Atovaquone Oral Suspension
(Atovaquone)Atovaquone Oral Suspension Prescribing Information
Atovaquone oral suspension, USP is a bright yellow, citrus-flavored, oral suspension containing 750 mg of atovaquone per 5 mL. Atovaquone oral suspension, USP is supplied in 210-mL bottles.
Atovaquone oral suspension is contraindicated in patients who develop or have a history of hypersensitivity reactions (e.g., angioedema, bronchospasm, throat tightness, urticaria) to atovaquone or any of the components of atovaquone oral suspension.
The following adverse reaction is discussed in another section of the labeling:
- Hepatotoxicity [see Warnings and Precautions (5.2)].
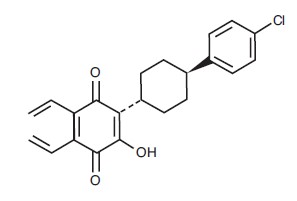
Atovaquone oral suspension, USP is a quinone antimicrobial drug. The chemical name of atovaquone is
22H
19ClO
3. The compound has the following structural formula:
Atovaquone oral suspension, USP is a formulation of micro-fine particles of atovaquone, USP.
Each 5 mL of atovaquone oral suspension, USP contains 750 mg of atovaquone and the inactive ingredients benzyl alcohol, flavor natural citrus, poloxamer 188, purified water, saccharin sodium, and xanthan gum.
Atovaquone oral suspension, USP (bright yellow, citrus-flavored) containing 750 mg atovaquone per 5 mL.
- Bottle of 210 mL with child-resistant cap (NDC 10702-223-21). Store at 15°C to 25°C (59°F to 77°F). Do not freeze.Dispense in tight container as defined in USP.
Atovaquone is a quinone antimicrobial drug