Aripiprazole Prescribing Information
Aripiprazole oral solution is indicated for the treatment of:
- Schizophrenia [see]
14.1 SchizophreniaAdultsThe efficacy of aripiprazole in the treatment of schizophrenia was evaluated in five short-term (4-week and 6-week), placebo-controlled trials of acutely relapsed inpatients who predominantly met DSM-III/IV criteria for schizophrenia. Four of the five trials were able to distinguish aripiprazole from placebo, but one study, the smallest, did not. Three of these studies also included an active control group consisting of either risperidone (one trial) or haloperidol (two trials), but they were not designed to allow for a comparison of aripiprazole and the active comparators.
In the four positive trials for aripiprazole, four primary measures were used for assessing psychiatric signs and symptoms. Efficacy was evaluated using the total score on the Positive and Negative Syndrome Scale (PANSS). The PANSS is a 30 item scale that measures positive symptoms of schizophrenia (7 items), negative symptoms of schizophrenia (7 items), and general psychopathology (16 items), each rated on a scale of 1 (absent) to 7 (extreme); total PANSS scores range from 30 to 210. The Clinical Global Impression (CGI) assessment reflects the impression of a skilled observer, fully familiar with the manifestations of schizophrenia, about the overall clinical state of the patient.
In a 4-week trial (n=414) comparing two fixed doses of aripiprazole (15 mg/day or 30 mg/day) to placebo, both doses of aripiprazole were superior to placebo in the PANSS total score (Study 1 in Table 26), PANSS positive subscale, and CGI-severity score. In addition, the 15 mg dose was superior to placebo in the PANSS negative subscale.
In a 4-week trial (n=404) comparing two fixed doses of aripiprazole (20 mg/day or 30 mg/day) to placebo, both doses of aripiprazole were superior to placebo in the PANSS total score (Study 2 in Table 26), PANSS positive subscale, PANSS negative subscale, and CGI-severity score.
In a 6-week trial (n=420) comparing three fixed doses of aripiprazole (10 mg/day, 15 mg/day, or 20 mg/day) to placebo, all three doses of aripiprazole were superior to placebo in the PANSS total score (Study 3 in Table 26), PANSS positive subscale, and the PANSS negative subscale.
In a 6-week trial (n=367) comparing three fixed doses of aripiprazole (2 mg/day, 5 mg/day, or 10 mg/day) to placebo, the 10 mg dose of aripiprazole was superior to placebo in the PANSS total score (Study 4 in Table 26), the primary outcome measure of the study. The 2 mg and 5 mg doses did not demonstrate superiority to placebo on the primary outcome measure.
Thus, the efficacy of 10 mg, 15 mg, 20 mg, and 30 mg daily doses was established in two studies for each dose. Among these doses, there was no evidence that the higher dose groups offered any advantage over the lowest dose group of these studies.
An examination of population subgroups did not reveal any clear evidence of differential responsiveness on the basis of age, gender, or race.
A longer-term trial enrolled 310 inpatients or outpatients meeting DSM-IV criteria for schizophrenia who were, by history, symptomatically stable on other antipsychotic medications for periods of 3 months or longer. These patients were discontinued from their antipsychotic medications and randomized to aripiprazole 15 mg/day or placebo for up to 26 weeks of observation for relapse. Relapse during the double-blind phase was defined as CGI-Improvement score of ≥5 (minimally worse), scores ≥5 (moderately severe) on the hostility or uncooperativeness items of the PANSS, or ≥20% increase in the PANSS total score. Patients receiving aripiprazole 15 mg/day experienced a significantly longer time to relapse over the subsequent 26 weeks compared to those receiving placebo (Study 5 in Figure 6).
Pediatric PatientsThe efficacy of aripiprazole in the treatment of schizophrenia in pediatric patients (13 to 17 years of age) was evaluated in one 6-week, placebo-controlled trial of outpatients who met DSM-IV criteria for schizophrenia and had a PANSS score ≥70 at baseline. In this trial (n=302) comparing two fixed doses of aripiprazole (10 mg/day or 30 mg/day) to placebo, aripiprazole was titrated starting from 2 mg/day to the target dose in 5 days in the 10 mg/day treatment arm and in 11 days in the 30 mg/day treatment arm. Both doses of aripiprazole were superior to placebo in the PANSS total score (Study 6 in Table 26), the primary outcome measure of the study. The 30 mg/day dosage was not shown to be more efficacious than the 10 mg/day dose. Although maintenance efficacy in pediatric patients has not been systematically evaluated, maintenance efficacy can be extrapolated from adult data along with comparisons of aripiprazole pharmacokinetic parameters in adult and pediatric patients.
Table 26: Schizophrenia StudiesStudyNumberTreatment GroupPrimary Efficacy Measure: PANSSMeanBaselineScore (SD)LS MeanChange fromBaseline (SE)Placebo-subtracted Difference*(95% CI)Study 1
Aripiprazole (15 mg/day)†
98.5 (17.2)
-15.5 (2.4)
-12.6 (-18.9, -6.2)
Aripiprazole (30 mg/day)†
99 (19.2)
-11.4 (2.39)
-8.5 (-14.8, -2.1)
Placebo
100.2 (16.5)
-2.9 (2.36)
--
Study 2
Aripiprazole (20 mg/day)†
92.6 (19.5)
-14.5 (2.23)
-9.6 (-15.4, -3.8)
Aripiprazole (30 mg/day)†
94.2 (18.5)
-13.9 (2.24)
-9.0 (-14.8, -3.1)
Placebo
94.3 (18.5)
-5.0 (2.17)
--
Study 3
Aripiprazole (10 mg/day)†
92.7 (19.5)
-15.0 (2.38)
-12.7 (-19, -6.41)
Aripiprazole (15 mg/day)†
93.2 (21.6)
-11.7 (2.38)
-9.4 (-15.71, -3.08)
Aripiprazole (20 mg/day)†
92.5 (20.9)
-14.4 (2.45)
-12.1 (-18.53, -5.68)
Placebo
92.3 (21.8)
-2.3 (2.35)
--
Study 4
Aripiprazole (2 mg/day)
90.7 (14.5)
-8.2 (1.9)
-2.9 (-8.29, 2.47)
Aripiprazole (5 mg/day)
92.0 (12.6)
-10.6 (1.93)
-5.2 (-10.7, 0.19)
Aripiprazole (10 mg/day)†
90 (11.9)
-11.3 (1.88)
-5.9 (-11.3, -0.58)
Placebo
90.8 (13.3)
-5.3 (1.97)
--
Study 6
(Pediatric,
13 to 17 years)
Aripiprazole (10 mg/day)†
93.6 (15.7)
-26.7 (1.91)
-5.5 (-10.7, -0.21)
Aripiprazole (30 mg/day)†
94 (16.1)
-28.6 (1.92)
-7.4 (-12.7, -2.13)
Placebo
94.6 (15.6)
-21.2 (1.93)
--
SD: standard deviation; SE: standard error; LS Mean: least-squares mean; CI: unadjusted confidence interval.
*Difference (drug minus placebo) in least-squares mean change from baseline.
†Doses statistically significantly superior to placebo.
Figure 6: Kaplan-Meier Estimation of Cumulative Proportion of Patients with Relapse (Schizophrenia Study 5)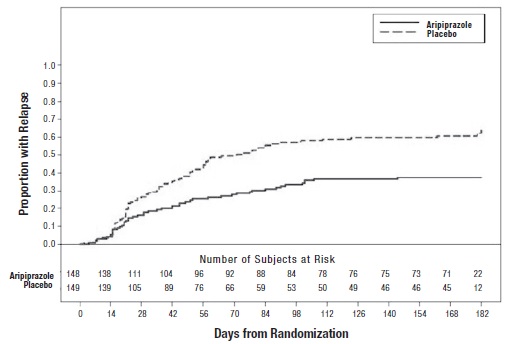

4 - Acute Treatment of Manic and Mixed Episodes associated with Bipolar I Disorder [see]
14.2 Bipolar DisorderAcute Treatment of Manic and Mixed Episodes
AdultsMonotherapyThe efficacy of aripiprazole as monotherapy in the acute treatment of manic episodes was established in four 3-week, placebo-controlled trials in hospitalized patients who met the DSM-IV criteria for bipolar I disorder with manic or mixed episodes. These studies included patients with or without psychotic features and two of the studies also included patients with or without a rapid-cycling course.
The primary instrument used for assessing manic symptoms was the Young Mania Rating Scale (Y-MRS), an 11-item clinician-rated scale traditionally used to assess the degree of manic symptomatology in a range from 0 (no manic features) to 60 (maximum score). A key secondary instrument included the Clinical Global Impression-Bipolar (CGI-BP) Scale.
In the four positive, 3-week, placebo-controlled trials (n=268; n=248; n=480; n=485) which evaluated aripiprazole in a range of 15 mg to 30 mg, once daily (with a starting dose of 30 mg/day in two studies and 15 mg/day in two studies), aripiprazole was superior to placebo in the reduction of Y-MRS total score (Studies 1 to 4 in Table 27) and CGI-BP Severity of Illness score (mania). In the two studies with a starting dose of 15 mg/day, 48% and 44% of patients were on 15 mg/day at endpoint. In the two studies with a starting dose of 30 mg/day, 86% and 85% of patients were on 30 mg/day at endpoint.
Adjunctive TherapyThe efficacy of adjunctive aripiprazole with concomitant lithium or valproate in the treatment of manic or mixed episodes was established in a 6-week, placebo-controlled study (n=384) with a 2-week lead-in mood stabilizer monotherapy phase in adult patients who met DSM-IV criteria for bipolar I disorder. This study included patients with manic or mixed episodes and with or without psychotic features.
Patients were initiated on open-label lithium (0.6 mEq/L to 1 mEq/L) or valproate (50 mcg/mL to 125 mcg/mL) at therapeutic serum levels, and remained on stable doses for 2 weeks. At the end of 2 weeks, patients demonstrating inadequate response (Y-MRS total score ≥16 and ≤25% improvement on the Y-MRS total score) to lithium or valproate were randomized to receive either aripiprazole (15 mg/day or an increase to 30 mg/day as early as day 7) or placebo as adjunctive therapy with open-label lithium or valproate. In the 6-week, placebo-controlled phase, adjunctive aripiprazole starting at 15 mg/day with concomitant lithium or valproate (in a therapeutic range of 0.6 mEq/L to 1 mEq/L or 50 mcg/mL to 125 mcg/mL, respectively) was superior to lithium or valproate with adjunctive placebo in the reduction of the Y-MRS total score (Study 5 in Table 27) and CGI-BP Severity of Illness score (mania). Seventy-one percent of the patients co-administered valproate and 62% of the patients co-administered lithium were on 15 mg/day at 6-week endpoint.
Pediatric PatientsThe efficacy of aripiprazole in the treatment of bipolar I disorder in pediatric patients (10 to 17 years of age) was evaluated in one 4-week, placebo-controlled trial (n=296) of outpatients who met DSM-IV criteria for bipolar I disorder manic or mixed episodes with or without psychotic features and had a Y-MRS score ≥20 at baseline. This double-blind, placebo-controlled trial compared two fixed doses of aripiprazole (10 mg/day or 30 mg/day) to placebo. The aripiprazole dose was started at 2 mg/day, which was titrated to 5 mg/day after 2 days, and to the target dose in 5 days in the 10 mg/day treatment arm, and in 13 days in the 30 mg/day treatment arm. Both doses of aripiprazole were superior to placebo in change from baseline to week 4 on the Y-MRS total score (Study 6 in Table 27).
Table 27: Bipolar StudiesStudy NumberTreatment GroupPrimary Efficacy Measure: Y-MRSMean Baseline Score (SD)LS Mean Change from Baseline (SE)Placebo-subtracted Difference*(95% CI)Study 1
Aripiprazole (30 / 15 mg/day)†
29.0 (5.9)
-12.52 (1.05)
-5.33 (-7.9, -2.76)
Placebo
28.5 (4.6)
-7.19 (1.07)
--
Study 2
Aripiprazole (30 / 15 mg/day)†
27.8 (5.7)
-8.15 (1.23)
-4.8 (-7.8, -1.8)
Placebo
29.1 (6.9)
-3.35 (1.22)
--
Study 3
Aripiprazole (15 mg/day to 30 mg/day)†
28.5 (5.6)
-12.64 (0.84)
-3.63 (-5.75 , -1.51)
Placebo
28.9 (5.9)
9.01 (0.81)
--
Study 4
Aripiprazole (15 mg/day to 30 mg/day)†
28 (5.8)
-11.98 (0.8)
-2.28 (-4.44 , -0.11)
Placebo
28.3 (5.8)
-9.7 (0.83)
--
Study 5
Aripiprazole (15 or 30 mg/day)†
+ Lithium/Valproate
23.2 (5.7)
-13.31 (0.5)
-2.62 (-4.29 , -0.95)
Placebo + Lithium/Valproate
23 (4.9)
-10.7 (0.69)
--
Study 6
(Pediatric,
10 to 17 years)
Aripiprazole (10 mg/day)†
29.8 (6.5)
-14.2 (0.89)
-5.99 (-8.49, -3.5)
Aripiprazole (30 mg/day)†
29.5 (6.3)
-16.5 (0.87)
-8.26 (-10.7, -5.77)
Placebo
30.7 (6.8)
-8.2 (0.91)
--
SD: standard deviation; SE: standard error; LS Mean: least-squares mean; CI: unadjusted confidence interval.
*Difference (drug minus placebo) in least-squares mean change from baseline.
†Doses statistically significantly superior to placebo.
Maintenance Treatment of Bipolar I DisorderMonotherapy Maintenance TherapyA maintenance trial was conducted in adult patients meeting DSM-IV criteria for bipolar I disorder with a recent manic or mixed episode who had been stabilized on open-label aripiprazole and who had maintained a clinical response for at least 6 weeks. The first phase of this trial was an open-label stabilization period in which inpatients and outpatients were clinically stabilized and then maintained on open-label aripiprazole (15 mg/day or 30 mg/day, with a starting dose of 30 mg/day) for at least 6 consecutive weeks. One hundred sixty-one outpatients were then randomized in a double-blind fashion, to either the same dose of aripiprazole they were on at the end of the stabilization and maintenance period or placebo and were then monitored for manic or depressive relapse. During the randomization phase, aripiprazole was superior to placebo on time to the number of combined affective relapses (manic plus depressive), the primary outcome measure for this study (Study 7 in Figure 7). A total of 55 mood events were observed during the double-blind treatment phase. Nineteen were from the aripiprazole group and 36 were from the placebo group. The number of observed manic episodes in the aripiprazole group (6) were fewer than that in the placebo group (19), while the number of depressive episodes in the aripiprazole group (9) was similar to that in the placebo group (11).
An examination of population subgroups did not reveal any clear evidence of differential responsiveness on the basis of age and gender; however, there were insufficient numbers of patients in each of the ethnic groups to adequately assess inter-group differences.
Figure 7: Kaplan-Meier Estimation of Cumulative Proportion of Patients with Relapse (Bipolar Study 7)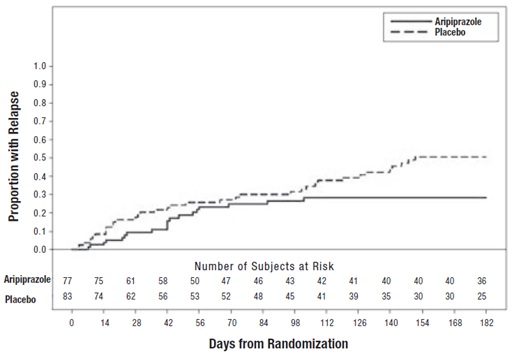 Adjunctive Maintenance Therapy
Adjunctive Maintenance TherapyAn adjunctive maintenance trial was conducted in adult patients meeting DSM-IV criteria for bipolar I disorder with a recent manic or mixed episode. Patients were initiated on open-label lithium (0.6 mEq/L to 1.0 mEq/L) or valproate (50 mcg/mL to 125 mcg/mL) at therapeutic serum levels, and remained on stable doses for 2 weeks. At the end of 2 weeks, patients demonstrating inadequate response (Y-MRS total score ≥16 and ≤35% improvement on the Y-MRS total score) to lithium or valproate received aripiprazole with a starting dose of 15 mg/day with the option to increase to 30 mg or reduce to 10 mg as early as day 4, as adjunctive therapy with open-label lithium or valproate. Prior to randomization, patients on the combination of single-blind aripiprazole and lithium or valproate were required to maintain stability (Y-MRS and MADRS total scores ≤12) for 12 consecutive weeks. Three hundred thirty-seven patients were then randomized in a double-blind fashion, to either the same dose of aripiprazole they were on at the end of the stabilization period or placebo plus lithium or valproate and were then monitored for manic, mixed, or depressive relapse for a maximum of 52 weeks. Aripiprazole was superior to placebo on the primary endpoint, time from randomization to relapse to any mood event (Study 8 in Figure 8). A mood event was defined as hospitalization for a manic, mixed, or depressive episode, study discontinuation due to lack of efficacy accompanied by Y-MRS score >16 and/or a MADRS >16, or an SAE of worsening disease accompanied by Y-MRS score >16 and/or a MADRS >16. A total of 68 mood events were observed during the double-blind treatment phase. Twenty-five were from the aripiprazole group and 43 were from the placebo group. The number of observed manic episodes in the aripiprazole group (7) were fewer than that in the placebo group (19), while the number of depressive episodes in the aripiprazole group (14) was similar to that in the placebo group (18). The Kaplan-Meier curves of the time from randomization to relapse to any mood event during the 52-week, double-blind treatment phase for aripiprazole and placebo groups are shown in Figure 8.
Figure 8: Kaplan-Meier Estimation of Cumulative Proportion of Patients with Relapse to Any Mood Event (Bipolar Study 8)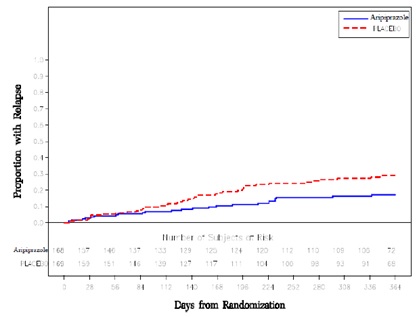
An examination of population subgroups did not reveal any clear evidence of differential responsiveness on the basis of age and gender; however, there were insufficient numbers of patients in each of the ethnic groups to adequately assess inter-group differences.

4 
4 - Adjunctive Treatment of Major Depressive Disorder [see]
14.3 Adjunctive Treatment of Major Depressive DisorderAdultsThe efficacy of aripiprazole in the adjunctive treatment of major depressive disorder (MDD) was demonstrated in two short-term (6-week), placebo-controlled trials of adult patients meeting DSM-IV criteria for MDD who had had an inadequate response to prior antidepressant therapy (1 to 3 courses) in the current episode and who had also demonstrated an inadequate response to 8 weeks of prospective antidepressant therapy (paroxetine controlled-release, venlafaxine extended-release, fluoxetine, escitalopram, or sertraline). Inadequate response for prospective treatment was defined as less than 50% improvement on the 17-item version of the Hamilton Depression Rating Scale (HAMD17), minimal HAMD17 score of 14, and a Clinical Global Impressions Improvement rating of no better than minimal improvement. Inadequate response to prior treatment was defined as less than 50% improvement as perceived by the patient after a minimum of 6 weeks of antidepressant therapy at or above the minimal effective dose.
The primary instrument used for assessing depressive symptoms was the Montgomery-Asberg Depression Rating Scale (MADRS), a 10-item clinician-rated scale used to assess the degree of depressive symptomatology. The key secondary instrument was the Sheehan Disability Scale (SDS), a 3-item self-rated instrument used to assess the impact of depression on three domains of functioning with each item scored from 0 (not at all) to 10 (extreme).
In the two trials (n=381, n=362), aripiprazole was superior to placebo in reducing mean MADRS total scores (Studies 1, 2 in Table 28). In one study, aripiprazole was also superior to placebo in reducing the mean SDS score.
In both trials, patients received aripiprazole adjunctive to antidepressants at a dose of 5 mg/day. Based on tolerability and efficacy, doses could be adjusted by 5 mg increments, one week apart. Allowable doses were: 2 mg/day, 5 mg/day, 10 mg/day, 15 mg/day, and for patients who were not on potent CYP2D6 inhibitors fluoxetine and paroxetine, 20 mg/day. The mean final dose at the end point for the two trials was 10.7 mg/day and 11.4 mg/day.
An examination of population subgroups did not reveal evidence of differential response based on age, choice of prospective antidepressant, or race. With regard to gender, a smaller mean reduction on the MADRS total score was seen in males than in females.
Table 28: Adjunctive Treatment of Major Depressive Disorder StudiesStudy NumberTreatment GroupPrimary Efficacy Measure: MADRSMean Baseline Score (SD)LS Mean Change from Baseline (SE)Placebo-subtracted Difference*(95% CI)Study 1
Aripiprazole (5 to 20 mg/day)†+ Antidepressant
Placebo + Antidepressant
25.2 (6.2)
27.0 (5.5)
-8.49 (0.66)
-5.65 (0.64)
-2.84 (-4.53, -1.15)
--
Study 2
Aripiprazole (5 to 20 mg/day)†+ Antidepressant
Placebo + Antidepressant
26.0 (6.0)
26.0 (6.5)
-8.78 (0.63)
-5.77 (0.67)
-3.01 (-4.66, -1.37)
--
SD: standard deviation; SE: standard error; LS Mean: least-squares mean; CI: unadjusted confidence interval.
*Difference (drug minus placebo) in least-squares mean change from baseline.
†Doses statistically significantly superior to placebo.
- Irritability Associated with Autistic Disorder [see]
14.4 Irritability Associated with Autistic DisorderPediatric PatientsThe efficacy of aripiprazole in the treatment of irritability associated with autistic disorder was established in two 8-week, placebo-controlled trials in pediatric patients (6 to 17 years of age) who met the DSM-IV criteria for autistic disorder and demonstrated behaviors such as tantrums, aggression, self-injurious behavior, or a combination of these problems. Over 75% of these subjects were under 13 years of age.
Efficacy was evaluated using two assessment scales: the Aberrant Behavior Checklist (ABC) and the Clinical Global Impression-Improvement (CGI-I) scale. The primary outcome measure in both trials was the change from baseline to endpoint in the Irritability subscale of the ABC (ABC-I). The ABC-I subscale measured symptoms of irritability in autistic disorder.
The results of these trials are as follows:
In one of the 8-week, placebo-controlled trials, children and adolescents with autistic disorder (n=98), aged 6 to 17 years, received daily doses of placebo or aripiprazole 2 mg/day to 15 mg/day. Aripiprazole, starting at 2 mg/day with increases allowed up to 15 mg/day based on clinical response, significantly improved scores on the ABC-I subscale and on the CGI-I scale compared with placebo. The mean daily dose of aripiprazole at the end of 8-week treatment was 8.6 mg/day (Study 1 in Table 29).
In the other 8-week, placebo-controlled trial in children and adolescents with autistic disorder (n=218), aged 6 to 17 years, three fixed doses of aripiprazole (5 mg/day, 10 mg/day, or 15 mg/day) were compared to placebo. Aripiprazole dosing started at 2 mg/day and was increased to 5 mg/day after one week. After a second week, it was increased to 10 mg/day for patients in the 10 mg and 15 mg dose arms, and after a third week, it was increased to 15 mg/day in the 15 mg/day treatment arm (Study 2 in Table 29). All three doses of aripiprazole significantly improved scores on the ABC-I subscale compared with placebo.
Table 29: Irritability Associated with Autistic Disorder Studies (Pediatric)Study NumberTreatment GroupPrimary Efficacy Measure: ABC-IMean Baseline Score (SD)LS Mean Change from Baseline (SE)Placebo-subtracted Difference*(95% CI)Study 1
Aripiprazole (2 to 15 mg/day)†
29.6 (6.37)
-12.9 (1.44)
-7.9 (-11.7, -4.1)
Placebo
30.2 (6.52)
-5.0 (1.43)
--
Study 2
Aripiprazole (5 mg/day)†
28.6 (7.56)
-12.4 (1.36)
-4.0 (-7.7, -0.4)
Aripiprazole (10 mg/day)†
28.2 (7.36)
-13.2 (1.25)
-4.8 (-8.4, -1.3)
Aripiprazole (15 mg/day)†
28.9 (6.41)
-14.4 (1.31)
-6.0 (-9.6, -2.3)
Placebo
28.0 (6.89)
-8.4 (1.39)
--
SD: standard deviation; SE: standard error; LS Mean: least-squares mean; CI: unadjusted confidence interval.
*Difference (drug minus placebo) in least-squares mean change from baseline.
†Doses statistically significantly superior to placebo.
- Treatment of Tourette’s Disorder [see]
14.5 Tourette’s DisorderPediatric PatientsThe efficacy of aripiprazole in the treatment of Tourette’s disorder was established in one 8-week (7 to 17 years of age) and one 10-week (6 to 18 years of age), placebo-controlled trials in pediatric patients (6 to 18 years of age) who met the DSM-IV criteria for Tourette’s disorder and had a Total Tic score (TTS) ≥ 20 to 22 on the Yale Global Tic Severity Scale (YGTSS). The YGTSS is a fully validated scale designed to measure current tic severity. Efficacy was evaluated using two assessment scales: 1) the Total Tic score (TTS) of the YGTSS and 2) the Clinical Global Impressions Scale for Tourette’s Syndrome (CGI-TS), a clinician-determined summary measure that takes into account all available patient information. Over 65% of these patients were under 13 years of age.
The primary outcome measure in both trials was the change from baseline to endpoint in the TTS of the YGTSS. Ratings for the TTS are made along 5 different dimensions on a scale of 0 to 5 for motor and vocal tics each. Summation of these 10 scores provides a TTS (i.e. 0 to 50).
The results of these trials are as follows:
In the 8-week, placebo-controlled, fixed-dose trial, children and adolescents with Tourette’s disorder (n=133), aged 7 to 17 years, were randomized 1:1:1 to low dose aripiprazole, high dose aripiprazole, or placebo. The target doses for the low and high dose aripiprazole groups were based on weight. Patients < 50 kg in the low dose aripiprazole group started at 2 mg per day with a target dose of 5 mg per day after 2 days. Patients ≥ 50 kg in the low dose aripiprazole group, started at 2 mg per day increased to 5 mg per day after 2 days, with a subsequent increase to a target dose of 10 mg per day at day 7. Patients <50 kg in the high dose aripiprazole group started at 2 mg per day increased to 5 mg per day after 2 days, with a subsequent increase to a target dose of 10 mg per day at day 7. Patients ≥ 50 kg in the high dose aripiprazole group, started at 2 mg per day increased to 5 mg per day after 2 days, with a subsequent increase to a dose of 10 mg per day at day 7 and were allowed weekly increases of 5 mg per day up to a target dose 20 mg per day at Day 21. Aripiprazole (both high and low dose groups) demonstrated statistically significantly improved scores on the YGTSS TTS (Study 1 in Table 30) and on the CGI-TS scale compared with placebo. The estimated improvements on the YGTSS TTS over the course of the study are displayed in Figure 9.
Figure 9: Least Square Means of Change from Baseline in YGTSS TTS by Week (Tourette’s Disorder Study 1)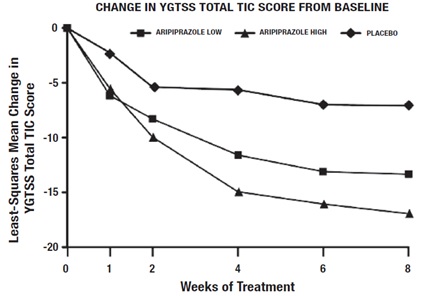
In the 10-week, placebo-controlled, flexible-dose trial in children and adolescents with Tourette’s disorder (n=61), aged 6 to 18 years, patients received daily doses of placebo or aripiprazole, starting at 2 mg/day with increases allowed up to 20 mg/day based on clinical response. Aripiprazole demonstrated statistically significantly improved scores on the YGTSS TTS scale compared with placebo (Study 2 in Table 30). The mean daily dose of aripiprazole at the end of 10-week treatment was 6.54 mg/day.
Table 30: Tourette’s Disorder Studies (Pediatric)Study NumberTreatment GroupPrimary Efficacy Measure: YGTSS TTSMean Baseline Score (SD)LS Mean Change from Baseline (SE)Placebo-subtracted Difference*(95% CI)Study 1
Aripiprazole (low dose)†
29.2 (5.63)
-13.4 (1.59)
-6.3 (-10.2, -2.3)
Aripiprazole (high dose)†
31.2 (6.4)
-16.9 (1.61)
-9.9 (-13.8, -5.9)
Placebo
30.7 (5.95)
-7.1 (1.55)
--
Study 2
Aripiprazole (2 to 20 mg/day)†
28.3 (5.51)
-15 (1.51)
-5.3 (-9.8, -0.9)
Placebo
29.5 (5.6)
-9.6 (1.64)
--
SD: standard deviation; SE: standard error; LS Mean: least-squares mean; CI: unadjusted confidence interval.
*Difference (drug minus placebo) in least-squares mean change from baseline.
†Doses statistically significantly superior to placebo.

4
Aripiprazole oral solution (1 mg/mL) is a clear, colorless to pale yellow solution, supplied in child-resistant bottles along with a calibrated oral dosing cup.
Aripiprazole oral solution is contraindicated in patients with a history of a hypersensitivity reaction to aripiprazole. Reactions have ranged from pruritus/urticaria to anaphylaxis
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The following adverse reactions are discussed in more detail in other sections of the labeling:
- Increased Mortality in Elderly Patients with Dementia-Related Psychosis [see Boxed WarningandWarnings and Precautions (5.1)]
- Cerebrovascular Adverse Events, Including Stroke [see Warnings and Precautions (5.2)]
- Suicidal Thoughts and Behaviors in Children, Adolescents, and Young Adults[see Boxed WarningandWarnings and Precautions (5.3)]
- Neuroleptic Malignant Syndrome (NMS) [see Warnings and Precautions (5.4)]
- Tardive Dyskinesia [see Warnings and Precautions (5.5)]
- Metabolic Changes [see Warnings and Precautions (5.6)]
- Pathological Gambling and Other Compulsive Behaviors [see Warnings and Precautions (5.7)]
- Orthostatic Hypotension [see Warnings and Precautions (5.8)]
- Falls [see Warnings and Precautions (5.9)]
- Leukopenia, Neutropenia, and Agranulocytosis[see Warnings and Precautions (5.10)]
- Seizures/Convulsions [see Warnings and Precautions (5.11)]
- Potential for Cognitive and Motor Impairment [see Warnings and Precautions (5.12)]
- Body Temperature Regulation [see Warnings and Precautions (5.13)]
- Suicide [see Warnings and Precautions (5.14)]
- Dysphagia [see Warnings and Precautions (5.15)]
The most common adverse reactions in adult patients in clinical trials (≥10%) were nausea, vomiting, constipation, headache, dizziness, akathisia, anxiety, insomnia, and restlessness.
The most common adverse reactions in the pediatric clinical trials (≥10%) were somnolence, headache, vomiting, extrapyramidal disorder, fatigue, increased appetite, insomnia, nausea, nasopharyngitis, and weight increased.
Aripiprazole has been evaluated for safety in 13,543 adult patients who participated in multiple-dose, clinical trials in schizophrenia, bipolar disorder, major depressive disorder, Dementia of the Alzheimer’s type, Parkinson’s disease, and alcoholism, and who had approximately 7,619 patient-years of exposure to oral aripiprazole. A total of 3,390 patients were treated with oral aripiprazole for at least 180 days and 1,933 patients treated with oral aripiprazole had at least 1 year of exposure.
Aripiprazole has been evaluated for safety in 1,686 patients (6 to 18 years) who participated in multiple-dose, clinical trials in schizophrenia, bipolar mania, autistic disorder, or Tourette’s disorder and who had approximately 1,342 patient-years of exposure to oral aripiprazole. A total of 959 pediatric patients were treated with oral aripiprazole for at least 180 days and 556 pediatric patients treated with oral aripiprazole had at least 1 year of exposure.
The conditions and duration of treatment with aripiprazole (monotherapy and adjunctive therapy with antidepressants or mood stabilizers) included (in overlapping categories) double-blind, comparative and noncomparative open-label studies, inpatient and outpatient studies, fixed- and flexible-dose studies, and short- and longer-term exposure.
Aripiprazole, USP is an atypical antipsychotic drug that is available as an aripiprazole oral solution. Aripiprazole, USP is 7-[4-[4-(2,3-dichlorophenyl)-1-piperazinyl]butoxy]-3,4-dihydrocarbostyril. The empirical formula is C23H27Cl2N3O2 and its molecular weight is 448.38. The chemical structure is:
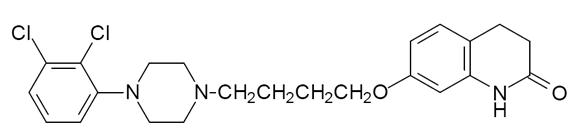
Aripiprazole oral solution is a clear, colorless to pale yellow solution available in a concentration of 1 mg/mL. The inactive ingredients for this solution include edetate disodium, fructose, glycerin, malic acid, methylparaben, propylene glycol, propylparaben, purified water, sodium hydroxide and sucrose. The oral solution is flavored with natural orange, cream and other natural and artificial flavors.