N=274 |
PASI b -75, n (%) | 15 (5.3) | 186 (33.1) | 8 (5.8) | 79 (28.8) |
sPGA c of Clear or Almost Clear, n (%) | 11 (3.9) | 122 (21.7) | 6 (4.4) | 56 (20.4) |
a N is number of randomized and treated subjects. b PASI=Psoriasis Area and Severity Index. c sPGA=Static Physician Global Assessment. d BID = twice daily. | ||||
The median time to loss of PASI-75 response among the subjects re-randomized to placebo at Week 32 during the Randomized Treatment Withdrawal Phase was 5.1 weeks.
A randomized, double-blind, placebo-controlled trial (PSOR-3 [NCT03123471]) was conducted in 303 adult subjects with moderate to severe plaque psoriasis of the scalp. Enrolled subjects had a Scalp Physician Global Assessment (ScPGA) score of ≥ 3, Scalp Surface Area (SSA) involvement of ≥ 20%, an inadequate response or intolerance to at least one topical therapy for plaque psoriasis of the scalp, and moderate to severe plaque psoriasis (BSA involvement of ≥ 10%, sPGA of ≥ 3 [moderate or severe disease], and PASI score ≥ 12).
Subjects were randomized 2:1 to receive either apremilast 30 mg twice daily (n =201) or placebo twice daily (n = 102) for 16 weeks. The primary endpoint was the proportion of subjects who achieved an ScPGA response at Week 16 (defined as ScPGA score of clear [0] or almost clear [1] with at least a 2-point reduction from baseline at Week 16). Secondary endpoints included the proportion of subjects with Whole Body Itch Numeric Rating Scale (NRS) response (defined as ≥ 4-point reduction from baseline) and the proportion of subjects with a Scalp Itch NRS response (defined as ≥ 4-point reduction from baseline).
Subjects had a mean age of 46.9 years, 61.7% were men and 75.6 % were white. At baseline, 76.9% of subjects had moderate plaque psoriasis of the scalp (ScPGA of 3), 23.1% had severe plaque psoriasis of the scalp (ScPGA of 4), 71.6% of subjects were biologic naïve, and 58.8% had failed 1 or 2 topical treatments. At baseline, the mean Whole- Body Itch NRS score was 7.2 and the mean Scalp Itch NRS score was 6.7 with the scales ranging from 0 to 10. The mean baseline SSA involvement was 60.6% and the mean baseline BSA involvement was 19.8%.
The proportion of subjects who achieved an ScPGA response, Whole Body Itch NRS response, and Scalp Itch NRS response at Week 16 are presented in Table 9.
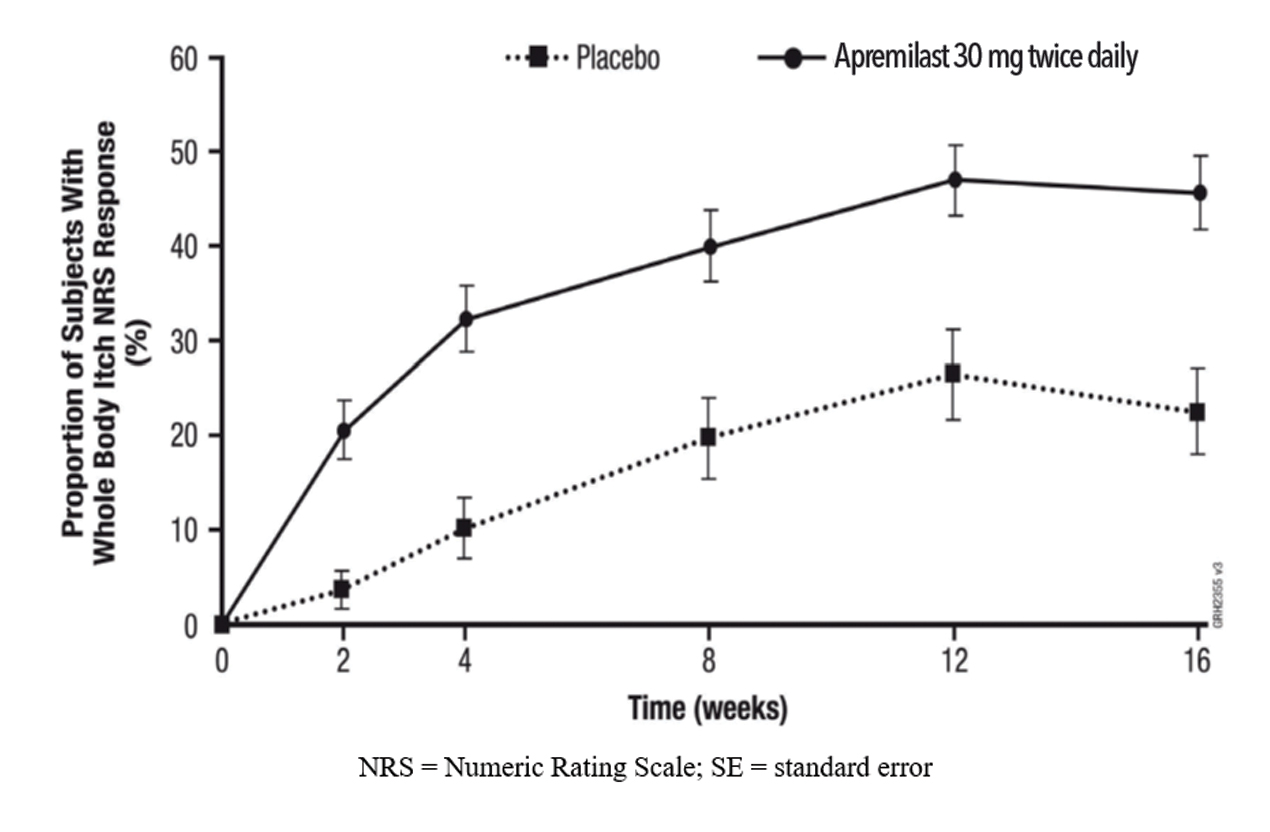
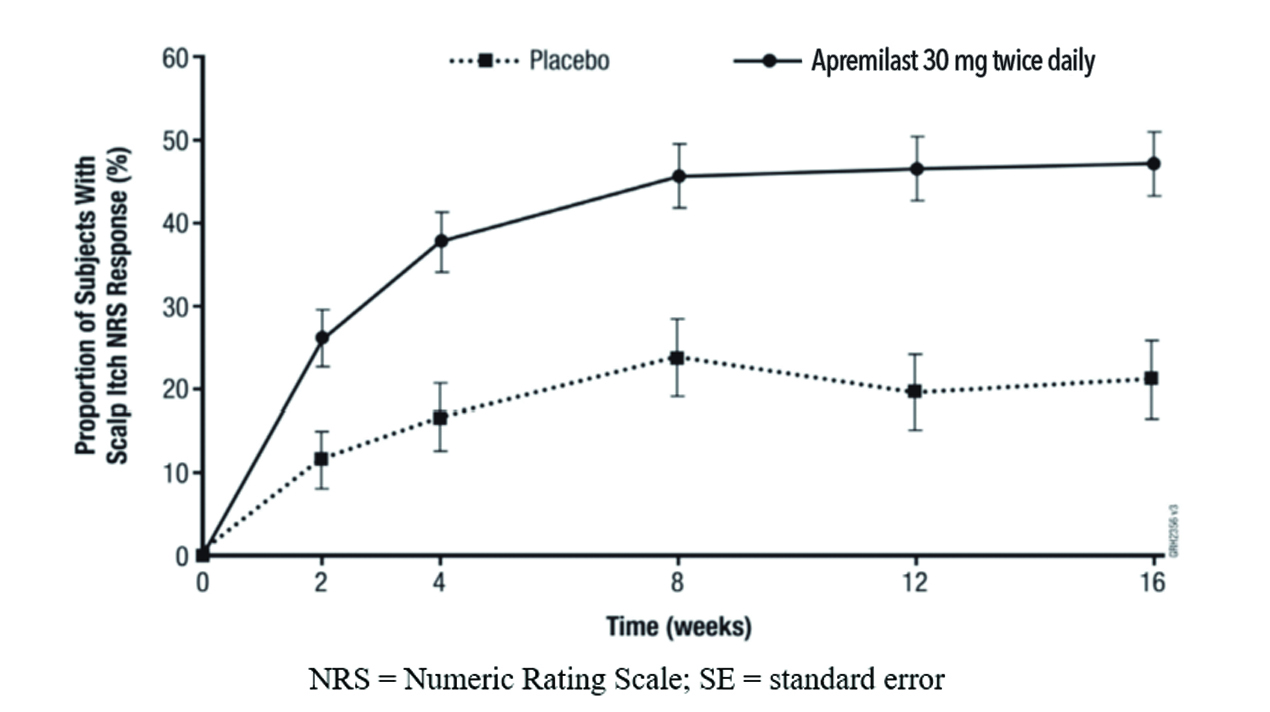
Figure 1 displays the proportion of subjects achieving Whole Body Itch NRS response at each visit, while Figure 2 displays the proportion of subjects achieving Scalp Itch NRS response at each visit.
Trial PSOR-3 | |||
Placebo | Apremilast 30 mg twice daily | Treatment Differencea,b (95% CIc) | |
Number of subjects randomized | N=102 | N=201 | |
ScPGA responsed | 13.7% | 43.3% | 29.6% (19.5%, 39.7%) |
Number of subjects with baseline Whole Body Itch NRS Score ≥4 | N=94 | N=185 | |
Whole Body Itch NRS response | 22.5% | 45.5% | 23.0% (11.5%, 34.6%) |
Number of subjects with baseline Scalp Itch NRS Score ≥4 | N=90 | N=175 | |
Scalp Itch NRS response | 21.1% | 47.1% | 26.2% (13.9%, 38.5%) |
a Apremilast – Placebo. b Adjusted difference in proportions is the weighted average of the treatment differences across baseline ScPGA scores with the Cochran-Mantel-Haenszel weights. c CI = confidence interval. d ScPGA score of clear [0] or almost clear [1] with at least a 2-point reduction from baseline. | |||