Betaxolol
Betaxolol Prescribing Information
Betaxolol is indicated in the management of hypertension. It may be used alone or concomitantly with other antihypertensive agents, particularly thiazide-type diuretics.
The initial dose of betaxolol in hypertension is ordinarily 10 mg once daily either alone or added to diuretic therapy. The full antihypertensive effect is usually seen within 7 to 14 days. If the desired response is not achieved the dose can be doubled after 7 to 14 days. Increasing the dose beyond 20 mg has not been shown to produce a statistically significant additional antihypertensive effect; but the 40-mg dose has been studied and is well tolerated. An increased effect (reduction) on heart rate should be anticipated with increasing dosage. If monotherapy with betaxolol does not produce the desired response, the addition of a diuretic agent or other antihypertensive should be considered (see,
The following drugs have been coadministered with betaxolol and have not altered its pharmacokinetics: cimetidine, nifedipine, chlorthalidone, and hydrochlorothiazide. Concomitant administration of betaxolol with the oral anticoagulant warfarin has been shown not to potentiate the anticoagulant effect of warfarin.
Catecholamine-depleting drugs (eg, reserpine) may have an additive effect when given with beta-blocking agents. Patients treated with a beta-adrenergic receptor blocking agent plus a catecholamine depletor should therefore be closely observed for evidence of hypotension or marked bradycardia, which may produce vertigo, syncope, or postural hypotension.
Should it be decided to discontinue therapy in patients receiving beta-blockers and clonidine concurrently, the beta-blocker should be discontinued slowly over several days before the gradual withdrawal of clonidine.
Literature reports suggest that oral calcium antagonists may be used in combination with beta-adrenergic blocking agents when heart function is normal, but should be avoided in patients with impaired cardiac function. Hypotension, AV conduction disturbances, and left ventricular failure have been reported in some patients receiving beta-adrenergic blocking agents when an oral calcium antagonist was added to the treatment regimen. Hypotension was more likely to occur if the calcium antagonist were a dihydropyridine derivative, eg, nifedipine, while left ventricular failure and AV conduction disturbances, including complete heart block, were more likely to occur with either verapamil or diltiazem.
Both digitalis glycosides and beta-blockers slow atrioventricular conduction and decrease heart rate. Concomitant use can increase the risk of bradycardia.
Amiodarone is an antiarrhythmic agent with negative chronotropic properties that may be additive to those seen with beta blockers.
Disopyramide is a Type I antiarrhythmic drug with potent negative inotropic and chronotropic effects. Disopyramide has been associated with severe bradycardia, asystole and heart failure when administered with beta blockers.
Particular care should be taken when using anesthetic agents which depress the myocardium, such as ether, cyclopropane, and trichloroethylene (see Warnings, Major surgery).
Betaxolol is contraindicated in patients with known hypersensitivity to the drug.
Betaxolol is contraindicated in patients with sinus bradycardia, heart block greater than first degree, cardiogenic shock, and overt cardiac failure. (see
Sympathetic stimulation may be a vital component supporting circulatory function in congestive heart failure, and beta-adrenergic receptor blockade carries the potential hazard of further depressing myocardial contractility and precipitating more severe heart failure. In hypertensive patients who have congestive heart failure controlled by digitalis and diuretics, beta-blockers should be administered cautiously. Both digitalis and beta-adrenergic receptor blocking agents slow AV conduction.
Continued depression of the myocardium with beta-blocking agents over a period of time can, in some cases, lead to cardiac failure. Therefore at the first sign or symptom of cardiac failure, discontinuation of betaxolol should be considered. In some cases beta-blocker therapy can be continued while cardiac failure is treated with cardiac glycosides, diuretics, and other agents, as appropriate.
Abrupt cessation of therapy with certain beta-blocking agents in patients with coronary artery disease has been followed by exacerbations of angina pectoris and, in some cases, myocardial infarction has been reported. Therefore, such patients should be warned against interruption of therapy without the physician's advice. Even in the absence of overt angina pectoris, when discontinuation of betaxolol is planned, the patient should be carefully observed and therapy should be reinstituted, at least temporarily, if withdrawal symptoms occur.
Chronically administered beta-blocking therapy should not be routinely withdrawn prior to major surgery, however the impaired ability of the heart to respond to reflex adrenergic stimuli may augment the risks of general anesthesia and surgical procedures (see Precautions, Drug Interactions). Titrate betaxolol dose to maintain effective heart rate control while avoiding frank hypotension and bradycardia.
Beta-blockers should be used with caution in diabetic patients. Beta-blockers may mask tachycardia occurring with hypoglycemia (patients should be warned of this), although other manifestations such as dizziness and sweating may not be significantly affected. Unlike nonselective beta-blockers, betaxolol does not prolong insulin-induced hypoglycemia.
Beta-adrenergic blockade may mask certain clinical signs of hyperthyroidism (eg, tachycardia). Abrupt withdrawal of beta-blockade might precipitate a thyroid storm; therefore, patients known or suspected of being thyrotoxic from whom betaxolol is to be withdrawn should be monitored closely (See
Betaxolol should not be given to patients with untreated pheochromocytoma.
Most adverse reactions have been mild and transient and are typical of beta-adrenergic blocking agents, eg, bradycardia, fatigue, dyspnea, and lethargy. Withdrawal of therapy in U.S. and European controlled clinical trials has been necessary in about 3.5% of patients, principally because of bradycardia, fatigue, dizziness, headache, and impotence.
Frequency estimates of adverse events were derived from controlled studies in which adverse reactions were volunteered and elicited in U.S. studies and volunteered and/or elicited in European studies.
In the U.S., the placebo-controlled hypertension studies lasted for 4 weeks, while the active-controlled hypertension studies had a 22- to 24-week double-blind phase. The following doses were studied: betaxolol—5, 10, 20, and 40 mg once daily; atenolol—25, 50, and 100 mg once daily; and propranolol—40, 80, and 160 mg b.i.d.
Betaxolol, like other beta-blockers, has been associated with the development of antinuclear antibodies (ANA) (e.g., lupus erythematosus). In controlled clinical studies, conversion of ANA from negative to positive occurred in 5.3% of the patients treated with betaxolol, 6.3% of the patients treated with atenolol, 4.9% of the patients treated with propranolol, and 3.2% of the patients treated with placebo.
Betaxolol adverse events reported with a 2% or greater frequency, and selected events with lower frequency, in U.S. controlled studies are:
Dose Range Body System/Adverse Reaction | Betaxolol (N=509) 5-40 mg q.d.* (%) | Propranolol (N=73) 40-160 mg b.i.d. (%) | Atenolol (N=75) 25-100 mg q.d. (%) | Placebo (N=109) (%) |
Cardiovascular
Symptomatic bradycardia Edema | 8.1 0.8 1.8 | 4.1 1.4 0 | 12.0 0 0 | 0 0 1.8 |
Central Nervous System Headache Dizziness Fatigue Lethargy | 6.5 4.5 2.9 2.8 | 4.1 11.0 9.6 4.1 | 5.3 2.7 4.0 2.7 | 15.6 5.5 0 0.9 |
Psychiatric Insomnia Nervousness Bizarre dreams Depression | 1.2 0.8 1.0 0.8 | 8.2 1.4 2.7 2.7 | 2.7 2.7 1.3 4.0 | 0 0 0 0 |
Autonomic Impotence | 1.2† | 0 | 0 | 0 |
Respiratory Dyspnea Pharyngitis Rhinitis Upper respiratory infection | 2.4 2.0 1.4 2.6 | 2.7 0 0 0 | 1.3 4.0 4.0 0 | 0.9 0.9 0.9 5.5 |
Gastrointestinal Dyspepsia Nausea Diarrhea | 4.7 1.6 2.0 | 6.8 1.4 6.8 | 2.7 4.0 8.0 | 0.9 0 0.9 |
Musculoskeletal Chest pain Arthralgia | 2.4 3.1 | 1.4 0 | 2.7 4.0 | 0.9 1.8 |
Skin Rash | 1.2 | 0 | 0 | 0 |
*Five patients received 80 mg q.d. †N=336 males; impotence is a known possible adverse effect of this pharmacological class. | ||||
Of the above adverse reactions associated with the use of betaxolol, only bradycardia was clearly dose related, but there was a suggestion of dose relatedness for fatigue, lethargy, and dyspepsia.
In Europe, the placebo-controlled study lasted for 4 weeks, while the comparative studies had a 4-52-week double-blind phase. The following doses were studied: betaxolol 20 and 40 mg once daily and atenolol 100 mg once daily.
From European controlled hypertension clinical trials, the following adverse events reported by 2% or more patients and selected events with lower frequency are presented:
Dose Range Body System/Adverse Reaction | Betaxolol (N=155) 20-40 mg q.d. (%) | Atenolol (N=81) 100 mg q.d. (%) | Placebo (N=60) (%) |
Cardiovascular Bradycardia (heartrate <50 BPM) Symptomatic bradycardia Palpitation Edema Cold extremities | 5.8 1.9 1.9 1.3 1.9 | 5.0 2.5 3.7 1.2 0 | 0 0 1.7 0 0 |
Central Nervous System Headache Dizziness Fatigue Asthenia Insomnia Paresthesia | 14.8 14.8 9.7 7.1 5.0 1.9 | 9.9 17.3 18.5 0 3.7 2.5 | 23.3 15.0 0 16.7 3.3 0 |
Gastrointestinal Nausea Dyspepsia Diarrhea | 5.8 3.9 1.9 | 1.2 7.4 3.7 | 0 3.3 0 |
Musculoskeletal Chest pain Joint pain Myalgia | 7.1 5.2 3.2 | 6.2 4.9 3.7 | 5.0 1.7 3.3 |
The only adverse event whose frequency clearly rose with increasing dose was bradycardia. Elderly patients were especially susceptible to bradycardia, which in some cases responded to dose-reduction (see
Beta-adrenoceptor blockade can cause reduction of intraocular pressure. Since betaxolol hydrochloride is marketed as an ophthalmic solution for treatment of glaucoma, patients should be told that betaxolol may interfere with the glaucoma-screening test. Withdrawal may lead to a return of increased intraocular pressure. Patients receiving beta-adrenergic blocking agents orally and beta-blocking ophthalmic solutions should be observed for potential additive effects either on the intraocular pressure or on the known systemic effects of beta-blockade.
The value of using beta-blockers in psoriatic patients should be carefully weighed since they have been reported to cause an aggravation in psoriasis.
Betaxolol is primarily metabolized in the liver to metabolites that are inactive and then excreted by the kidneys; clearance is somewhat reduced in patients with renal failure but little changed in patients with hepatic disease. Dosage reductions have not routinely been necessary when hepatic insufficiency is present (see
Patients, especially those with evidence of coronary artery insufficiency, should be warned against interruption or discontinuation of betaxolol therapy without the physician's advice.
Although cardiac failure rarely occurs in appropriately selected patients, patients being treated with beta-adrenergic blocking agents should be advised to consult a physician at the first sign or symptom of failure.
Patients should know how they react to this medicine before they operate automobiles and machinery or engage in other tasks requiring alertness. Patients should contact their physician if any difficulty in breathing occurs, and before surgery of any type.
Patients should inform their physicians, ophthalmologists, or dentists that they are taking betaxolol. Patients with diabetes should be warned that beta-blockers may mask tachycardia occurring with hypoglycemia.
The following drugs have been coadministered with betaxolol and have not altered its pharmacokinetics: cimetidine, nifedipine, chlorthalidone, and hydrochlorothiazide. Concomitant administration of betaxolol with the oral anticoagulant warfarin has been shown not to potentiate the anticoagulant effect of warfarin.
Catecholamine-depleting drugs (eg, reserpine) may have an additive effect when given with beta-blocking agents. Patients treated with a beta-adrenergic receptor blocking agent plus a catecholamine depletor should therefore be closely observed for evidence of hypotension or marked bradycardia, which may produce vertigo, syncope, or postural hypotension.
Should it be decided to discontinue therapy in patients receiving beta-blockers and clonidine concurrently, the beta-blocker should be discontinued slowly over several days before the gradual withdrawal of clonidine.
Literature reports suggest that oral calcium antagonists may be used in combination with beta-adrenergic blocking agents when heart function is normal, but should be avoided in patients with impaired cardiac function. Hypotension, AV conduction disturbances, and left ventricular failure have been reported in some patients receiving beta-adrenergic blocking agents when an oral calcium antagonist was added to the treatment regimen. Hypotension was more likely to occur if the calcium antagonist were a dihydropyridine derivative, eg, nifedipine, while left ventricular failure and AV conduction disturbances, including complete heart block, were more likely to occur with either verapamil or diltiazem.
Both digitalis glycosides and beta-blockers slow atrioventricular conduction and decrease heart rate. Concomitant use can increase the risk of bradycardia.
Amiodarone is an antiarrhythmic agent with negative chronotropic properties that may be additive to those seen with beta blockers.
Disopyramide is a Type I antiarrhythmic drug with potent negative inotropic and chronotropic effects. Disopyramide has been associated with severe bradycardia, asystole and heart failure when administered with beta blockers.
Particular care should be taken when using anesthetic agents which depress the myocardium, such as ether, cyclopropane, and trichloroethylene (see Warnings, Major surgery).
Although it is known that patients on beta-blockers may be refractory to epinephrine in the treatment of anaphylactic shock, beta-blockers can, in addition, interfere with the modulation of allergic reaction and lead to an increased severity and/or frequency of attacks. Severe allergic reactions including anaphylaxis have been reported in patients exposed to a variety of allergens either by repeated challenge, or accidental contact, and with diagnostic or therapeutic agents while receiving beta-blockers. Such patients may be unresponsive to the usual doses of epinephrine used to treat allergic reaction.
Lifetime studies with betaxolol HCl in mice at oral dosages of 6, 20, and 60 mg/kg/day (up to 90 × the maximum recommended human dose [MRHD] based on 60-kg body weight) and in rats at 3, 12, or 48 mg/kg/day (up to 72 × MRHD) showed no evidence of a carcinogenic effect. In a variety of in vitro and in vivo bacterial and mammalian cell assays, betaxolol HCl was nonmutagenic. Betaxolol did not adversely affect fertility or mating performance of male or female rats at doses up to 256 mg/kg/day (380 × MRHD).
In a study in which pregnant rats received betaxolol at doses of 4, 40, or 400 mg/kg/day, the highest dose (600 × MRHD) was associated with increased postimplantation loss, reduced litter size and weight, and an increased incidence of skeletal and visceral abnormalities, which may have been a consequence of drug-related maternal toxicity. Other than a possible increased incidence of incomplete descent of testes and sternebral reductions, betaxolol at 4 mg/kg/day and 40 mg/kg/day (6 × MRHD and 60 × MRHD) caused no fetal abnormalities. In a second study with a different strain of rat, 200 mg betaxolol/kg/day (300 × MRHD) was associated with maternal toxicity and an increase in resorptions, but no teratogenicity. In a study in which pregnant rabbits received doses of 1, 4, 12, or 36 mg betaxolol/kg/day (54 × MRHD), a marked increase in post-implantation loss occurred at the highest dose, but no drug-related teratogenicity was observed. The rabbit is more sensitive to betaxolol than other species because of higher bioavailability resulting from saturation of the first-pass effect. In a peri- and postnatal study in rats at doses of 4, 32, and 256 mg betaxolol/kg/day (380 × MRHD), the highest dose was associated with a marked increase in total litter loss within 4 days postpartum. In surviving offspring, growth and development were also affected.
There are no adequate and well-controlled studies in pregnant women. Betaxolol should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Beta-blockers reduce placental perfusion, which may result in intrauterine fetal death, immature and premature deliveries. In addition, adverse effects (especially hypoglycemia and bradycardia) may occur in fetus.
The beta-blocker action persists in the neonate for several days after birth to a treated mother: there is an increased risk of cardiac and pulmonary complications in the neonate in the postnatal period. Bradycardia, respiratory distress and hypoglycemia have also been reported. Accordingly, attentive surveillance of the neonate (heart rate and blood glucose for the first 3 to 5 days of life) in a specialized setting is recommended.
Since betaxolol is excreted in human milk in sufficient amounts to have pharmacological effects in the infant, caution should be exercised when betaxolol is administered to a nursing mother.
Safety and effectiveness in pediatric patients have not been established.
Betaxolol may produce bradycardia more frequently in elderly patients. In general, patients 65 years of age and older had a higher incidence rate of bradycardia (heart rate < 50 BPM) than younger patients in U.S. clinical trials. In a double-blind study in Europe, 19 elderly patients (mean age = 82) received betaxolol 20 mg daily. Dosage reduction to 10 mg or discontinuation was required for 6 patients due to bradycardia (See
The following selected (potentially important) adverse events have been reported at an incidence of less than 2% in U.S. controlled and open, long-term clinical studies, European controlled clinical trials, or in marketing experience. It is not known whether a causal relationship exists between betaxolol and these events; they are listed to alert the physician to a possible relationship:
The following drugs have been coadministered with betaxolol and have not altered its pharmacokinetics: cimetidine, nifedipine, chlorthalidone, and hydrochlorothiazide. Concomitant administration of betaxolol with the oral anticoagulant warfarin has been shown not to potentiate the anticoagulant effect of warfarin.
Catecholamine-depleting drugs (eg, reserpine) may have an additive effect when given with beta-blocking agents. Patients treated with a beta-adrenergic receptor blocking agent plus a catecholamine depletor should therefore be closely observed for evidence of hypotension or marked bradycardia, which may produce vertigo, syncope, or postural hypotension.
Should it be decided to discontinue therapy in patients receiving beta-blockers and clonidine concurrently, the beta-blocker should be discontinued slowly over several days before the gradual withdrawal of clonidine.
Literature reports suggest that oral calcium antagonists may be used in combination with beta-adrenergic blocking agents when heart function is normal, but should be avoided in patients with impaired cardiac function. Hypotension, AV conduction disturbances, and left ventricular failure have been reported in some patients receiving beta-adrenergic blocking agents when an oral calcium antagonist was added to the treatment regimen. Hypotension was more likely to occur if the calcium antagonist were a dihydropyridine derivative, eg, nifedipine, while left ventricular failure and AV conduction disturbances, including complete heart block, were more likely to occur with either verapamil or diltiazem.
Both digitalis glycosides and beta-blockers slow atrioventricular conduction and decrease heart rate. Concomitant use can increase the risk of bradycardia.
Amiodarone is an antiarrhythmic agent with negative chronotropic properties that may be additive to those seen with beta blockers.
Disopyramide is a Type I antiarrhythmic drug with potent negative inotropic and chronotropic effects. Disopyramide has been associated with severe bradycardia, asystole and heart failure when administered with beta blockers.
Particular care should be taken when using anesthetic agents which depress the myocardium, such as ether, cyclopropane, and trichloroethylene (see
Chronically administered beta-blocking therapy should not be routinely withdrawn prior to major surgery, however the impaired ability of the heart to respond to reflex adrenergic stimuli may augment the risks of general anesthesia and surgical procedures (see Precautions, Drug Interactions). Titrate betaxolol dose to maintain effective heart rate control while avoiding frank hypotension and bradycardia.
Betaxolol is a β1-selective (cardioselective) adrenergic receptor blocking agent available as 10-mg and 20-mg tablets for oral administration. Betaxolol is chemically described as 2-propanol,1-[4-[2-(cyclopropylmethoxy)ethyl]phenoxy]-3-[(1-methylethyl)amino]-,hydrochloride,(±). It has the following chemical structure:
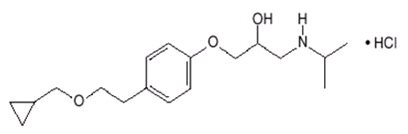
Betaxolol hydrochloride is a water-soluble white crystalline powder with a molecular formula of C18H29NO3•HCl and a molecular weight of 343.9. It is freely soluble in water, ethanol, chloroform, and methanol, and has a pKa of 9.4.
The inactive ingredients are anhydrous lactose, carnauba wax, hypromellose, microcrystalline cellulose, polyethylene glycol, polysorbate 80, pregelatanized starch (corn), sodium starch glycolate, stearic acid and titanium dioxide.