Atomoxetine Hydrochloride Prescribing Information
Atomoxetine increased the risk of suicidal ideation in short-term studies in children and adolescents with Attention-Deficit/Hyperactivity Disorder (ADHD). Pooled analyses of short-term (6 to 18 weeks) placebo-controlled trials of atomoxetine in children and adolescents have revealed a greater risk of suicidal ideation early during treatment in those receiving atomoxetine. There were a total of 12 trials (11 in ADHD and 1 in enuresis) involving over 2200 patients (including 1357 patients receiving atomoxetine and 851 receiving placebo). The average risk of suicidal ideation in patients receiving atomoxetine was 0.4% (5/1357 patients), compared to none in placebo-treated patients. There was 1 suicide attempt among these approximately 2200 patients, occurring in a patient treated with atomoxetine.
The following symptoms have been reported with atomoxetine: anxiety, agitation, panic attacks, insomnia, irritability, hostility, aggressiveness, impulsivity, akathisia (psychomotor restlessness), hypomania and mania. Although a causal link between the emergence of such symptoms and the emergence of suicidal impulses has not been established, there is a concern that such symptoms may represent precursors to emerging suicidality. Thus, patients being treated with atomoxetine should be observed for the emergence of such symptoms.
Consideration should be given to changing the therapeutic regimen, including possibly discontinuing the medication, in patients who are experiencing emergent suicidality or symptoms that might be precursors to emerging suicidality, especially if these symptoms are severe or abrupt in onset, or were not part of the patient’s presenting symptoms.
Dosage and Administration, Screen for Bipolar Disorder Prior to Starting Atomoxetine Capsules (
Warnings and Precautions, Emergence of New Psychotic or Manic Symptoms (
Warnings and Precautions, Screening Patients for Bipolar Disorder (
Warnings and Precautions, Aggressive Behavior or Hostility (
Each capsule contains atomoxetine hydrochloride, USP equivalent to 10 mg (Opaque White, Opaque White), 18 mg (Opaque Gold, Opaque White), 25 mg (Opaque Blue, Opaque White), 40 mg (Opaque Blue, Opaque Blue), 60 mg (Opaque Blue, Opaque Gold), 80 mg (Opaque Orange, Opaque White), or 100 mg (Opaque Orange, Opaque Orange) of atomoxetine.
Atomoxetine is a selective norepinephrine reuptake inhibitor. Atomoxetine hydrochloride, USP is the
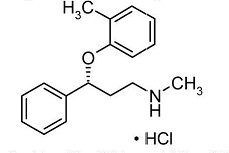
Atomoxetine hydrochloride, USP is a white to practically white solid, which has a solubility of 27.8 mg/mL in water. Atomoxetine capsules, USP are intended for oral administration only.
Each capsule contains atomoxetine hydrochloride, USP equivalent to 10, 18, 25, 40, 60, 80, or 100 mg of atomoxetine. The capsules also contain pregelatinized starch. The capsule shells contain gelatin and titanium dioxide. The capsule shells may also contain one or more of the following: FD&C Blue No. 2 (25 mg, 40 mg, and 60 mg), iron oxide red (80 mg and 100 mg), and iron oxide yellow (18 mg, 25 mg, 40 mg, 60 mg, 80 mg and 100 mg). The capsules are imprinted with edible black ink (comprised of ammonia solution, iron oxide black, potassium hydroxide, propylene glycol, and shellac.).
The precise mechanism by which atomoxetine produces its therapeutic effects in Attention–Deficit/Hyperactivity Disorder (ADHD) is unknown, but is thought to be related to selective inhibition of the pre–synaptic norepinephrine transporter, as determined in