Baclofen
Baclofen Prescribing Information
Baclofen oral solution is indicated for the treatment of spasticity resulting from multiple sclerosis, particularly for the relief of flexor spasms and concomitant pain, clonus, and muscular rigidity.
Baclofen oral solution may also be of some value in patients with spinal cord injuries and other spinal cord diseases.
Baclofen oral solution is not indicated in the treatment of skeletal muscle spasm resulting from rheumatic disorders.
Oral Solution: 10 mg/5 mL baclofen as a clear, colorless solution
Baclofen oral solution is contraindicated in patients with hypersensitivity to baclofen.
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Adverse Reactions from Abrupt Withdrawal of Baclofen Oral Solution [see Warnings and Precautions ()]
5.1 Adverse Reactions from Abrupt Withdrawal of Baclofen Oral SolutionAbrupt discontinuation of baclofen, regardless of the cause, has resulted in adverse reactions that include hallucinations, seizures, high fever, altered mental status, exaggerated rebound spasticity, and muscle rigidity, that in rare cases has advanced to rhabdomyolysis, multiple organ-system failure, and death. Therefore, reduce the dosage slowly when baclofen oral solution is discontinued, unless the clinical situation justifies a rapid withdrawal.
- Neonatal Withdrawal Symptoms[see Warnings and Precautions ()]
5.2 Neonatal Withdrawal SymptomsWithdrawal symptoms in neonates whose mothers were treated with oral baclofen throughout pregnancy have been reported starting hours to days after delivery. The symptoms of withdrawal in these infants have included increased muscle tone, tremor, jitteriness, and seizure. If the potential benefit justifies the potential risk to the fetus and baclofen oral solution is continued during pregnancy, gradually reduce the dosage and discontinue baclofen oral solution before delivery. If slow withdrawal is not feasible, advise the parents or caregivers of the exposed neonate of the potential for neonatal withdrawal.
- Drowsiness and Sedation[see Warnings and Precautions ()]
5.3 Drowsiness and SedationDrowsiness and sedation have been reported in up to 63% of patients taking baclofen, the active ingredient in baclofen oral solution
[see Adverse Reactions ]. Patients should avoid operation of automobiles or other dangerous machinery and activities made hazardous by decreased alertness when starting baclofen oral solution or increasing the dose until they know how the drug affects them. Advise patients that the central nervous system depressant effects of baclofen oral solution may be additive to those of alcohol and other CNS depressants. - Poor Tolerability in Stroke Patients[see Warnings and Precautions ()]
5.4 Poor Tolerability in Stroke PatientsBaclofen oral solution should be used with caution in patients who have had a stroke. Baclofen has not significantly benefited patients with stroke. These patients have also shown poor tolerability to the drug.
- Exacerbation of Psychotic Disorders, Schizophrenia, or Confusional States[see Warnings and Precautions ()]
5.5 Exacerbation of Psychotic Disorders, Schizophrenia, or Confusional StatesBaclofen oral solution should be used with caution in patients suffering from psychotic disorders, schizophrenia, or confusional states. If treated with baclofen oral solution, these patients should be kept under careful surveillance because exacerbations of these conditions have been observed with oral baclofen administration.
- Exacerbation of Autonomic Dysreflexia[see Warnings and Precautions ()]
5.6 Exacerbation of Autonomic DysreflexiaBaclofen oral solution should be used with caution in patients with a history of autonomic dysreflexia. The presence of nociceptive stimuli or abrupt withdrawal of baclofen oral solution may cause an autonomic dysreflexic episode.
- Exacerbation of Epilepsy[see Warnings and Precautions ()]
5.7 Exacerbation of EpilepsyBaclofen oral solution should be used with caution in patients with epilepsy. Deterioration in seizure control has been reported in patients taking baclofen.
- Posture and Balance Effects[see Warnings and Precautions ()]
5.8 Posture and Balance EffectsBaclofen oral solution should be used with caution in patients where spasticity is utilized to sustain upright posture and balance in locomotion or whenever spasticity is utilized to obtain increased function.
- Ovarian Cysts[see Warnings and Precautions ()]
5.9 Ovarian CystsA dose-related increase in incidence of ovarian cysts was observed in female rats treated chronically with oral baclofen. Ovarian cysts have been found by palpation in about 4% of the multiple sclerosis patients who were treated with oral baclofen for up to one year. In most cases, these cysts disappeared spontaneously while patients continued to receive the drug. Ovarian cysts are estimated to occur spontaneously in approximately 1% to 5% of the normal female population.
Baclofen oral solution is a gamma-aminobutyric acid (GABA-ergic) agonist available as 10 mg/5 mL solution for oral administration. Its chemical name is 4-amino-3-(4-chlorophenyl)-butanoic acid, and its structural formula is:
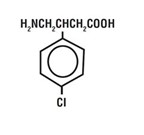
Molecular formula is C
1OH
12CINO
2.
Molecular Weight is 213.66.
Baclofen USP is a white to off-white, odorless or practically odorless crystalline powder. It is slightly soluble in water, very slightly soluble in methanol, and insoluble in chloroform.
The baclofen oral solution inactive ingredients are: glycerin, methylparaben, propylparaben, purified water, and sucralose. May also contain sodium hydroxide or hydrochloric acid for pH adjustment.
The efficacy of baclofen oral solution is based upon a bioavailability study in healthy adults comparing baclofen oral tablets to baclofen oral solution
A pharmacokinetic study in heathy adult male subjects under fasting conditions at 20 mg dose level demonstrated similar bioavailability for baclofen oral solution (5 mg/5 mL) and oral tablets. The peak plasma concentrations were achieved in about 0.75 hours from oral solution and the apparent elimination half-life is about 5.7 hours. Baclofen is excreted primarily by the kidney in unchanged form, and there is relatively large intersubject variation in absorption and/or elimination.