Argatroban - Argatroban injection
(Argatroban)Argatroban - Argatroban injection Prescribing Information
- Argatroban Injection 250 mg/2.5 mL (100 mg/mL) must be diluted 100-fold by mixing with 0.9% Sodium Chloride Injection, 5% Dextrose Injection, or Lactated Ringer’s Injection to a final concentration of 1 mg/mL. ()
2.1 Preparation for Intravenous AdministrationArgatroban Injection 250 mg/2.5 mL (100 mg/mL) must be diluted 100-fold prior to infusion. Argatroban should not be mixed with other drugs prior to dilution.
Argatroban Injection 250 mg/2.5 mL (100 mg/mL)Argatroban 250 mg/2.5 mL (100 mg/mL) should be diluted in 0.9% Sodium Chloride Injection, 5% Dextrose Injection, or Lactated Ringer’s Injection to a final concentration of 1 mg/mL. The contents of each 2.5-mL vial should be diluted 100-fold by mixing with 250 mL of diluent. Use 250 mg (2.5 mL) per 250 mL of diluent or 500 mg (5 mL) per 500 mL of diluent.
The constituted solution must be mixed by repeated inversion of the diluent bag for 1 minute. Upon preparation, the solution may show slight but brief haziness due to the formation of microprecipitates that rapidly dissolve upon mixing. Use of diluent at room temperature is recommended. Colder temperatures can slow down the rate of dissolution of precipitates. The final solution must be clear before use. The pH of the intravenous solution prepared as recommended is 3.2 to 7.5. Solutions prepared as recommended are stable at controlled room temperature, 20º to 25ºC (68º to 77ºF) (see USP) in ambient indoor light for 24 hours; therefore, light-resistant measures such as foil protection for intravenous lines are unnecessary. Solutions are physically and chemically stable for up to 96 hours when protected from light and stored at controlled room temperature, 20º to 25ºC (68º to 77ºF) (see USP) or at refrigerated conditions, 5º±3ºC (41º±5ºF). Prepared solutions should not be exposed to direct sunlight. No significant potency losses have been noted following simulated delivery of the solution through intravenous tubing.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.
Before administering argatroban, discontinue heparin therapy and obtain a baseline activated partial thromboplastin time (aPTT). The recommended initial dose of argatroban for adult patients without hepatic impairment is 2 mcg/kg/min, administered as a continuous infusion (see Table 1).
Table 1 Recommended Doses and Infusion Rates for 2 mcg/kg/min Dose of Argatroban for Patients With HIT* and Without Hepatic Impairment (1 mg/mL Final Concentration) | ||
Body Weight (kg) | Dose (mcg/min) | Infusion Rate (mL/hr) |
50 | 100 | 6 |
60 | 120 | 7 |
70 | 140 | 8 |
80 | 160 | 10 |
90 | 180 | 11 |
100 | 200 | 12 |
110 | 220 | 13 |
120 | 240 | 14 |
130 | 260 | 16 |
140 | 280 | 17 |
*with or without thrombosis
Check the aPTT 2 hours after initiation of therapy and after any dose change to confirm that the patient has attained the desired therapeutic range.
The dose for patients with or at risk for heparin-induced thrombocytopenia undergoing percutaneous coronary intervention is started at 25 mcg/kg/min and a bolus of 350 mcg/kg administered via a large bore intravenous line over 3 to 5 minutes. (
If the ACT is greater than 450 seconds, decrease the infusion rate to 15 mcg/kg/min, and check the ACT 5 to 10 minutes later (Table 3).
Continue titrating the dose until a therapeutic ACT (between 300 and 450 seconds) has been achieved; continue the same infusion rate for the duration of the PCI procedure.
In case of dissection, impending abrupt closure, thrombus formation during the procedure, or inability to achieve or maintain an ACT over 300 seconds, additional bolus doses of 150 mcg/kg may be administered and the infusion dose increased to 40 mcg/kg/min. Check the ACT after each additional bolus or change in the rate of infusion.
Table 2 Recommended Starting and Maintenance Doses (Within the Target ACT Range) of Argatroban Injection in Patients Undergoing PCI Without Hepatic Impairment (1 mg/mL Final Concentration) | ||||
Body Weight (kg) | Starting Bolus Dose (350 mcg/kg) | Starting and Maintenance Continuous Infusion Dosing For ACT 300-450 seconds 25 mcg/kg/min | ||
Bolus Dose (mcg) | Bolus Volume (mL) | Continuous Infusion Dose (mcg/min) | Continuous Infusion Rate (mL/hr) | |
50 | 17500 | 18 | 1250 | 75 |
60 | 21000 | 21 | 1500 | 90 |
70 | 24500 | 25 | 1750 | 105 |
80 | 28000 | 28 | 2000 | 120 |
90 | 31500 | 32 | 2250 | 135 |
100 | 35000 | 35 | 2500 | 150 |
110 | 38500 | 39 | 2750 | 165 |
120 | 42000 | 42 | 3000 | 180 |
130 | 45500 | 46 | 3250 | 195 |
140 | 49000 | 49 | 3500 | 210 |
NOTE: 1 mg = 1000 mcg; 1 kg = 2.2 lbs
Table 3 Recommended Dose Adjustments of Argatroban Injection for Patients Outside of ACT Target Range Undergoing PCI Without Hepatic Impairment (1 mg/mL Final Concentration) | ||||||
Body Weight (kg) | If ACT Less than 300 seconds Dosage Adjustment† 30 mcg/kg/min | If ACT Greater than 450 seconds Dosage Adjustment* 15 mcg/kg/min | ||||
Additional Bolus Dose (mcg) | Bolus Volume (mL) | Continuous Infusion Dose (mcg/min) | Continuous Infusion Rate (mL/hr) | Continuous Infusion Dose (mcg/min) | Continuous Infusion Rate (mL/hr) | |
50 | 7500 | 8 | 1500 | 90 | 750 | 45 |
60 | 9000 | 9 | 1800 | 108 | 900 | 54 |
70 | 10500 | 11 | 2100 | 126 | 1050 | 63 |
80 | 12000 | 12 | 2400 | 144 | 1200 | 72 |
90 | 13500 | 14 | 2700 | 162 | 1350 | 81 |
100 | 15000 | 15 | 3000 | 180 | 1500 | 90 |
110 | 16500 | 17 | 3300 | 198 | 1650 | 99 |
120 | 18000 | 18 | 3600 | 216 | 1800 | 108 |
130 | 19500 | 20 | 3900 | 234 | 1950 | 117 |
140 | 21000 | 21 | 4200 | 252 | 2100 | 126 |
NOTE: 1 mg = 1000 mcg; 1 kg = 2.2 lbs
†Additional intravenous bolus dose of 150 mcg/kg should be administered if ACT less than 300 seconds.
* No bolus dose is given if ACT greater than 450 seconds
- Injection: 250 mg/2.5 mL (100 mg/mL) single-dose vial
Argatroban is contraindicated in:
- Patients with major bleeding, [see]5.1 Risk of Hemorrhage
Hemorrhage can occur at any site in the body in patients receiving argatroban. Unexplained fall in hematocrit or blood pressure may indicate hemorrhage. Intracranial and retroperitoneal hemorrhage [
see Adverse Reactions (6.1)]have been reported. The risk of hemorrhage with argatroban may be increased in severe hypertension; immediately following lumbar puncture, spinal anesthesia, major surgery (especially involving the brain, spinal cord, or eye), hematologic conditions associated with increased bleeding tendencies such as congenital or acquired bleeding disorders, and gastrointestinal lesions such as ulcerations.Concomitant use of argatroban with antiplatelet agents, thrombolytics, and other anticoagulants may increase the risk of bleeding.
- Patients with a history of hypersensitivity to argatroban. Airway, skin, and generalized hypersensitivity reactions have been reported [see]
6.1 Clinical Trials ExperienceAdverse Reactions in Patients with HIT (With or Without Thrombosis)Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The following safety information is based on all 568 patients treated with argatroban in Study 1 and Study 2. The safety profile of the patients from these studies is compared with that of 193 historical controls in which the adverse reactions were collected retrospectively. Adverse reactions are separated into hemorrhagic and non-hemorrhagic reactions.
Major bleeding was defined as bleeding that was overt and associated with a hemoglobin decrease ≥ 2 g/dL, that led to a transfusion of ≥ 2 units, or that was intracranial, retroperitoneal, or into a major prosthetic joint. Minor bleeding was overt bleeding that did not meet the criteria for major bleeding.
Table 4 gives an overview of the most frequently observed hemorrhagic reactions, presented separately by major and minor bleeding, sorted by decreasing occurrence among argatroban-treated patients with HIT (with or without thrombosis).
Table 4Major and Minor Hemorrhagic Adverse Reactions in Patients With HIT*Major Hemorrhagic ReactionsaArgatroban-treated Patients(Study 1 and Study 2)(n = 568) %Historical
Controlc(n = 193) %Overall bleeding
5.3
6.7
Gastrointestinal
2.3
1.6
Genitourinary and hematuria
0.9
0.5
Decrease in hemoglobin and hematocrit
0.7
0
Multisystem hemorrhage and DIC
0.5
1
Limb and BKA stump
0.5
0
Intracranial hemorrhage
0b
0.5
Minor Hemorrhagic ReactionsaArgatroban-treated Patients(Study 1 and Study 2)(n = 568) %Historical Controlc(n = 193) %Gastrointestinal
14.4
18.1
Genitourinary and hematuria
11.6
0.8
Decrease in hemoglobin and hematocrit
10.4
0
Groin
5.4
3.1
Hemoptysis
2.9
0.8
Brachial
2.4
0.8
* with or without thrombosis
a) Patients may have experienced more than 1 adverse reaction.
b) One patient experienced intracranial hemorrhage 4 days after discontinuation of argatroban and following therapy with urokinase and
oral anticoagulation.
c) The historical control group consisted of patients with a clinical diagnosis of HIT (with or without thrombosis) that were considered eligible by an independent medical panel.
DIC = disseminated intravascular coagulation.
BKA = below the knee amputation.Table 5 gives an overview of the most frequently observed non-hemorrhagic reactions sorted by decreasing frequency of occurrence (≥2%) among argatroban-treated HIT/HITTS patients.
Table 5Non-hemorrhagic Adverse Reactions in PatientsaWith HITbArgatroban-treated Patients(Study 1 and Study 2)(n = 568) %Historical
Controlc(n = 193) %Dyspnea
8.1
8.8
Hypotension
7.2
2.6
Fever
6.9
2.1
Diarrhea
6.2
1.6
Sepsis
6.0
12.4
Cardiac arrest
5.8
3.1
Nausea
4.8
0.5
Ventricular tachycardia
4.8
3.1
Pain
4.6
3.1
Urinary tract infection
4.6
5.2
Vomiting
4.2
0
Infection
3.7
3.6
Pneumonia
3.3
9.3
Atrial fibrillation
3.0
11.4
Coughing
2.8
1.6
Abnormal renal function
2.8
4.7
Abdominal pain
2.6
1.6
Cerebrovascular disorder
2.3
4.1
a) Patients may have experienced more than 1 adverse reaction.
b) With or without thrombosis
c) The historical control group consisted of patients with a clinical diagnosis of HIT (with or without thrombosis) that were considered eligible by an independent medical panel.Adverse Reactions in Patients with or at Risk for HIT Undergoing PCIThe following safety information is based on 91 patients initially treated with argatroban and 21 patients subsequently re-exposed to argatroban for a total of 112 PCIs with argatroban anticoagulation. Adverse reactions are separated into hemorrhagic (Table 6) and non-hemorrhagic (Table 7) reactions.
Major bleeding was defined as bleeding that was overt and associated with a hemoglobin decrease ≥5 g/dL, that led to a transfusion of ≥2 units, or that was intracranial, retroperitoneal, or into a major prosthetic joint. The rate of major bleeding events in patients treated with argatroban in the PCI trials was 1.8%.
Table 6Major and Minor Hemorrhagic Adverse Reactions in Patients With HIT Undergoing PCIMajor Hemorrhagic ReactionsaArgatroban-treated
Patients(n = 112)b%Retroperitoneal
0.9
Gastrointestinal
0.9
Intracranial
0
Minor Hemorrhagic ReactionsaArgatroban-treated
Patients(n = 112)b%Groin (bleeding or hematoma)
3.6
Gastrointestinal (includes hematemesis)
2.6
Genitourinary (includes hematuria)
1.8
Decrease in hemoglobin and/or hematocrit
1.8
CABG (coronary arteries)
1.8
Access site
0.9
Hemoptysis
0.9
Other
0.9
a) Patients may have experienced more than 1 adverse reaction.
b) 91 patients who underwent 112 interventions.
CABG = coronary artery bypass graft.Table 7 gives an overview of the most frequently observed non-hemorrhagic adverse reactions (>2%), sorted by decreasing frequency of occurrence among argatroban-treated PCI patients.
Table 7Non-hemorrhagic Adverse Reactionsain Patients With HIT Undergoing PCIArgatroban Proceduresa(n = 112)b%Chest pain
15.2
Hypotension
10.7
Back pain
8.0
Nausea
7.1
Vomiting
6.3
Headache
5.4
Bradycardia
4.5
Abdominal pain
3.6
Fever
3.6
Myocardial infarction
3.6
a) Patients may have experienced more than 1 adverse reaction.
b) 91 patients who underwent 112 interventions.There were 22 serious adverse reactions in 17 PCI patients (19.6% in 112 interventions). Table 8 lists the serious adverse reactions occurring in argatroban-treated patients with or at risk for HIT undergoing PCI.
Table 8Serious Adverse Reactions in Patients With HIT Undergoing PCIaCoded TermArgatroban Proceduresb(n = 112)Myocardial infarction
4 (3.5%)
Angina pectoris
2 (1.8%)
Coronary thrombosis
2 (1.8%)
Myocardial ischemia
2 (1.8%)
Occlusion coronary
2 (1.8%)
Chest pain
1 (0.9%)
Fever
1 (0.9%)
Retroperitoneal hemorrhage
1 (0.9%)
Aortic stenosis
1 (0.9%)
Arterial thrombosis
1 (0.9%)
Gastrointestinal hemorrhage
1 (0.9%)
Gastrointestinal disorder (GERD)
1 (0.9%)
Cerebrovascular disorder
1 (0.9%)
Lung edema
1 (0.9%)
Vascular disorder
1 (0.9%)
a) Individual reactions may also have been reported elsewhere (see Table 6 and 7).
b) 91 patients underwent 112 procedures. Some patients may have experienced more than 1 reaction.Intracranial Bleeding in Other PopulationsIncreased risks for intracranial bleeding have been observed in investigational studies of argatroban for other uses. In a study of patients with acute myocardial infarction receiving both argatroban and thrombolytic therapy (streptokinase or tissue plasminogen activator), the overall frequency of intracranial bleeding was 1% (8 out of 810 patients). Intracranial bleeding was not observed in 317 subjects or patients who did not receive concomitant thrombolysis
[seeDrug Interactions (7.4)].The safety and effectiveness of argatroban for cardiac indications other than PCI in patients with HIT have not been established. Intracranial bleeding was also observed in a prospective, placebo-controlled study of argatroban in patients who had onset of acute stroke within 12 hours of study entry. Symptomatic intracranial hemorrhage was reported in 5 of 117 patients (4.3%) who received argatroban at 1 to 3 mcg/kg/min and in none of the 54 patients who received placebo. Asymptomatic intracranial hemorrhage occurred in 5 (4.3%) and 2 (3.7%) of the patients, respectively.
Allergic ReactionsOne hundred fifty-six allergic reactions or suspected allergic reactions were observed in 1,127 individuals who were treated with argatroban in clinical pharmacology studies or for various clinical indications. About 95% (148/156) of these reactions occurred in patients who concomitantly received thrombolytic therapy (e.g., streptokinase) or contrast media.
Allergic reactions or suspected allergic reactions in populations other than patients with HIT (with or without thrombosis) include (in descending order of frequency):
- Airway reactions (coughing, dyspnea): 10% or more
- Skin reactions (rash, bullous eruption): 1 to <10%
- General reactions (vasodilation): 1 to 10%
Limited data are available on the potential formation of drug-related antibodies. Plasma from 12 healthy volunteers treated with argatroban over 6 days showed no evidence of neutralizing antibodies. No loss of anticoagulant activity was noted with repeated administration of argatroban to more than 40 patients.
Hemorrhage can occur. Unexplained fall in hematocrit or blood pressure may indicate hemorrhage, (
)5.1 Risk of HemorrhageHemorrhage can occur at any site in the body in patients receiving argatroban. Unexplained fall in hematocrit or blood pressure may indicate hemorrhage. Intracranial and retroperitoneal hemorrhage [
see Adverse Reactions (6.1)]have been reported. The risk of hemorrhage with argatroban may be increased in severe hypertension; immediately following lumbar puncture, spinal anesthesia, major surgery (especially involving the brain, spinal cord, or eye), hematologic conditions associated with increased bleeding tendencies such as congenital or acquired bleeding disorders, and gastrointestinal lesions such as ulcerations.Concomitant use of argatroban with antiplatelet agents, thrombolytics, and other anticoagulants may increase the risk of bleeding.
Hepatic impairment: Adjust starting dose and titrate carefully in patients with HIT who have moderate or severe hepatic impairment. Avoid use in PCI in patients with clinically significant hepatic impairment. (
)5.2 Use in Hepatic ImpairmentWhen administering argatroban to patients with hepatic impairment, start with a lower dose and carefully titrate until the desired level of anticoagulation is achieved. Achievement of steady state aPTT levels may take longer and require more argatroban dose adjustments in patients with hepatic impairment compared to patients with normal hepatic function
[see Use in Specific Populations (8.6)]. Also, upon cessation of argatroban infusion in the hepatically impaired patient, full reversal of anticoagulant effects may require longer than 4 hours due to decreased clearance and increased elimination half-life of argatroban[see Dosage and Administration (2.4), Clinical Pharmacology (12.3)].Avoid the use of high doses of argatroban in patients undergoing PCI who have clinically significant hepatic disease or AST/ALT levels ≥3 times the upper limit of normal.
The following adverse reaction is also discussed in other sections of the labeling:
- Risk of Hemorrhage[see.]5.1 Risk of Hemorrhage
Hemorrhage can occur at any site in the body in patients receiving argatroban. Unexplained fall in hematocrit or blood pressure may indicate hemorrhage. Intracranial and retroperitoneal hemorrhage [
see Adverse Reactions (6.1)]have been reported. The risk of hemorrhage with argatroban may be increased in severe hypertension; immediately following lumbar puncture, spinal anesthesia, major surgery (especially involving the brain, spinal cord, or eye), hematologic conditions associated with increased bleeding tendencies such as congenital or acquired bleeding disorders, and gastrointestinal lesions such as ulcerations.Concomitant use of argatroban with antiplatelet agents, thrombolytics, and other anticoagulants may increase the risk of bleeding.
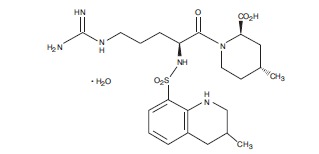
Argatroban is a synthetic direct thrombin inhibitor and the chemical name is 1-[5-[(aminoiminomethyl)amino]-1-oxo-2-[[(1,2,3,4-tetrahydro-3-methyl-8- quinolinyl)sulfonyl]amino]pentyl]-4-methyl-2-piperidinecarboxylic acid, monohydrate. Argatroban has 4 asymmetric carbons. One of the asymmetric carbons has an
The molecular formula of argatroban is C23H36N6O5S•H2O. Its molecular weight is 526.66 g/mol. The structural formula is shown below:
Argatroban is a white, odorless crystalline powder that is freely soluble in glacial acetic acid, slightly soluble in ethanol, and insoluble in acetone, ethyl acetate, and ether.
Argatroban Injection 250 mg/2.5 mL (100 mg/mL) is a sterile clear, colorless to pale yellow, slightly viscous solution. Argatroban Injection 250 mg/2.5 mL (100 mg/mL) is available in 250-mg (in 2.5-mL) single-dose amber vials, with white flip-top caps. Each mL of sterile, nonpyrogenic solution contains 100 mg Argatroban. Inert ingredients (per vial): 1300 mg Propylene glycol, 760 mg Dehydrated alcohol.