Betoptic S - Betaxolol Hydrochloride suspension/ Drops
(Betaxolol Hydrochloride)Betoptic S - Betaxolol Hydrochloride suspension/ Drops Prescribing Information
BETOPTIC S® (betaxolol hydrochloride ophthalmic suspension) 0.25% is indicated for the treatment of elevated intraocular pressure (IOP) in patients with chronic open-angle glaucoma or ocular hypertension.
Instill one drop of BETOPTIC S in the affected eye(s) twice daily. Shake well before using.
BETOPTIC S may be used alone or in combination with other IOP lowering medications. Advise patients requiring concomitant topical ophthalmic medications to administer these at least 10 minutes before instilling BETOPTIC S.
Ophthalmic suspension containing 2.5 mg/mL of betaxolol as base (0.25%) in 10 mL and 15 mL bottles.
BETOPTIC S is contraindicated in patients with:
- sinus bradycardia
- greater than a first degree atrioventricular (AV) block
- cardiogenic shock
- patients with overt cardiac failure
- hypersensitivity to any component of this product
BETOPTIC S contains betaxolol hydrochloride, a cardioselective beta-adrenergic receptor inhibitor, in a sterile resin suspension formulation. Betaxolol hydrochloride is a white, crystalline powder, with a molecular weight of 343.89 g/mol. The chemical structure is presented below.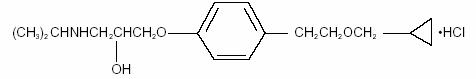
Empirical Formula: C18H29NO3•HCl
Chemical Name:
(±)-1-[p-[2-(cyclopropylmethoxy) ethyl]phenoxy]-3-(isopropylamino)-2-propanol hydrochloride.
Each mL of BETOPTIC S contains:
BETOPTIC S has pH of approximately 7.6 and an osmolality of approximately 290 mOsmol/kg.
In controlled double-masked studies, the magnitude and duration of the ocular hypotensive effect of BETOPTIC S (betaxolol hydrochloride ophthalmic suspension) 0.25% and BETOPTIC (betaxolol hydrochloride ophthalmic solution) 0.5% were clinically equivalent.