Baclofen Prescribing Information
Abrupt discontinuation of intrathecal baclofen, regardless of the cause, has resulted in sequelae that include high fever, altered mental status, exaggerated rebound spasticity, and muscle rigidity, that in rare cases has advanced to rhabdomyolysis, multiple organ-system failure and death.
Prevention of abrupt discontinuation of intrathecal baclofen requires careful attention to programming and monitoring of the infusion system, refill scheduling and procedures, and pump alarms. Patients and caregivers should be advised of the importance of keeping scheduled refill visits and should be educated on the early symptoms of baclofen withdrawal. Special attention should be given to patients at apparent risk (e.g. spinal cord injuries at T-6 or above, communication difficulties, history of withdrawal symptoms from oral or intrathecal baclofen). Consult the technical manual of the implantable infusion system for additional postimplant clinician and patient information (see
Baclofen Injection is indicated for use in the management of severe spasticity. Patients should first respond to a screening dose of intrathecal baclofen prior to consideration for long term infusion via an implantable pump. For spasticity of spinal cord origin, chronic infusion of Baclofen Injection via an implantable pump should be reserved for patients unresponsive to oral baclofen therapy, or those who experience intolerable CNS side effects at effective doses. Patients with spasticity due to traumatic brain injury should wait at least one year after the injury before consideration of long term intrathecal baclofen therapy. Baclofen Injection is intended for use by the intrathecal route in single bolus test doses (via spinal catheter or lumbar puncture) and, for chronic use, only in implantable pumps approved by the FDA specifically for the administration of Baclofen Injection into the intrathecal space.
Baclofen Injection therapy may be considered an alternative to destructive neurosurgical procedures. Prior to implantation of a device for chronic intrathecal infusion of Baclofen Injection, patients must show a response to Baclofen Injection in a screening trial (see Dosage and Administration).
Hypersensitivity to baclofen. Baclofen Injection is not recommended for intravenous, intramuscular, subcutaneous or epidural administration.
Adverse experiences reported during all U.S. studies (both controlled and uncontrolled) are shown in the following table. Eight of 474 patients who received chronic infusion via implanted pumps had adverse experiences which led to a discontinuation of long term treatment in the pre- and post-marketing studies.
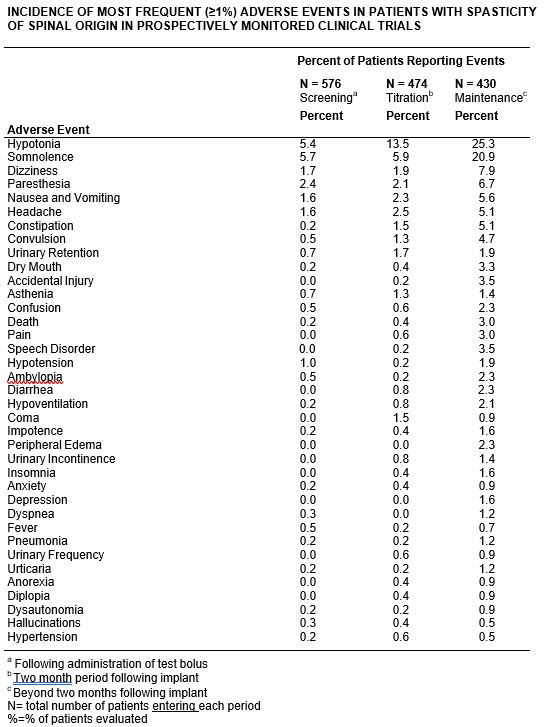
In addition to the more common (1% or more) adverse events reported in the prospectively followed 576 domestic patients in pre- and post-marketing studies, experience from an additional 194 patients exposed to Baclofen Injection from foreign studies has been reported. The following adverse events, not described in the table, and arranged in decreasing order of frequency, and classified by body system, were reported:
The nine adverse events leading to discontinuation were: infection (3), CSF leaks (2), meningitis (2), drainage (1), and unmanageable trunk control (1).
Because of the open, uncontrolled nature of the experience, a causal linkage between events observed and the administration of Baclofen Injection cannot be reliably assessed in many cases. Nonetheless, many of the more commonly reported reactions— somnolence, dizziness, headache, nausea, hypotension, hypotonia and coma— appear clearly drug-related.
The most frequent (≥1%) adverse events reported during all clinical trials are shown in the following table. Nine patients discontinued long term treatment due to adverse events.
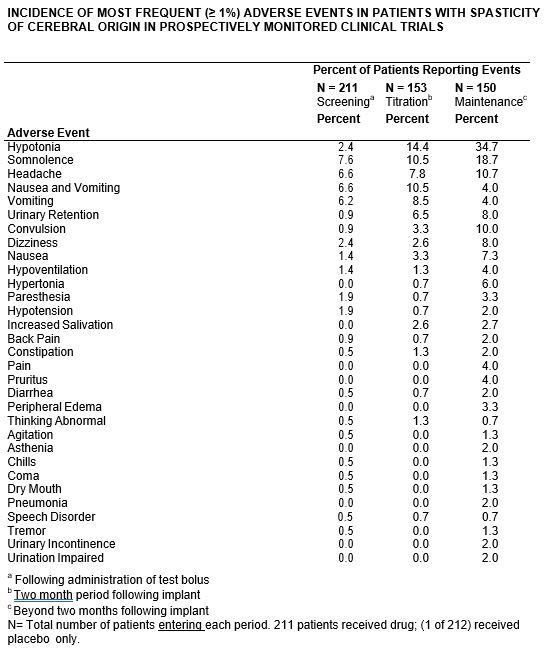
The more common (1% or more) adverse events reported in the prospectively followed 211 patients exposed to Baclofen Injection have been reported. In the total cohort, the following adverse events, not described in the table, and arranged in decreasing order of frequency, and classified by body system, were reported:
The following adverse events have been reported during post-approval use of Baclofen Injection. Because these events are reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency.
There is inadequate systematic experience with the use of Baclofen Injection in combination with other medications to predict specific drug-drug interactions. Interactions attributed to the combined use of Baclofen Injection and epidural morphine include hypotension and dyspnea.