Asenapine - Asenapine Maleate tablet Prescribing Information
Indications and Usage (1 INDICATIONS AND USAGE Asenapine sublingual Tablets are indicated for: • Schizophrenia in adults [see Clinical Studies ( 14.1 )] • Bipolar I disorder [see Clinical Studies ( 14.2 )] • Acute monotherapy of manic or mixed episodes, in adults and pediatric patients 10 to 17 years of age • Adjunctive treatment to lithium or valproate in adults • Maintenance monotherapy treatment in adults Asenapine is an atypical antipsychotic indicated for :
| 01/2017 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Dosage and Administration (2.3 Bipolar I Disorder Acute Treatment of Manic or Mixed Episodes : Monotherapy in Adults : The recommended starting and treatment dose of asenapine is 5 mg to 10 mg twice daily. The safety of doses above 10 mg twice daily has not been evaluated in clinical trials[see Clinical Studies ( 14.2 )] . Monotherapy in Pediatric P atients: The recommended dose of asenapine is 2.5 mg to 10 mg twice daily in pediatric patients 10 to 17 years of age, and dose may be adjusted for individual response and tolerability. The starting dose of asenapine is 2.5 mg twice daily. After 3 days, the dose can be increased to 5 mg twice daily, and from 5 mg to 10 mg twice daily after 3 additional days. Pediatric patients aged 10 to 17 years appear to be more sensitive to dystonia with initial dosing with asenapine when the recommended escalation schedule is not followed[see Use in Specific Populations ( 8.4 )] . The safety of doses greater than 10 mg twice daily has not been evaluated in clinical trials[see Use in Specific Populations ( 8.4 ) and Clinical Pharmacology ( 12.3 )] .Adjunctive Therapy in Adults : The recommended starting dose of asenapine is 5 mg twice daily when administered as adjunctive therapy with either lithium or valproate. Depending on the clinical response and tolerability in the individual patient, the dose can be increased to 10 mg twice daily. The safety of doses above 10 mg twice daily as adjunctive therapy with lithium or valproate has not been evaluated in clinical trials.For patients on asenapine, whether used as monotherapy or as adjunctive therapy with lithium or valproate, it is generally recommended that responding patients continue treatment beyond the acute episode. Maintenance Treatment of Bipolar I Disorder: Monotherapy in Adults: Continue on the asenapine dose that the patient received during stabilization (5 mg to 10 mg twice daily). Depending on the clinical response and tolerability in the individual patient, a dose of 10 mg twice daily can be decreased to 5 mg twice daily. The safety of doses above 10 mg twice daily has not been evaluated in clinical trials [see Clinical Studies ( 14.2 )]. | 01/2017 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Contraindications (4 CONTRAINDICATIONS Asenapine is contraindicated in patients with: • Severe hepatic impairment (Child-Pugh C) [see Specific Populations ( 8.7 ), Clinical Pharmacology ( 12.3 )] .• A history of hypersensitivity reactions to asenapine. Reactions have included anaphylaxis, angioedema, hypotension, tachycardia, swollen tongue, dyspnea, wheezing and rash [see Warnings and Precautions ( 5.6 ), Adverse Reactions ( 6 )].
| 01/2017 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Warnings and Precautions ( 5.1 Increased Mortality in Elderly Patients with Dementia-Related Psychosis Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Analyses of 17 placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in drug-treated patients of between 1.6 to 1.7 times the risk of death in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in the placebo group. Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (e.g., heart failure, sudden death) or infectious (e.g., pneumonia) in nature. Asenapine is not approved for the treatment of patients with dementia-related psychosis [see Boxed Warning and Warnings and Precautions ( 5.2 ) ]. 5.2 Cerebrovascular Adverse Events, Including Stroke, In Elderly Patients with Dementia-Related Psychosis In placebo-controlled trials in elderly subjects with dementia, patients randomized to risperidone, aripiprazole, and olanzapine had a higher incidence of stroke and transient ischemic attack, including fatal stroke. Asenapine is not approved for the treatment of patients with dementia-related psychosis [see Boxed Warning , Warnings and Precautions ( 5.1 )]. 5.3 Neuroleptic Malignant Syndrome A potentially fatal symptom complex sometimes referred to as Neuroleptic Malignant Syndrome (NMS) has been reported in association with administration of antipsychotic drugs. Clinical manifestations of NMS are hyperpyrexia, muscle rigidity, delirium, and autonomic instability. Additional signs may include elevated creatine phosphokinase, myoglobinuria (rhabdomyolysis), and acute renal failure. If NMS is suspected, immediately discontinue asenapine and provide intensive symptomatic treatment and monitoring. 5.4 Tardive Dyskinesia Tardive dyskinesia, a syndrome consisting of potentially irreversible, involuntary, dyskinetic movements, may develop in patients treated with antipsychotic drugs, including asenapine. The risk appears to be highest among the elderly, especially elderly women, but it is not possible to predict which patients are likely to develop the syndrome. Whether antipsychotic drug products differ in their potential to cause tardive dyskinesia is unknown. The risk of tardive dyskinesia and the likelihood that it will become irreversible increase with the duration of treatment and the cumulative dose. The syndrome can develop after a relatively brief treatment period, even at low doses. It may also occur after discontinuation of treatment. There is no known treatment for tardive dyskinesia, although the syndrome may remit, partially or completely, if antipsychotic treatment is discontinued. Antipsychotic treatment itself, however, may suppress (or partially suppress) the signs and symptoms of the syndrome, possibly masking the underlying process. The effect that symptomatic suppression has upon the long-term course of tardive dyskinesia is unknown. Given these considerations, asenapine should be prescribed in a manner most likely to reduce the risk of tardive dyskinesia. Chronic antipsychotic treatment should generally be reserved for patients: 1) who suffer from a chronic illness that is known to respond to antipsychotic drugs; and 2) for whom alternative, effective, but potentially less harmful treatments are not available or appropriate. In patients who do require chronic treatment, use the lowest dose and the shortest duration of treatment producing a satisfactory clinical response should be sought. Periodically reassess the need for continued treatment. If signs and symptoms of TD appear in a patient on asenapine, drug discontinuation should be considered. However, some patients may require treatment with asenapine despite the presence of the syndrome. 5.5 Metabolic Changes Atypical antipsychotic drugs, including asenapine, have caused metabolic changes, including hyperglycemia, diabetes mellitus, dyslipidemia, and body weight gain. Although all of the drugs in the class to date have been shown to produce some metabolic changes, each drug has its own specific risk profile. Hyperglycemia and Diabetes Mellitus Hyperglycemia, in some cases extreme and associated with ketoacidosis or hyperosmolar coma or death, has been reported in patients treated with atypical antipsychotics. There have been reports of hyperglycemia in patients treated with asenapine. Assess fasting plasma glucose before or soon after initiation of antipsychotic medication, and monitor periodically during long-term treatment. Adult Patients : Pooled data from the short-term placebo-controlled schizophrenia and bipolar mania trials are presented inTable 1. TABLE 1 :Changes in Fasting Glucose in Adult Patients
N* = Number of patients who had assessments at both Baseline and Endpoint. N** = Number of patients at risk at Baseline with assessments at both Baseline and Endpoint. §Includes patients treated with flexible dose of asenapine 5 or 10 mg twice daily (N=90). †Includes patients treated with flexible dose of asenapine 5 or 10 mg twice daily (N=379). In a 52-week, double-blind, comparator-controlled trial that included primarily patients with schizophrenia, the mean increase from baseline of fasting glucose was 2.4 mg/dL. Pediatric Patients: Data from the short-term, placebo-controlled trial in pediatric patients with bipolar I disorder are shown inTable 2. TABLE 2 :Changes in Fasting Glucose in Pediatric Subjects
N* = Number of subjects who had assessments at both Baseline and Endpoint. Dyslipidemia Atypical antipsychotics cause adverse alterations in lipids. Before or soon after initiation of antipsychotic medication, obtain a fasting lipid profile at baseline and monitor periodically during treatment. Adult Patients: Pooled data from the short-term, placebo-controlled schizophrenia and bipolar mania trials are presented inTable 3 . TABLE 3 : Changes in Lipids in Adult Patients
N* = Number of subjects who had assessments at both Baseline and Endpoint. §Includes subjects treated with flexible dose of asenapine 5 or 10 mg twice daily (N=90). †Includes patients treated with flexible dose of asenapine 5 or 10 mg twice daily (N=379) In short-term schizophrenia trials, the proportion of patients with total cholesterol elevations ≥240 mg/dL (at Endpoint) was 8.3% for asenapine-treated patients versus 7% for placebo-treated patients. The proportion of patients with elevations in triglycerides ≥200 mg/dL (at Endpoint) was 13.2% for asenapine-treated patients versus 10.5% for placebo-treated patients. In short-term, placebo-controlled bipolar mania trials, the proportion of patients with total cholesterol elevations ≥240 mg/dL (at Endpoint) was 7.8% for asenapine-treated patients versus 7.9% for placebo-treated patients. The proportion of patients with elevations in triglycerides ≥200 mg/dL (at Endpoint) was 13.1% for asenapine-treated patients versus 8.6% for placebo-treated patients. Pediatric Patients: Data from the short-term, placebo-controlled bipolar mania trial are presented inTable 4. TABLE 4 : Changes in Fasting Lipids in Pediatric Subjects
N* = Number of patients who had assessments at both Baseline and Endpoint Weight Gain Weight gain has been observed in patients treated with atypical antipsychotics, including asenapine. Monitor weight at baseline and frequently thereafter. Adult Patients: Pooled data on mean changes in body weight and the proportion of subjects meeting a weight gain criterion of ≥7% of body weight from the short-term, placebo-controlled schizophrenia and bipolar mania trials are presented inTable 5 . Table 5 : Change in Body Weight in Adult Patients from Baseline
N* = Number of subjects who had assessments at both Baseline and Endpoint. §Includes subjects treated with flexible dose of asenapine 5 or 10 mg twice daily (N=90). †Includes patients treated with flexible dose of asenapine 5 or 10 mg twice daily (N=379). Adult Patients: In a 52-week, double-blind, comparator-controlled adult trial that included primarily patients with schizophrenia, the mean weight gain from baseline was 0.9 kg. The proportion of patients with a ≥7% increase in body weight (at Endpoint) was 14.7%.Table 6 provides the mean weight change from baseline and the proportion of patients with a weight gain of ≥7% categorized by Body Mass Index (BMI) at baseline.T able 6 : Weight Change Results Categorized by BMI at Baseline: Comparator-Controlled 52-Week Study in Adults with Schizophrenia
Pediatric Patients: Data on mean changes in body weight and the proportion of pediatric patients meeting a weight gain criterion of ≥7% of body weight from the short-term, placebo-controlled bipolar mania trial are presented inTable 7 . To adjust for normal growth, z-scores were derived (measured in standard deviations [SD]), which normalize for the natural growth of pediatric patients by comparisons to age- and sex-matched population standards.The distance of a z-score from 0 represents the distance of a percentile from the median, measured in standard deviations (SD). After adjusting for age and sex, the mean change from baseline to endpoint in weight z-score for asenapine 2.5 mg, 5 mg, and 10 mg twice daily, was 0.11, 0.08 and 0.09 SD versus 0.02 SD for placebo, respectively. When treating pediatric patients, weight gain should be monitored and assessed against that expected for normal growth. Table 7: Change in Body Weight in Pediatric Subjects from Baseline
N* = Number of subjects who had assessments at both Baseline and Endpoint. 5.000000000000000e+00 7 Orthostatic Hypotension, Syncope, and Other Hemodynamic Effects Atypical antipsychotics cause orthostatic hypotension and syncope. Generally, the risk is greatest during initial dose titration and when increasing the dose. In short-term schizophrenia adult trials, syncope was reported in 0.2% (1/572) of patients treated with therapeutic doses (5 mg or 10 mg twice daily) of asenapine, compared to 0.3% (1/378) of patients treated with placebo. In short-term bipolar mania adult trials, syncope was reported in 0.2% (1/620) of patients treated with therapeutic doses (5 mg or 10 mg twice daily) of asenapine, compared to 0% (0/329) of patients treated with placebo. During adult pre-marketing clinical trials with asenapine, including long-term trials without comparison to placebo, syncope was reported in 0.6% (11/1953) of patients treated with asenapine. In a 3-week, bipolar mania pediatric trial, syncope was reported in 1% (1/104) of patients treated with asenapine 2.5 mg twice daily, 1% (1/99) of patients treated with asenapine 5 mg twice daily, and 0% (0/99) for patients treated with asenapine 10 mg twice daily compared to 0% (0/101) for patients treated with placebo. Orthostatic vital signs should be monitored in patients who are vulnerable to hypotension (elderly patients, patients with dehydration, hypovolemia, concomitant treatment with antihypertensive medications, patients with known cardiovascular disease (history of myocardial infarction or ischemic heart disease, heart failure, or conduction abnormalities), and patients with cerebrovascular disease. Asenapine should be used cautiously when treating patients who receive treatment with other drugs that can induce hypotension, bradycardia, respiratory or central nervous system depression [see Drug Interactions ( 7.1 )]. Monitoring of orthostatic vital signs should be considered in all such patients, and a dose reduction should be considered if hypotension occurs.5.000000000000000e+00 9 Leukopenia, Neutropenia, and Agranulocytosis In clinical trial and postmarketing experience, leukopenia and neutropenia have been reported temporally related to antipsychotic agents, including asenapine. Agranulocytosis (including fatal cases) has been reported with other agents in the class. Possible risk factors for leukopenia/neutropenia include pre-existing low white blood cell count (WBC) or absolute neutrophil count (ANC) and history of drug induced leukopenia/neutropenia. In patients with a pre-existing low WBC or ANC or a history of drug-induced leukopenia or neutropenia, perform a complete blood count (CBC) during the first few months of therapy. In such patients, consider discontinuation of asenapine at the first sign of a clinically significant decline in WBC in the absence of other causative factors. Monitor patients with clinically significant neutropenia for fever or other symptoms or signs of infection and treat promptly if such symptoms or signs occur. Discontinue asenapine in patients with severe neutropenia (absolute neutrophil count <1000/mm3) and follow their WBC until recovery. 5.000000000000000e+00 10 QT Prolongation The effects of asenapine on the QT/QTc interval were evaluated in a dedicated adult QT study. This trial involved asenapine doses of 5 mg, 10 mg, 15 mg, and 20 mg twice daily, and placebo, and was conducted in 151 clinically stable patients with schizophrenia, with electrocardiographic assessments throughout the dosing interval at baseline and steady state. At these doses, asenapine was associated with increases in QTc interval ranging from 2 to 5 msec compared to placebo. No patients treated with asenapine experienced QTc increases ≥60 msec from baseline measurements, nor did any patient experience a QTc of ≥500 msec. Electrocardiogram (ECG) measurements were taken at various time points during the asenapine clinical trial program (5 mg or 10 mg twice daily doses). Post-baseline QT prolongations exceeding 500 msec were reported at comparable rates for asenapine and placebo in these short-term trials. There were no reports of Torsade de Pointes or any other adverse reactions associated with delayed ventricular repolarization. The use of asenapine should be avoided in combination with other drugs known to prolong QTc including Class 1A antiarrhythmics (e.g., quinidine, procainamide) or Class 3 antiarrhythmics (e.g., amiodarone, sotalol), antipsychotic medications (e.g., ziprasidone, chlorpromazine, thioridazine), and antibiotics (e.g., gatifloxacin, moxifloxacin). Asenapine should also be avoided in patients with a history of cardiac arrhythmias and in other circumstances that may increase the risk of the occurrence of torsade de pointes and/or sudden death in association with the use of drugs that prolong the QTc interval, including bradycardia; hypokalemia or hypomagnesemia; and presence of congenital prolongation of the QT interval. 5.1 1 Hyperprolactinemia Like other drugs that antagonize dopamine D2 receptors, asenapine can elevate prolactin levels, and the elevation can persist during chronic administration. Hyperprolactinemia may suppress hypothalamic GnRH, resulting in reduced pituitary gonadotropin secretion. This, in turn, may inhibit reproductive function by impairing gonadal steroidogenesis in both female and male patients. Galactorrhea, amenorrhea, gynecomastia, and impotence have been reported in patients receiving prolactin-elevating compounds. Long-standing hyperprolactinemia when associated with hypogonadism may lead to decreased bone density in both female and male subjects. In asenapine adult pre-marketing clinical trials, the incidences of adverse events related to abnormal prolactin levels were 0.4% versus 0% for placebo. In a 3-week, bipolar mania pediatric trial, the incidence of adverse events related to abnormal prolactin levels were 0% in the asenapine 2.5 mg twice daily treatment group, 2% in the asenapine 5 mg twice daily treatment group, and 1% in the asenapine 10 mg twice daily treatment group versus to 1% for patients treated with placebo [see Adverse Reactions ( 6.1 )]. Tissue culture experiments indicate that approximately one-third of human breast cancers are prolactin-dependent in vitro , a factor of potential importance if the prescription of these drugs is considered in a patient with previously-detected breast cancer. Neither clinical studies nor epidemiologic studies conducted to date have shown an association between chronic administration of this class of drugs and tumorigenesis in humans, but the available evidence is too limited to be conclusive.5.1 2 Seizures Seizures were reported in 0% and 0.3% (0/572, 1/379) of adult patients treated with doses of 5 mg and 10 mg twice daily of asenapine, respectively, compared to 0% (0/503, 0/203) of patients treated with placebo in pre-marketing short-term schizophrenia and bipolar mania trials, respectively. During adult pre-marketing clinical trials with asenapine, including long-term trials without comparison to placebo, seizures were reported in 0.3% (5/1953) of patients treated with asenapine. There were no reports of seizures in pediatric patients treated with asenapine in a 3-week-term, bipolar mania trial. As with other antipsychotic drugs, asenapine should be used with caution in patients with a history of seizures or with conditions that potentially lower the seizure threshold. Conditions that lower the seizure threshold may be more prevalent in patients 65 years or older. 5.1 3 Potential for Cognitive and Motor Impairment Somnolence was reported in patients treated with asenapine. It was usually transient with the highest incidence reported during the first week of treatment. In short-term, fixed-dose, placebo-controlled schizophrenia adult trials, somnolence was reported in 15% (41/274) of patients on asenapine 5 mg twice daily and in 13% (26/208) of patients on asenapine 10 mg twice daily compared to 7% (26/378) of placebo patients. In short-term, placebo-controlled bipolar mania adult trials of therapeutic doses (5-10 mg twice daily), somnolence was reported in 23% (145/620) of patients on asenapine compared to 5% (18/329) of placebo patients. In the 3-week fixed-dose study, somnolence occurred at a lower rate in the 5mg twice daily dose 20% (24/122) versus the 10mg twice daily dose 26% (31/119) compared to 4% (5/126) in placebo patients. During adult pre-marketing clinical trials with asenapine, including long-term trials without comparison to placebo, somnolence was reported in 18% (358/1953) of patients treated with asenapine. Somnolence led to discontinuation in 0.6% (12/1953) of patients in short-term, placebo-controlled trials. In a 3-week, placebo-controlled, bipolar I pediatric trial, the incidence of somnolence (including sedation and hypersomnia) for placebo, asenapine 2.5 mg twice daily, 5 mg twice daily, and 10 mg twice daily, was 12% (12/101), 46% (48/104), 53% (52/99), and 49% (49/99), respectively. Somnolence led to discontinuation in 0%, 3%, 1%, and 2% of patients treated with placebo, and asenapine 2.5 mg twice daily, 5 mg twice daily, and 10 mg twice daily, respectively. Patients should be cautioned about operating hazardous machinery, including motor vehicles, until they are reasonably certain that asenapine therapy does not affect them adversely. 5.1 4 Body Temperature Regulation Atypical antipsychotics may disrupt the body’s ability to reduce core body temperature. In the pre-marketing short-term placebo-controlled trials for both schizophrenia and acute bipolar I disorder, the incidence of adverse reactions suggestive of body temperature increases was low (≤1%) and comparable to placebo (0%). During pre-marketing clinical trials with asenapine, including long-term trials without comparison to placebo, the incidence of adverse reactions suggestive of body temperature increases (pyrexia and feeling hot) was ≤1%. Strenuous exercise, exposure to extreme heat, dehydration, and anticholinergic medications may contribute to an elevation in core body temperature; use asenapine with caution in patient who may experience these conditions. 5.1 5 Dysphagia Esophageal dysmotility and aspiration have been associated with antipsychotic drug use. Dysphagia has been reported with asenapine. Asenapine and other antipsychotic drugs should be used cautiously in patients at risk for aspiration. | 01/2017 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Warnings and Precautions (5.8 Falls Asenapine may cause somnolence, postural hypotension, motor and sensory instability, which may lead to falls and, consequently, fractures or other injuries. For patients with diseases, conditions, or medications that could exacerbate these effects, complete fall risk assessments when initiating antipsychotic treatment and recurrently for patients on long-term antipsychotic therapy. | 02/2017 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Asenapine sublingual Tablets are indicated for:
• Schizophrenia in adults
The efficacy of asenapine in the treatment of schizophrenia in adults was evaluated in three fixed-dose, short-term (6 week), randomized, double-blind, placebo-controlled, and active-controlled (haloperidol, risperidone, and olanzapine) trials of adult patients who met DSM-IV criteria for schizophrenia and were having an acute exacerbation of their schizophrenic illness. In two of the three trials asenapine demonstrated superior efficacy to placebo. In a third trial, asenapine could not be distinguished from placebo; however, an active control in that trial was superior to placebo.
In the two positive trials for asenapine, the primary efficacy rating scale was the Positive and Negative Syndrome Scale (PANSS). The PANSS is a 30 item scale that measures positive symptoms of schizophrenia (7 items), negative symptoms of schizophrenia (7 items), and general psychopathology (16 items), each rated on a scale of 1 (absent) to 7 (extreme); total PANSS scores range from 30 to 210. The primary endpoint was change from baseline to endpoint on the PANSS total score. The results of the asenapine trials in schizophrenia follow:
In trial 1, a 6-week trial (n=174), comparing asenapine (5 mg twice daily) to placebo, asenapine 5 mg twice daily was statistically superior to placebo on the PANSS total score (Trial 1 in Table 13).
In trial 2, a 6-week trial (n=448), comparing two fixed doses of asenapine (5 mg and 10 mg twice daily) to placebo, asenapine 5 mg twice daily was statistically superior to placebo on the PANSS total score. Asenapine 10 mg twice daily showed no added benefit compared to 5 mg twice daily and was not significantly different from placebo (Trial 2 in Table 13).
An examination of population subgroups did not reveal any clear evidence of differential responsiveness on the basis of age, sex or race.
Table 1 3 : Short-Term Schizophrenia Trials Establishing Efficacy in Adults | ||||
Trial Number | Treatment Group | Primary Efficacy Measure: PANSS Total Score | ||
Mean Baseline Score (SD) | LS Mean Change from Baseline (SE) | Placebo-subtracted Difference a (95% CI) | ||
| Trial 1 | asenapine 5 mg* twice daily | 96.5 (16.4) | -14.4 (2.6) | -9.7 (-17.6, -1.8) |
| Placebo | 92.4 (14.9) | -4.6 (2.5) | -- | |
| Trial 2 | asenapine 5 mg* twice daily | 89.2 (12.0) | -16.2 (1.7) | -5.5 (-10.7, -0.2) |
| asenapine 10 mg twice daily | 89.1 (12.9) | -14.9 (1.7) | -4.1 (-9.4, 1.2) | |
| Placebo | 88.9 (11.7) | -10.7 (1.6) | -- | |
| SD: standard deviation; SE: standard error; LS Mean: least-squares mean; CI: confidence interval, not adjusted for multiple comparisons. aDifference (drug minus placebo) in least-squares mean change from baseline. * Doses that are demonstrated to be effective. | ||||
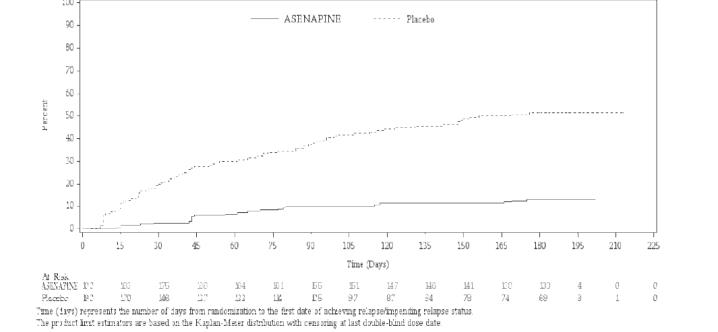
Maintenance of efficacy has been demonstrated in a placebo-controlled, double-blind, multicenter, flexible dose (5 mg or 10 mg twice daily based on tolerability) clinical trial with a randomized withdrawal design. All patients were initially administered 5 mg twice daily for 1 week and then titrated up to 10 mg twice daily. A total of 700 patients entered open-label treatment with asenapine for a period of 26 weeks. Of these, a total of 386 patients who met pre-specified criteria for continued stability (mean length of stabilization was 22 weeks) were randomized to a double-blind, placebo-controlled, randomized withdrawal phase. Asenapine was statistically superior to placebo in time to relapse or impending relapse defined as increase in PANSS ≥20% from baseline and a Clinical Global Impression–Severity of Illness (CGI-S) score ≥4 (at least 2 days within 1 week) or PANSS score ≥5 on “hostility” or “uncooperativeness” items and CGI-S score ≥4 (≥2 days within a week), or PANSS score ≥5 on any two of the following items: “unusual thought content”, “conceptual disorganization”, or “hallucinatory behavior” items, and CGI-S score ≥4 (≥2 days within 1 week) or investigator judgment of worsening symptoms or increased risk of violence to self (including suicide) or other persons. The Kaplan-Meier curves of the time to relapse or impending relapse during the double-blind, placebo-controlled, randomized withdrawal phase of this trial for asenapine and placebo are shown in Figure 4.
The primary rating instrument used for assessing manic symptoms in these trials was the Young Mania Rating Scale (YMRS), an 11-item clinician-rated scale traditionally used to assess the degree of manic symptomatology in a range from 0 (no manic features) to 60 (maximum score). Patients were also assessed on the Clinical Global Impression – Bipolar (CGI-BP) scale. In both trials, all patients randomized to asenapine were initially administered 10 mg twice daily, and the dose could be adjusted within the dose range of 5 to 10 mg twice daily from Day 2 onward based on efficacy and tolerability. Ninety percent of patients remained on the 10 mg twice daily dose. Asenapine was statistically superior to placebo on the YMRS total score and the CGI-BP Severity of Illness score (mania) in both studies (Trials 1 and 2 in Table 14).
In another 3-week, randomized, double-blind, placebo-controlled trial (n=359), comparing two fixed doses of asenapine (5 mg and 10 mg twice daily) to placebo, both doses were statistically superior to placebo on the YMRS total score and CGI-BP Severity of Illness overall score. (Trial 3 in Table 14).
An examination of subgroups did not reveal any clear evidence of differential responsiveness on the basis of age, sex, or race.
Maintenance of efficacy has been demonstrated in a placebo-controlled, double-blind, multicenter, flexible dose (5 mg or 10 mg twice daily based on tolerability) clinical trial with a randomized withdrawal design. All patients were initially administered 5 or 10 mg twice daily, and the option to titrate down to 5 mg twice daily was provided based on tolerability. A total of 549 patients entered open-label treatment with asenapine for a period of 12 to 16 weeks. Of these, a total of 252 patients who met pre-specified criteria for continued stability were randomized to and treated in a double-blind, placebo-controlled, randomized withdrawal phase. Asenapine was statistically superior to placebo in time to relapse defined as 1) YMRS or MADRS score ≥ 16; 2) requirement or initiation of any non-study medication to treat mixed, manic, or depressive symptoms, including an antipsychotic, antidepressant, or mood-stabilizing agent; 3) requirement or initiation of psychiatric hospitalization; 4) investigator judgment to discontinue the study due to a mood event. The Kaplan-Meier curves of the time to relapse during the double-blind, placebo-controlled, randomized withdrawal phase of this trial for asenapine and placebo are shown in Figure 5.
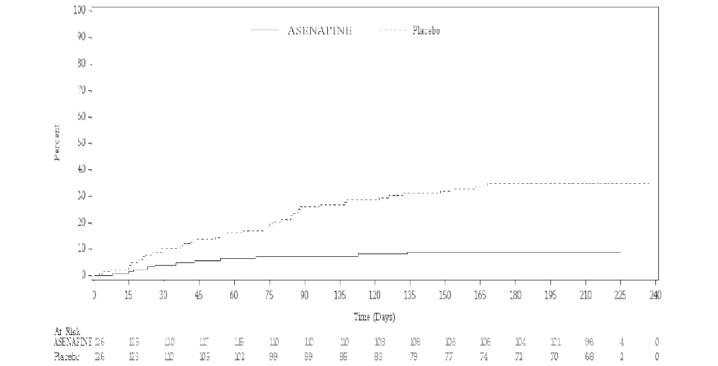
Time (days) represents the number of days from randomization in the double-blind period to the first date of achieving relapse status.
The product limit estimators are based on the Kaplan-Meier distribution with censoring at last contact date.
Asenapine was statistically superior to placebo in improving YMRS total score and the CGI-BP Severity of Illness overall score as measured by the change from baseline to week 3 (Trial 3 Pediatric in Table 14). An examination of subgroups did not reveal any clear evidence of differential responsiveness on the basis of age, sex, and race.
Table 1 4 : Acute Bipolar I Trials Establishing Efficacy in Adults and Pediatric Patients 10 to 17 Years | ||||
Study Number | Treatment Group | Primary Efficacy Measure: YMRS Total Score | ||
Mean Baseline Score (SD) | LS Mean Change from Baseline (SE) | Placebo-subtracted Difference a (95% CI) | ||
| Trial 1 | asenapine 5-10 mg* twice daily | 29.4 (6.7) | -11.5 (0.8) | -3.7 (-6.6, -0.7) |
| Placebo | 28.3 (6.3) | -7.8 (1.1) | -- | |
| Trial 2 | asenapine 5-10 mg* twice daily | 28.3 (5.5) | -10.8 (0.8) | -5.3 (-8.0, -2.5) |
| Placebo | 29.0 (6.1) | -5.5 (1.0) | -- | |
| Trial 3 | asenapine 5 mg* twice daily asenapine 10 mg* twice daily Placebo | 29.7 (5.9) 30.2 (5.4) 30.0 (5.6) | -14.4 (1.0) -14.9 (1.0) -10.9 (1.0) | -3.5 (-6.3, -0.7) -4.0 (-6.9, -1.2) -- |
| Trial 4 (Pediatric 10 to17 years) | asenapine 2.5 mg* twice daily | 29.5 (5.7) | -12.8 (0.8) | -3.2 (-5.6, -0.8) |
| asenapine 5 mg* twice daily | 30.4 (5.9) | -14.9 (0.8) | -5.3 (-7.7, -2.9) | |
| asenapine 10 mg* twice daily | 30.1 (5.7) | -15.8 (0.9) | -6.2 (-8.6, -3.8) | |
| Placebo | 30.1 (5.7) | - 9.6 (0.9) | -- | |
| Trial 5 (Adjunctive) | asenapine 5-10 mg* twice daily + lithium/ Valproate | 28.0 (5.6) | -10.3 (0.8) | -2.4 (-4.4, -0.3) |
| Lithium/Valproate | 28.2 (5.8) | -7.9 (0.8) | -- | |
| SD: standard deviation; SE: standard error; LS Mean: least-squares mean; CI: confidence interval, not adjusted for multiple comparisons. aDifference (drug minus placebo) in least-squares mean change from baseline. * Doses that are demonstrated to be effective. | ||||
Starting Dose | Recommended Dose | Maximum Dose | |
Schizophrenia – acute treatment in adults (2.2 Schizophrenia The recommended dose of asenapine is 5 mg given twice daily. In short-term controlled trials, there was no suggestion of added benefit with a 10 mg twice daily dose, but there was a clear increase in certain adverse reactions. If tolerated, daily dosage can be increased to 10 mg twice daily after one week. The safety of doses above 10 mg twice daily has not been evaluated in clinical studies [see Clinical Studies ( 14.1 )] . | 5 mg sublingually twice daily | 5 mg sublingually twice daily | 10 mg sublingually twice daily |
Schizophrenia – maintenance treatment in adults (2.2 Schizophrenia The recommended dose of asenapine is 5 mg given twice daily. In short-term controlled trials, there was no suggestion of added benefit with a 10 mg twice daily dose, but there was a clear increase in certain adverse reactions. If tolerated, daily dosage can be increased to 10 mg twice daily after one week. The safety of doses above 10 mg twice daily has not been evaluated in clinical studies [see Clinical Studies ( 14.1 )] . | 5 mg sublingually twice daily | 5-10 mg sublingually twice daily | 10 mg sublingually twice daily |
Bipolar mania – adults: acute and maintenance monotherapy (2.3 Bipolar I Disorder Acute Treatment of Manic or Mixed Episodes : Monotherapy in Adults : The recommended starting and treatment dose of asenapine is 5 mg to 10 mg twice daily. The safety of doses above 10 mg twice daily has not been evaluated in clinical trials[see Clinical Studies ( 14.2 )] . Monotherapy in Pediatric P atients: The recommended dose of asenapine is 2.5 mg to 10 mg twice daily in pediatric patients 10 to 17 years of age, and dose may be adjusted for individual response and tolerability. The starting dose of asenapine is 2.5 mg twice daily. After 3 days, the dose can be increased to 5 mg twice daily, and from 5 mg to 10 mg twice daily after 3 additional days. Pediatric patients aged 10 to 17 years appear to be more sensitive to dystonia with initial dosing with asenapine when the recommended escalation schedule is not followed[see Use in Specific Populations ( 8.4 )] . The safety of doses greater than 10 mg twice daily has not been evaluated in clinical trials[see Use in Specific Populations ( 8.4 ) and Clinical Pharmacology ( 12.3 )] .Adjunctive Therapy in Adults : The recommended starting dose of asenapine is 5 mg twice daily when administered as adjunctive therapy with either lithium or valproate. Depending on the clinical response and tolerability in the individual patient, the dose can be increased to 10 mg twice daily. The safety of doses above 10 mg twice daily as adjunctive therapy with lithium or valproate has not been evaluated in clinical trials.For patients on asenapine, whether used as monotherapy or as adjunctive therapy with lithium or valproate, it is generally recommended that responding patients continue treatment beyond the acute episode. Maintenance Treatment of Bipolar I Disorder: Monotherapy in Adults: Continue on the asenapine dose that the patient received during stabilization (5 mg to 10 mg twice daily). Depending on the clinical response and tolerability in the individual patient, a dose of 10 mg twice daily can be decreased to 5 mg twice daily. The safety of doses above 10 mg twice daily has not been evaluated in clinical trials [see Clinical Studies ( 14.2 )]. | 5-10 mg sublingually twice daily | 5-10 mg sublingually twice daily | 10 mg sublingually twice daily |
Bipolar mania –pediatric patients (10 to 17 years): monotherapy (2.3 Bipolar I Disorder Acute Treatment of Manic or Mixed Episodes : Monotherapy in Adults : The recommended starting and treatment dose of asenapine is 5 mg to 10 mg twice daily. The safety of doses above 10 mg twice daily has not been evaluated in clinical trials[see Clinical Studies ( 14.2 )] . Monotherapy in Pediatric P atients: The recommended dose of asenapine is 2.5 mg to 10 mg twice daily in pediatric patients 10 to 17 years of age, and dose may be adjusted for individual response and tolerability. The starting dose of asenapine is 2.5 mg twice daily. After 3 days, the dose can be increased to 5 mg twice daily, and from 5 mg to 10 mg twice daily after 3 additional days. Pediatric patients aged 10 to 17 years appear to be more sensitive to dystonia with initial dosing with asenapine when the recommended escalation schedule is not followed[see Use in Specific Populations ( 8.4 )] . The safety of doses greater than 10 mg twice daily has not been evaluated in clinical trials[see Use in Specific Populations ( 8.4 ) and Clinical Pharmacology ( 12.3 )] .Adjunctive Therapy in Adults : The recommended starting dose of asenapine is 5 mg twice daily when administered as adjunctive therapy with either lithium or valproate. Depending on the clinical response and tolerability in the individual patient, the dose can be increased to 10 mg twice daily. The safety of doses above 10 mg twice daily as adjunctive therapy with lithium or valproate has not been evaluated in clinical trials.For patients on asenapine, whether used as monotherapy or as adjunctive therapy with lithium or valproate, it is generally recommended that responding patients continue treatment beyond the acute episode. Maintenance Treatment of Bipolar I Disorder: Monotherapy in Adults: Continue on the asenapine dose that the patient received during stabilization (5 mg to 10 mg twice daily). Depending on the clinical response and tolerability in the individual patient, a dose of 10 mg twice daily can be decreased to 5 mg twice daily. The safety of doses above 10 mg twice daily has not been evaluated in clinical trials [see Clinical Studies ( 14.2 )]. | 2.5 mg sublingually twice daily | 2.5-10 mg sublingually twice daily | 10 mg sublingually twice daily |
Bipolar mania – adults: as an adjunct to lithium or valproate (2.3 Bipolar I Disorder Acute Treatment of Manic or Mixed Episodes : Monotherapy in Adults : The recommended starting and treatment dose of asenapine is 5 mg to 10 mg twice daily. The safety of doses above 10 mg twice daily has not been evaluated in clinical trials[see Clinical Studies ( 14.2 )] . Monotherapy in Pediatric P atients: The recommended dose of asenapine is 2.5 mg to 10 mg twice daily in pediatric patients 10 to 17 years of age, and dose may be adjusted for individual response and tolerability. The starting dose of asenapine is 2.5 mg twice daily. After 3 days, the dose can be increased to 5 mg twice daily, and from 5 mg to 10 mg twice daily after 3 additional days. Pediatric patients aged 10 to 17 years appear to be more sensitive to dystonia with initial dosing with asenapine when the recommended escalation schedule is not followed[see Use in Specific Populations ( 8.4 )] . The safety of doses greater than 10 mg twice daily has not been evaluated in clinical trials[see Use in Specific Populations ( 8.4 ) and Clinical Pharmacology ( 12.3 )] .Adjunctive Therapy in Adults : The recommended starting dose of asenapine is 5 mg twice daily when administered as adjunctive therapy with either lithium or valproate. Depending on the clinical response and tolerability in the individual patient, the dose can be increased to 10 mg twice daily. The safety of doses above 10 mg twice daily as adjunctive therapy with lithium or valproate has not been evaluated in clinical trials.For patients on asenapine, whether used as monotherapy or as adjunctive therapy with lithium or valproate, it is generally recommended that responding patients continue treatment beyond the acute episode. Maintenance Treatment of Bipolar I Disorder: Monotherapy in Adults: Continue on the asenapine dose that the patient received during stabilization (5 mg to 10 mg twice daily). Depending on the clinical response and tolerability in the individual patient, a dose of 10 mg twice daily can be decreased to 5 mg twice daily. The safety of doses above 10 mg twice daily has not been evaluated in clinical trials [see Clinical Studies ( 14.2 )]. | 5 mg sublingually twice daily | 5-10 mg sublingually twice daily | 10 mg sublingually twice daily |
- Do not swallow tablet. Asenapine sublingual tablets should be placed under the tongue and left to dissolve completely. The tablet will dissolve in saliva within seconds. Eating and drinking should be avoided for 10 minutes after administration. (,2.1Administration Instructions
Asenapine is a sublingual tablet. To ensure optimal absorption, patients should be instructed to place the tablet under the tongue and allow it to dissolve completely. The tablet will dissolve in saliva within seconds. Asenapine sublingual tablets should not be split, crushed, chewed, or swallowed
[seeClinical Pharmacology(12.3)]. Patients should be instructed to not eat or drink for 10 minutes after administration[see Clinical Pharmacology (12.3)].)17PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Instructions for Use).Dosage and AdministrationCounsel patients on proper sublingual administration of asenapine sublingual tablets and advise them to read the FDA-approved patient labeling . When initiating treatment with asenapine sublingual tablets, provide dosage escalation instructions
[see Dosage and Administration (2)].Hypersensitivity ReactionsCounsel patients on the signs and symptoms of a serious allergic reaction (e.g., difficulty breathing, itching, swelling of the face, tongue or throat, feeling lightheaded etc.) and to seek immediate emergency assistance if they develop any of these signs and symptoms
[seeContraindications (4),Warnings and Precautions (5.6) and Adverse Reactions (6)].Application Site ReactionsInform patients that application site reactions, primarily in the sublingual area, including oral ulcers, blisters, peeling/sloughing and inflammation have been reported. Instruct patients to monitor for these reactions
[see Adverse Reactions (6.2)].Inform patients that numbness or tingling of the mouth or throat may occur directly after administration of asenapine sublingual tablets and usually resolves within 1 hour[see Adverse Reactions (6.1)].Neuroleptic Malignant SyndromeCounsel patients about a potentially fatal symptom complex sometimes referred to as Neuroleptic Malignant Syndrome (NMS) that has been reported in association with administration of antipsychotic drugs. Patients should contact their healthcare provider or report to the emergency room if they experience the following signs and symptoms of NMS, including hyperpyrexia, muscle rigidity, altered mental status, and evidence of autonomic instability (irregular pulse or blood pressure, tachycardia, diaphoresis, and cardiac dysrhythmia)
[see Warnings and Precautions (5.3)].Tardive DyskinesiaCounsel patients on the signs and symptoms of tardive dyskinesia and to contact their healthcare provider if these abnormal movements occur [
see Warnings and Precautions (5.4)].Metabolic Changes (Hyperglycemia and Diabetes Mellitus,Dyslipidemia,and Weight Gain)Educate patients about the risk of metabolic changes, how to recognize symptoms of hyperglycemia (high blood sugar) and diabetes mellitus, and the need for specific monitoring, including blood glucose, lipids, and weight
[see Warnings and Precautions (5.5)].Orthostatic HypotensionEducate patients about the risk of orthostatic hypotension (symptoms include feeling dizzy or lightheaded upon standing) especially early in treatment, and also at times of re-initiating treatment or increases in dose
[see Warnings and Precautions (5.7)].Leukopenia/NeutropeniaAdvise patients with a pre-existing low WBC or a history of drug induced leukopenia/neutropenia they should have their CBC monitored while taking asenapine sublingual tablets
[see Warnings and Precautions(5.9)].HyperprolactinemiaCounsel patients on the signs and symptoms of hyperprolactinemia and to contact their healthcare provider if these abnormalities occur [
see Warnings and Precautions (5.11)].Interference with Cognitive and Motor PerformanceCaution patients about performing activities requiring mental alertness, such as operating hazardous machinery or operating a motor vehicle, until they are reasonably certain that asenapine therapy does not affect them adversely
[see Warnings and Precautions (5.13)].Heat Exposure and DehydrationCounsel patients regarding appropriate care in avoiding overheating and dehydration
[see Warnings and Precautions (5.14)].Concomitant MedicationsAdvise patients to inform their health care provider if they are taking, or plan to take, any prescription or over-the-counter medications since there is a potential for interactions
[see Drug Interactions (7.1)].PregnancyAdvise patients that asenapine sublingual tablets may cause fetal harm as well as extrapyramidal and/or withdrawal symptoms in a neonate. Advise patients to notify their healthcare provider with a known or suspected pregnancy
[see Use in Specific Populations (8.1)].Pregnancy RegistryAdvise patients that there is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to asenapine during pregnancy
[see Use in Specific Populations (8.1)].Distributed by:
Greenstone, LLC
Peapack, NJ 07977
© 2019 Allergan. All rights reserved.
- Asenapine 2.5 mg tablets, black cherry flavor, are round, white to off-white sublingual tablets, with a hexagon on one side.
- Asenapine 5 mg tablets, black cherry flavor, are round, white to off-white sublingual tablets, with “5” on one side within a circle.
- Asenapine 10 mg tablets, black cherry flavor, are round, white to off-white sublingual tablets, with “10” on one side within a circle.
- Pregnancy:May cause extrapyramidal and/or withdrawal symptoms in neonates with third trimester exposure. ()8.1PregnancyPregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to asenapine during pregnancy. For more information contact the National Pregnancy Registry for Atypical Antipsychotics at 1-866-961-2388 or visit
http://womensmentalhealth.org/clinical-and-research-programs/pregnancyregistry/.Risk SummaryNeonates exposed to antipsychotic drugs during the third trimester of pregnancy are at risk for extrapyramidal and/or withdrawal symptoms. Studies have not been conducted with asenapine in pregnant women. There are no available human data informing the drug-associated risk. The background risk of major birth defects and miscarriage for the indicated populations are unknown. However, the background risk in the U.S. general population of major birth defects is 2-4% and of miscarriage is 15-20% of clinically recognized pregnancies. No teratogenicity was observed in animal reproduction studies with intravenous administration of asenapine to rats and rabbits during organogenesis at doses 0.7 and 0.4 times, respectively, the maximum recommended human dose (MRHD) of 10 mg sublingually twice daily. In a pre-and post-natal study in rats, intravenous administration of asenapine at doses up to 0.7 times the MRHD produced increases in post-implantation loss and early pup deaths, and decreases in subsequent pup survival and weight gain
[see Data].Advise pregnant women of the potential risk to a fetus.Clinical ConsiderationsFetal/Neonatal Adverse ReactionsExtrapyramidal and/or withdrawal symptoms, including agitation, hypertonia, hypotonia, tremor, somnolence, respiratory distress and feeding disorder have been reported in neonates who were exposed to antipsychotic drugs during the third trimester of pregnancy. These symptoms have varied in severity. Some neonates recovered within hours or days without specific treatment; others required prolonged hospitalization. Monitor neonates for extrapyramidal and/or withdrawal symptoms and manage symptoms appropriately.
DataAnimal DataIn animal studies, asenapine increased post-implantation loss and decreased pup weight and survival at doses similar to or less than recommended clinical doses. In these studies there was no increase in the incidence of structural abnormalities caused by asenapine.
Asenapine was not teratogenic in reproduction studies in rats and rabbits at intravenous doses up to 1.5 mg/kg in rats and 0.44 mg/kg in rabbits administered during organogenesis. These doses are 0.7 and 0.4 times, respectively, the maximum recommended human dose (MRHD) of 10 mg twice daily given sublingually on a mg/m2basis. Plasma levels of asenapine were measured in the rabbit study, and the area under the curve (AUC) at the highest dose tested was 2 times that in humans receiving the MRHD.
In a study in which rats were treated from day 6 of gestation through day 21 postpartum with intravenous doses of asenapine of 0.3, 0.9, and 1.5 mg/kg/day (0.15, 0.4, and 0.7 times the MRHD of 10 mg twice daily given sublingually on a mg/m2basis), increases in post-implantation loss and early pup deaths were seen at all doses, and decreases in subsequent pup survival and weight gain were seen at the two higher doses. A cross-fostering study indicated that the decreases in pup survival were largely due to prenatal drug effects. Increases in post-implantation loss and decreases in pup weight and survival were also seen when pregnant rats were dosed orally with asenapine.
- Pediatric Use:Safety and efficacy in the treatment of bipolar I disorder in patients less than 10 years of age, and patients with schizophrenia ages less than 12 years have not been evaluated. ()8.4Pediatric Use
Safety and efficacy of asenapine in pediatric patients below the age of 10 years of age have not been evaluated.
BipolarIDisorderThe safety and efficacy of asenapine as monotherapy in the treatment of bipolar I disorder were established in a 3-week, placebo-controlled, double-blind trial of 403 pediatric patients 10 to 17 years of age, of whom 302 patients received asenapine at fixed doses ranging from 2.5 mg to 10 mg twice daily
[see Dosage and Administration (2.3), Adverse Reactions (6.1), Clinical Pharmacology (12.3), and Clinical Studies (14.2)]. In a Phase 1 study, pediatric patients aged 10 to 17 years appeared to be more sensitive to dystonia with initial dosing with asenapine when the recommended dose escalation schedule was not followed. Similar safety findings were reported from a 50-week, open-label, uncontrolled safety trial in pediatric patients with bipolar I disorder treated with asenapine monotherapy. The safety and efficacy of asenapine as adjunctive therapy in the treatment of bipolar I disorder have not been established in the pediatric population. In general, the pharmacokinetics of asenapine in pediatric patients (10 to 17 years) and adults are similar[see Clinical Pharmacology (12.3)].SchizophreniaEfficacy of asenapine was not demonstrated in an 8-week, placebo-controlled, double-blind trial, in 306 adolescent patients aged 12 to 17 years with schizophrenia at doses of 2.5 and 5 mg twice daily. The most common adverse reactions (proportion of patients equal or greater than 5% and at least twice placebo) reported were somnolence, akathisia, dizziness, and oral hypoesthesia or paresthesia. The proportion of patients with an equal or greater than 7% increase in body weight at endpoint compared to baseline for placebo, asenapine 2.5 mg twice daily, and asenapine 5 mg twice daily was 3%, 10%, and 10%, respectively.
The clinically relevant adverse reactions identified in the pediatric schizophrenia trial were generally similar to those observed in the pediatric bipolar I and adult bipolar I and schizophrenia trials. No new major safety findings were reported from a 26-week, open-label, uncontrolled safety trial in pediatric patients with schizophrenia treated with asenapine monotherapy.
JuvenileAnimal DataSubcutaneous administration of asenapine to juvenile rats for 56 days from day 14 of age to day 69 of age at 0.4, 1.2, and 3.2 mg/kg/day (0.2, 0.6 and 1.5 times the maximum recommended human dose of 10 mg twice daily given sublingually on a mg/m2basis) resulted in significant reduction in body weight gain in animals of both sexes at all dose levels from the start of dosing until weaning. Body weight gain remained reduced in males to the end of treatment, however, recovery was observed once treatment ended. Neurobehavioral assessment indicated increased motor activity in animals at all dose levels following the completion of treatment, with the evidence of recovery in males. There was no recovery after the end of treatment in female activity pattern as late as day 30 following the completion of treatment (last retesting). Therefore, a No Observed Adverse Effect Level (NOAEL) for the juvenile animal toxicity of asenapine could not be determined. There were no treatment-related effects on the startle response, learning/memory, organ weights, microscopic evaluations of the brain and, reproductive performance (except for minimally reduced conception rate and fertility index in males and females administered 1.2 and 3.2 mg/kg/day).