Balsalazide Disodium - Balsalazide Disodium capsule (Balsalazide Disodium)
Balsalazide Disodium - Balsalazide Disodium capsule prescribing information
Balsalazide disodium capsules are indicated for the treatment of mildly to moderately active ulcerative colitis in patients 5 years of age and older.
Safety and effectiveness of balsalazide disodium capsules beyond 8 weeks in pediatric patients 5 years to 17 years of age and 12 weeks in adults have not been established.
- Administration Instructions:
• Evaluate renal function before initiating therapy with balsalazide disodium capsules. ()2.1 Important Preparation and Administration Instructions• Evaluate renal function before initiating therapy with balsalazide disodium capsules[see Warnings and Precaution ].• Swallow balsalazide disodium capsules whole. Do not cut, break, crush or chew the capsules.• For patients who cannot swallow intact capsules, balsalazide disodium capsules may also be administered by opening the capsule and sprinkling the capsule contents on applesauce. If the capsules are opened for sprinkling, color variation of the powder inside the capsules ranges from orange to yellow and is expected due to color variation of the active pharmaceutical ingredient.• Place a small amount (approximately 10 mL) of applesauce into a clean container.• Carefully open the capsules.• Sprinkle the capsule contents on the applesauce.• Mix the capsule contents with the applesauce. The contents may be chewed, if necessary.• Consume the entire amount of applesauce mixture immediately. Do not store the applesauce mixture for future use.• Teeth and/or tongue staining may occur in some patients when administered sprinkled on applesauce.• Drink an adequate amount of fluids[see Warnings and Precautions ].• Take balsalazide disodium capsules with or without food[see Clinical Pharmacology ].
• Swallow capsules whole. Do not cut, break, crush or chew. ()2.1 Important Preparation and Administration Instructions• Evaluate renal function before initiating therapy with balsalazide disodium capsules[see Warnings and Precaution ].• Swallow balsalazide disodium capsules whole. Do not cut, break, crush or chew the capsules.• For patients who cannot swallow intact capsules, balsalazide disodium capsules may also be administered by opening the capsule and sprinkling the capsule contents on applesauce. If the capsules are opened for sprinkling, color variation of the powder inside the capsules ranges from orange to yellow and is expected due to color variation of the active pharmaceutical ingredient.• Place a small amount (approximately 10 mL) of applesauce into a clean container.• Carefully open the capsules.• Sprinkle the capsule contents on the applesauce.• Mix the capsule contents with the applesauce. The contents may be chewed, if necessary.• Consume the entire amount of applesauce mixture immediately. Do not store the applesauce mixture for future use.• Teeth and/or tongue staining may occur in some patients when administered sprinkled on applesauce.• Drink an adequate amount of fluids[see Warnings and Precautions ].• Take balsalazide disodium capsules with or without food[see Clinical Pharmacology ].
• For patients who cannot swallow intact capsules, the capsules may be opened and sprinkled on applesauce, then chewed and swallowed immediately. ()2.1 Important Preparation and Administration Instructions• Evaluate renal function before initiating therapy with balsalazide disodium capsules[see Warnings and Precaution ].• Swallow balsalazide disodium capsules whole. Do not cut, break, crush or chew the capsules.• For patients who cannot swallow intact capsules, balsalazide disodium capsules may also be administered by opening the capsule and sprinkling the capsule contents on applesauce. If the capsules are opened for sprinkling, color variation of the powder inside the capsules ranges from orange to yellow and is expected due to color variation of the active pharmaceutical ingredient.• Place a small amount (approximately 10 mL) of applesauce into a clean container.• Carefully open the capsules.• Sprinkle the capsule contents on the applesauce.• Mix the capsule contents with the applesauce. The contents may be chewed, if necessary.• Consume the entire amount of applesauce mixture immediately. Do not store the applesauce mixture for future use.• Teeth and/or tongue staining may occur in some patients when administered sprinkled on applesauce.• Drink an adequate amount of fluids[see Warnings and Precautions ].• Take balsalazide disodium capsules with or without food[see Clinical Pharmacology ].
• Teeth and/or tongue staining may occur when administered sprinkled on applesauce. ()2.1 Important Preparation and Administration Instructions• Evaluate renal function before initiating therapy with balsalazide disodium capsules[see Warnings and Precaution ].• Swallow balsalazide disodium capsules whole. Do not cut, break, crush or chew the capsules.• For patients who cannot swallow intact capsules, balsalazide disodium capsules may also be administered by opening the capsule and sprinkling the capsule contents on applesauce. If the capsules are opened for sprinkling, color variation of the powder inside the capsules ranges from orange to yellow and is expected due to color variation of the active pharmaceutical ingredient.• Place a small amount (approximately 10 mL) of applesauce into a clean container.• Carefully open the capsules.• Sprinkle the capsule contents on the applesauce.• Mix the capsule contents with the applesauce. The contents may be chewed, if necessary.• Consume the entire amount of applesauce mixture immediately. Do not store the applesauce mixture for future use.• Teeth and/or tongue staining may occur in some patients when administered sprinkled on applesauce.• Drink an adequate amount of fluids[see Warnings and Precautions ].• Take balsalazide disodium capsules with or without food[see Clinical Pharmacology ].
• Drink an adequate amount of fluids. (,2.1 Important Preparation and Administration Instructions• Evaluate renal function before initiating therapy with balsalazide disodium capsules[see Warnings and Precaution ].• Swallow balsalazide disodium capsules whole. Do not cut, break, crush or chew the capsules.• For patients who cannot swallow intact capsules, balsalazide disodium capsules may also be administered by opening the capsule and sprinkling the capsule contents on applesauce. If the capsules are opened for sprinkling, color variation of the powder inside the capsules ranges from orange to yellow and is expected due to color variation of the active pharmaceutical ingredient.• Place a small amount (approximately 10 mL) of applesauce into a clean container.• Carefully open the capsules.• Sprinkle the capsule contents on the applesauce.• Mix the capsule contents with the applesauce. The contents may be chewed, if necessary.• Consume the entire amount of applesauce mixture immediately. Do not store the applesauce mixture for future use.• Teeth and/or tongue staining may occur in some patients when administered sprinkled on applesauce.• Drink an adequate amount of fluids[see Warnings and Precautions ].• Take balsalazide disodium capsules with or without food[see Clinical Pharmacology ].
)5.8 NephrolithiasisCases of nephrolithiasis have been reported with the use of mesalamine, the active moiety of balsalazide disodium capsules, including stones with 100% mesalamine content. Mesalamine-containing stones are radiotransparent and undetectable by standard radiography or computed tomography (CT). Ensure adequate fluid intake during treatment with balsalazide disodium capsules.
• Take balsalazide disodium capsules with or without food. ()2.1 Important Preparation and Administration Instructions• Evaluate renal function before initiating therapy with balsalazide disodium capsules[see Warnings and Precaution ].• Swallow balsalazide disodium capsules whole. Do not cut, break, crush or chew the capsules.• For patients who cannot swallow intact capsules, balsalazide disodium capsules may also be administered by opening the capsule and sprinkling the capsule contents on applesauce. If the capsules are opened for sprinkling, color variation of the powder inside the capsules ranges from orange to yellow and is expected due to color variation of the active pharmaceutical ingredient.• Place a small amount (approximately 10 mL) of applesauce into a clean container.• Carefully open the capsules.• Sprinkle the capsule contents on the applesauce.• Mix the capsule contents with the applesauce. The contents may be chewed, if necessary.• Consume the entire amount of applesauce mixture immediately. Do not store the applesauce mixture for future use.• Teeth and/or tongue staining may occur in some patients when administered sprinkled on applesauce.• Drink an adequate amount of fluids[see Warnings and Precautions ].• Take balsalazide disodium capsules with or without food[see Clinical Pharmacology ].
• Adults:The recommended dosage is 2.25 g (three 750 mg capsules) three times daily for 8 weeks. Some adult patients required treatment for up to 12 weeks in clinical trials. ()2.2 Recommended Dosage in Adults and Pediatric Patients 5 Years to 17 Years of AgeAdults:The recommended dosage in adults is 2.25 g (three 750 mg capsules) three times daily for up to 8 weeks. Some patients in the adult clinical trials required treatment for up to 12 weeks.
Pediatric Patients 5 Years to 17 Years of Age:The recommended dosage in pediatric patients 5 years to 17 years of age is
either:• 2.25 g (three 750 mg capsules) three times daily for up to 8 weeks;- OR:
• 750 mg (one capsule) three times daily for up to 8 weeks.
Use of balsalazide disodium capsules in the pediatric population for more than 8 weeks has not been evaluated in clinical trials
[see Clinical Studies ].• Pediatric Patients 5 Years to 17 Years of Age:The recommended dosage iseither:• 2.25 g (three 750 mg capsules) three times for up to 8 weeks.- OR:
• 750 mg (one capsule) three times daily for up to 8 weeks. ()2.2 Recommended Dosage in Adults and Pediatric Patients 5 Years to 17 Years of AgeAdults:The recommended dosage in adults is 2.25 g (three 750 mg capsules) three times daily for up to 8 weeks. Some patients in the adult clinical trials required treatment for up to 12 weeks.
Pediatric Patients 5 Years to 17 Years of Age:The recommended dosage in pediatric patients 5 years to 17 years of age is
either:• 2.25 g (three 750 mg capsules) three times daily for up to 8 weeks;- OR:
• 750 mg (one capsule) three times daily for up to 8 weeks.
Use of balsalazide disodium capsules in the pediatric population for more than 8 weeks has not been evaluated in clinical trials
[see Clinical Studies ].
Balsalazide Disodium Capsules, USP are available as light orange opaque capsules containing 750 mg balsalazide disodium, USP and "54 795" printed in black ink on the cap and body, containing a yellow-orange powder.
Clinical trials of balsalazide disodium capsules did not include sufficient numbers of subjects aged 65 years and over to determine whether they respond differently than younger subjects. Reports from uncontrolled clinical studies and postmarketing reporting systems suggested a higher incidence of blood dyscrasias, i.e., neutropenia and pancytopenia, in patients who were 65 years or older compared to younger patients taking mesalamine-containing products. Balsalazide disodium capsules are converted into mesalamine in the colon. Monitor complete blood cell counts and platelet counts in elderly patients during treatment with balsalazide disodium capsules. In general, consider the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy in elderly patients when prescribing balsalazide disodium capsules
Balsalazide disodium capsules are contraindicated in patients with known or suspected hypersensitivity to salicylates, aminosalicylates, or to any of the components of balsalazide disodium capsules or balsalazide metabolites
Some patients have experienced a hypersensitivity reaction to sulfasalazine may have a similar reaction to balsalazide disodium capsules or to other compounds that contain or are converted to mesalamine. Mesalamine-induced hypersensitivity reactions may present as internal organ involvement, including myocarditis, pericarditis, nephritis, hepatitis, pneumonitis and hematologic abnormalities. Evaluate patients immediately if signs or symptoms of a hypersensitivity reaction are present. Discontinue balsalazide disodium capsules if an alternative etiology for the signs and symptoms cannot be established.
The following adverse reactions have been identified during post-approval use of balsalazide, or other products which contain or are metabolized to mesalamine. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
• Urine discoloration occurring ex-vivo caused by contact of mesalamine, including inactive metabolite, with surfaces or water treated with hypochlorite-containing bleach
Each Balsalazide Disodium Capsule, USP contains 750 mg of balsalazide disodium, a prodrug that is enzymatically cleaved in the colon to produce mesalamine (5-aminosalicylic acid or 5-ASA), an aminosalicylate. Each capsule of balsalazide (750 mg) is equivalent to 267 mg of mesalamine. Balsalazide disodium has the chemical name (E)-5-[[4-[[(2-Carboxyethyl)amino]carbonyl] phenyl]azo]-2-hydroxybenzoic acid, disodium salt, dihydrate. Its structural formula is:
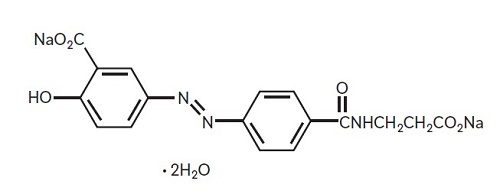
Molecular Weight: 437.31
Molecular Formula: C17H13N3Na2O6•2H2O
Balsalazide disodium is a yellow to orange crystalline powder. It is freely soluble in water and isotonic saline and DMSO, sparingly soluble in methanol and ethanol, and practically insoluble in all other organic solvents.
The inactive ingredients in Balsalazide Disodium Capsules, USP are colloidal silicon dioxide and magnesium stearate. Additionally, the capsule shell contains FD&C Blue #1, FD&C Red #40, FD&C Yellow #6, gelatin, and titanium dioxide. The black monogramming ink contains ammonium hydroxide, iron oxide black, isopropyl alcohol, n-butyl alcohol, propylene glycol, and shellac glaze. The sodium content of each capsule is approximately 79 mg.

• Renal Impairment:Assess renal function at the beginning of treatment and periodically during treatment. Evaluate the risks and benefits in patients with known renal impairment or taking nephrotoxic drugs; monitor renal function. Discontinue if renal function deteriorates. (,5.1 Renal ImpairmentRenal impairment, including minimal change disease, acute and chronic interstitial nephritis, and renal failure, has been reported in patients given products such as balsalazide disodium capsules that release mesalamine into the gastrointestinal tract. Evaluate renal function prior to initiation of balsalazide disodium capsules and periodically while on therapy. Evaluate the risks and benefits of using balsalazide disodium capsules in patients with known renal impairment, a history of renal disease or taking nephrotoxic drugs. Discontinue balsalazide disodium capsules if renal function deteriorates while on therapy
[see Drug Interactions , Use in Specific Populations ].,7.1 Nephrotoxic Agents, Including Non-Steroidal Anti-Inflammatory DrugsThe concurrent use of mesalamine with known nephrotoxic agents, including non-steroidal anti-inflammatory drugs (NSAIDs), may increase the risk of renal reactions. Monitor patients taking nephrotoxic drugs for changes in renal function and mesalamine-related adverse reactions
[see Warnings and Precautions ].)8.6 Renal ImpairmentMesalamine is known to be substantially excreted by the kidney, and the risk of adverse reactions to balsalazide disodium capsules, which is converted to mesalamine, may be greater in patients with impaired renal function. Evaluate renal function in all patients prior to initiation and periodically while on balsalazide disodium capsule therapy. Monitor patients with known renal impairment or history of renal disease or taking nephrotoxic drugs for decreased renal function and mesalamine-related adverse reactions. Discontinue balsalazide disodium capsules if renal function deteriorates while on therapy
[see Warnings and Precautions , Adverse Reactions , Drug Interactions ].• Mesalamine-Induced Acute Intolerance Syndrome:Symptoms may be difficult to distinguish from an exacerbation of ulcerative colitis; monitor for worsening symptoms; discontinue treatment if acute intolerance syndrome is suspected. ()5.2 Mesalamine-Induced Acute Intolerance SyndromeBalsalazide is converted to mesalamine, which has been associated with an acute intolerance syndrome that may be difficult to distinguish from an exacerbation of ulcerative colitis. Although the exact frequency of occurrence has not been determined, it has occurred in 3% of patients in controlled clinical trials of mesalamine or sulfasalazine. Symptoms include cramping, acute abdominal pain and bloody diarrhea, sometimes fever, headache, and rash. Monitor patients for worsening of these symptoms while on treatment. If acute intolerance syndrome is suspected, promptly discontinue treatment with balsalazide disodium capsules.
• Hypersensitivity Reactions, including Myocarditis and Pericarditis: Evaluate patients immediately and discontinue if a hypersensitivity reaction is suspected. ()5.3 Hypersensitivity ReactionsSome patients have experienced a hypersensitivity reaction to sulfasalazine may have a similar reaction to balsalazide disodium capsules or to other compounds that contain or are converted to mesalamine. Mesalamine-induced hypersensitivity reactions may present as internal organ involvement, including myocarditis, pericarditis, nephritis, hepatitis, pneumonitis and hematologic abnormalities. Evaluate patients immediately if signs or symptoms of a hypersensitivity reaction are present. Discontinue balsalazide disodium capsules if an alternative etiology for the signs and symptoms cannot be established.
• Hepatic Failure: Evaluate the risks and benefits in patients with known liver impairment. ()5.4 Hepatic FailureThere have been reports of hepatic failure in patients with pre-existing liver disease who have been administered mesalamine. Because balsalazide is converted to mesalamine, evaluate the risks and benefits of using balsalazide disodium capsules in patients with known liver impairment.
• Severe Cutaneous Adverse Reactions: Discontinue at the first signs or symptoms of severe cutaneous adverse reactions or other signs of hypersensitivity and consider further evaluation ()5.5 Severe Cutaneous Adverse ReactionsSevere cutaneous adverse reactions, including Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), drug reaction with eosinophilia and systemic symptoms (DRESS), and acute generalized exanthematous pustulosis (AGEP) have been reported with the use of mesalamine, the active moiety of balsalazide disodium capsules
[see Adverse Reactions ].Discontinue balsalazide disodium capsules at the first signs or symptoms of severe cutaneous adverse reactions or other signs of hypersensitivity and consider further evaluation.
• Upper Gastrointestinal Tract Obstruction: Avoid in patients with pyloric stenosis or other organic or functional obstruction. ()5.6 Upper Gastrointestinal Tract ObstructionPyloric stenosis or other organic or functional obstruction in the upper gastrointestinal tract may cause prolonged gastric retention of balsalazide disodium capsules, which would delay mesalamine release in the colon. Avoid balsalazide disodium capsules in patients at risk of upper gastrointestinal tract obstruction.
• Photosensitivity: Advise patients with pre-existing skin conditions to avoid sun exposure, wear protective clothing, and use a broad-spectrum sunscreen when outdoors. ()5.7 PhotosensitivityPatients with pre-existing skin conditions such as atopic dermatitis and atopic eczema have reported more severe photosensitivity reactions. Advise patients to avoid sun exposure, wear protective clothing, and use a broad-spectrum sunscreen when outdoors.
• Nephrolithiasis: Stones containing mesalamine, the active moiety in balsalazide disodium capsules, are undetectable by standard radiography or computed tomography (CT). Ensure adequate fluid intake during treatment with balsalazide disodium capsules. ()5.8 NephrolithiasisCases of nephrolithiasis have been reported with the use of mesalamine, the active moiety of balsalazide disodium capsules, including stones with 100% mesalamine content. Mesalamine-containing stones are radiotransparent and undetectable by standard radiography or computed tomography (CT). Ensure adequate fluid intake during treatment with balsalazide disodium capsules.
• Interference with Laboratory Tests: Use of mesalamine may lead to spuriously elevated test results when measuring urinary normetanephrine by liquid chromatography with electrochemical detection. ()5.9 Interference with Laboratory TestsUse of balsalazide disodium capsules, which is converted to mesalamine, may lead to spuriously elevated test results when measuring urinary normetanephrine by liquid chromatography with electrochemical detection because of the similarity in the chromatograms of normetanephrine and the main metabolite of mesalamine, N-acetyl-5-aminosalicylic acid (N-Ac-5-ASA). Consider an alternative, selective assay for normetanephrine.