Bisoprolol Fumarate And Hydrochlorothiazide
Bisoprolol Fumarate And Hydrochlorothiazide Prescribing Information
Bisoprolol fumarate and hydrochlorothiazide tablets are indicated in the management of hypertension.
Bisoprolol is an effective treatment of hypertension in once-daily doses of 2.5 to 40 mg, while hydrochlorothiazide is effective in doses of 12.5 to 50 mg. In clinical trials of bisoprolol/hydrochlorothiazide combination therapy using bisoprolol doses of 2.5 to 20 mg and hydrochlorothiazide doses of 6.25 to 25 mg, the antihypertensive effects increased with increasing doses of either component.
The adverse effects (see
In general, beta-blocking agents should be avoided in patients with overt congestive failure. However, in some patients with compensated cardiac failure, it may be necessary to utilize these agents. In such situations, they must be used cautiously.
Continued depression of the myocardium with beta-blockers can, in some patients, precipitate cardiac failure. At the first signs or symptoms of heart failure, discontinuation of bisoprolol fumarate and hydrochlorothiazide tablets should be considered. In some cases bisoprolol fumarate and hydrochlorothiazide tablets therapy can be continued while heart failure is treated with other drugs.
Exacerbations of angina pectoris and, in some instances, myocardial infarction or ventricular arrhythmia, have been observed in patients with coronary artery disease following abrupt cessation of therapy with beta-blockers. Such patients should, therefore, be cautioned against interruption or discontinuation of therapy without the physician’s advice. Even in patients without overt coronary artery disease, it may be advisable to taper therapy with bisoprolol fumarate and hydrochlorothiazide tablets over approximately 1 week with the patient under careful observation. If withdrawal symptoms occur, beta-blocking agent therapy should be reinstituted, at least temporarily.
Beta-blockers can precipitate or aggravate symptoms of arterial insufficiency in patients with peripheral vascular disease. Caution should be exercised in such individuals.
Chronically administered beta-blocking therapy should not be routinely withdrawn prior to major surgery; however, the impaired ability of the heart to respond to reflex adrenergic stimuli may augment the risks of general anesthesia and surgical procedures.
Beta-blockers may prevent early warning signs of hypoglycemia, such as tachycardia, and increase the risk for severe or prolonged hypoglycemia at any time during treatment, especially in patients with diabetes mellitus or children and patients who are fasting (i.e., surgery, not eating regularly, or are vomiting). If severe hypoglycemia occurs, patients should be instructed to seek emergency treatment. Also, latent diabetes mellitus may become manifest and diabetic patients given thiazides may require adjustment of their insulin dose.
Beta-adrenergic blockade may mask clinical signs of hyperthyroidism, such as tachycardia. Abrupt withdrawal of beta-blockade may be followed by an exacerbation of the symptoms of hyperthyroidism or may precipitate thyroid storm.
Cumulative effects of the thiazides may develop in patients with impaired renal function. In such patients, thiazides may precipitate azotemia. In subjects with creatinine clearance less than 40 mL/min, the plasma half-life of bisoprolol fumarate is increased up to threefold, as compared to healthy subjects. If progressive renal impairment becomes apparent, bisoprolol fumarate and hydrochlorothiazide tablets should be discontinued (See Pharmacokinetics and Metabolism).
Bisoprolol fumarate and hydrochlorothiazide tablets should be used with caution in patients with impaired hepatic function or progressive liver disease. Thiazides may alter fluid and electrolyte balance, which may precipitate hepatic coma. Also, elimination of bisoprolol fumarate is significantly slower in patients with cirrhosis than in healthy subjects (See Pharmacokinetics and Metabolism).
Hydrochlorothiazide, a sulfonamide, can cause an idiosyncratic reaction, resulting in acute angle-closure glaucoma and elevated intraocular pressure with or without a noticeable acute myopic shift and/or choroidal effusions. Symptoms may include acute onset of decreased visual acuity or ocular pain and typically occur within hours to weeks of drug initiation. Untreated, the acute angle-closure glaucoma may result in permanent visual field loss. The primary treatment is to discontinue hydrochlorothiazide as rapidly as possible. Prompt medical or surgical treatments may need to be considered if the intraocular pressure remains uncontrolled. Risk factors for developing acute angle-closure glaucoma may include a history of sulfonamide or penicillin allergy.
Bisoprolol fumarate and HCTZ have been used individually and in combination for the treatment of hypertension. The antihypertensive effects of these agents are additive; HCTZ 6.25 mg significantly increases the antihypertensive effect of bisoprolol fumarate. The incidence of hypokalemia with the bisoprolol fumarate and HCTZ 6.25 mg combination (B/H) is significantly lower than with HCTZ 25 mg. In clinical trials of bisoprolol fumarate and hydrochlorothiazide tablets, mean changes in serum potassium for patients treated with bisoprolol fumarate and hydrochlorothiazide tablets 2.5/6.25 mg, 5/6.25 mg or 10/6.25 mg or placebo were less than ± 0.1 mEq/L. Mean changes in serum potassium for patients treated with any dose of bisoprolol in combination with HCTZ 25 mg ranged from -0.1 to -0.3 mEq/L.
Bisoprolol fumarate is a beta1-selective (cardioselective) adrenoceptor blocking agent without significant membrane stabilizing or intrinsic sympathomimetic activities in its therapeutic dose range. At higher doses (≥20 mg) bisoprolol fumarate also inhibits beta2-adrenoreceptors located in bronchial and vascular musculature. To retain relative selectivity, it is important to use the lowest effective dose.
Hydrochlorothiazide is a benzothiadiazine diuretic. Thiazides affect renal tubular mechanisms of electrolyte reabsorption and increase excretion of sodium and chloride in approximately equivalent amounts. Natriuresis causes a secondary loss of potassium.
In healthy volunteers, both bisoprolol fumarate and hydrochlorothiazide are well absorbed following oral administration of bisoprolol fumarate and hydrochlorothiazide tablets. No change is observed in the bioavailability of either agent when given together in a single tablet. Absorption is not affected whether bisoprolol fumarate and hydrochlorothiazide tablets are taken with or without food. Mean peak bisoprolol fumarate plasma concentrations of about 9.0 ng/mL, 19 ng/mL and 36 ng/mL occur approximately 3 hours after the administration of the 2.5 mg/6.25 mg, 5 mg/6.25 mg and 10 mg/6.25 mg combination tablets, respectively. Mean peak plasma hydrochlorothiazide concentrations of 30 ng/mL occur approximately 2.5 hours following the administration of the combination. Dose proportional increases in plasma bisoprolol concentrations are observed between the 2.5 and 5, as well as between the 5 and 10 mg doses. The elimination T1/2of bisoprolol ranges from 7 to 15 hours, and that of hydrochlorothiazide ranges from 4 to 10 hours. The percent of dose excreted unchanged in urine is about 55% for bisoprolol and about 60% for hydrochlorothiazide.
The absolute bioavailability after a 10 mg oral dose of bisoprolol fumarate is about 80%. The first pass metabolism of bisoprolol fumarate is about 20%.
The pharmacokinetic profile of bisoprolol fumarate has been examined following single doses and at steady state. Binding to serum proteins is approximately 30%. Peak plasma concentrations occur within 2-4 hours of dosing with 2.5 to 20 mg, and mean peak values range from 9.0 ng/mL at 2.5 mg to 70 ng/mL at 20 mg. Once-daily dosing with bisoprolol fumarate results in less than twofold intersubject variation in peak plasma concentrations. Plasma concentrations are proportional to the administered dose in the range of 2.5 to 20 mg. The plasma elimination half-life is 9-12 hours and is slightly longer in elderly patients, in part because of decreased renal function. Steady state is attained within 5 days with once-daily dosing. In both young and elderly populations, plasma accumulation is low; the accumulation factor ranges from 1.1 to 1.3 and is what would be expected from the half-life and once-daily dosing. Bisoprolol is eliminated equally by renal and nonrenal pathways with about 50% of the dose appearing unchanged in the urine and the remainder in the form of inactive metabolites. In humans, the known metabolites are labile or have no known pharmacologic activity. Less than 2% of the dose is excreted in the feces. The pharmacokinetic characteristics of the two enantiomers are similar. Bisoprolol is not metabolized by cytochrome P450 II D6 (debrisoquin hydroxylase).
In subjects with creatinine clearance less than 40 mL/min, the plasma half-life is increased approximately threefold compared to healthy subjects.
In patients with liver cirrhosis, the rate of elimination of bisoprolol is more variable and significantly slower than that in healthy subjects, with a plasma half-life ranging from 8 to 22 hours.
In elderly subjects, mean plasma concentrations at steady state are increased, in part attributed to lower creatinine clearance. However, no significant differences in the degree of bisoprolol accumulation is found between young and elderly populations.
Hydrochlorothiazide is well absorbed (65%-75%) following oral administration. Absorption of hydrochlorothiazide is reduced in patients with congestive heart failure.
Peak plasma concentrations are observed within 1-5 hours of dosing and range from 70-490 ng/mL following oral doses of 12.5-100 mg. Plasma concentrations are linearly related to the administered dose. Concentrations of hydrochlorothiazide are 1.6-1.8 times higher in whole blood than in plasma. Binding to serum proteins has been reported to be approximately 40% to 68%. The plasma elimination half-life has been reported to be 6-15 hours. Hydrochlorothiazide is eliminated primarily by renal pathways. Following oral doses of 12.5-100 mg, 55%-77% of the administered dose appears in urine and greater than 95% of the absorbed dose is excreted in urine as unchanged drug. Plasma concentrations of hydrochlorothiazide are increased, and the elimination half-life is prolonged in patients with renal disease.
Findings in clinical hemodynamics studies with bisoprolol fumarate are similar to those observed with other beta-blockers. The most prominent effect is the negative chronotropic effect, giving a reduction in resting and exercise heart rate. There is a fall in resting and exercise cardiac output with little observed change in stroke volume, and only a small increase in right atrial pressure, or pulmonary capillary wedge pressure at rest or during exercise.
In normal volunteers, bisoprolol fumarate therapy resulted in a reduction of exercise- and isoproterenol-induced tachycardia. The maximal effect occurred within 1-4 hours post-dosing. Effects generally persisted for 24 hours at doses of 5 mg or greater.
In controlled clinical trials, bisoprolol fumarate given as a single daily dose has been shown to be an effective antihypertensive agent when used alone or concomitantly with thiazide diuretics (see CLINICAL STUDIES).
The mechanism of bisoprolol fumarate’s antihypertensive effect has not been completely established. Factors that may be involved include:
- Decreased cardiac output,
- Inhibition of renin release by the kidneys,
- Diminution of tonic sympathetic outflow from vasomotor centers in the brain.
Beta1-selectivity of bisoprolol fumarate has been demonstrated in both animal and human studies. No effects at therapeutic doses on beta2-adrenoreceptor density have been observed. Pulmonary function studies have been conducted in healthy volunteers, asthmatics, and patients with chronic obstructive pulmonary disease (COPD). Doses of bisoprolol fumarate ranged from 5 to 60 mg, atenolol from 50 to 200 mg, metoprolol from 100 to 200 mg, and propranolol from 40 to 80 mg. In some studies, slight, asymptomatic increases in airway resistance (AWR) and decreases in forced expiratory volume (FEV1) were observed with doses of bisoprolol fumarate 20 mg and higher, similar to the small increases in AWR noted with other cardioselective beta-blocking agents. The changes induced by beta-blockade with all agents were reversed by bronchodilator therapy.
Electrophysiology studies in man have demonstrated that bisoprolol fumarate significantly decreases heart rate, increases sinus node recovery time, prolongs AV node refractory periods, and, with rapid atrial stimulation, prolongs AV nodal conduction.
Acute effects of thiazides are thought to result from a reduction in blood volume and cardiac output, secondary to a natriuretic effect, although a direct vasodilatory mechanism has also been proposed. With chronic administration, plasma volume returns toward normal, but peripheral vascular resistance is decreased.
Thiazides do not affect normal blood pressure. Onset of action occurs within 2 hours of dosing, peak effect is observed at about 4 hours, and activity persists for up to 24 hours.
Bisoprolol fumarate and hydrochlorothiazide tablets are contraindicated in patients in cardiogenic shock, overt cardiac failure (see WARNINGS), second or third degree AV block, marked sinus bradycardia, anuria, and hypersensitivity to either component of this product or to other sulfonamide-derived drugs.
Bisoprolol fumarate and hydrochlorothiazide tablets may potentiate the action of other antihypertensive agents used concomitantly. Bisoprolol fumarate and hydrochlorothiazide tablets should not be combined with other beta-blocking agents. Patients receiving catecholamine-depleting drugs, such as reserpine or guanethidine, should be closely monitored because the added beta-adrenergic blocking action of bisoprolol fumarate may produce excessive reduction of sympathetic activity. In patients receiving concurrent therapy with clonidine, if therapy is to be discontinued, it is suggested that bisoprolol fumarate and hydrochlorothiazide tablets be discontinued for several days before the withdrawal of clonidine.
Bisoprolol fumarate and hydrochlorothiazide tablets should be used with caution when myocardial depressants or inhibitors of AV conduction, such as certain calcium antagonists (particularly of the phenylalkylamine [verapamil] and benzothiazepine [diltiazem] classes), or antiarrhythmic agents, such as disopyramide, are used concurrently.
Both digitalis glycosides and beta-blockers slow atrioventricular conduction and decrease heart rate. Concomitant use can increase the risk of bradycardia.
Bisoprolol fumarate and hydrochlorothiazide tablets are indicated for the treatment of hypertension. It combines two antihypertensive agents in a once-daily dosage: a synthetic beta1-selective (cardioselective) adrenoceptor blocking agent (bisoprolol fumarate) and a benzothiadiazine diuretic (hydrochlorothiazide).
Bisoprolol fumarate is chemically described as (±)-1-[4-[[2-(1-methylethoxy)ethoxy]methyl]phenoxy]-3-[(1-methylethyl)amino]-2-propanol(
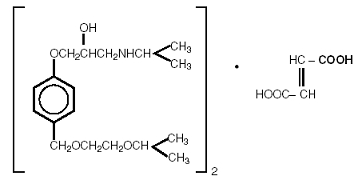
Bisoprolol fumarate is a white crystalline powder, approximately equally hydrophilic and lipophilic, and readily soluble in water, methanol, ethanol, and chloroform.
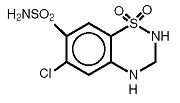
Hydrochlorothiazide (HCTZ) is 6-Chloro-3,4-dihydro-2
Each bisoprolol fumarate and hydrochlorothiazide tablet - 2.5 mg/6.25 mg tablet for oral administration contains:
Bisoprolol fumarate…………………………………………..2.5 mg
Hydrochlorothiazide………………………………………..6.25 mg
Each bisoprolol fumarate and hydrochlorothiazide tablet - 5 mg/6.25 mg tablet for oral administration contains:
Bisoprolol fumarate……………………………………………5 mg
Hydrochlorothiazide………………………………………..6.25 mg
Each bisoprolol fumarate and hydrochlorothiazide tablet - 10 mg/6.25 mg tablet for oral administration contains:
Bisoprolol fumarate…………………………………………...10 mg
Hydrochlorothiazide………………………………………..6.25 mg
Inactive ingredients include Corn Starch, Dibasic Calcium Phosphate, Hypromellose, Magnesium Stearate, Microcrystalline Cellulose, Polyethylene Glycol, Polysorbate 80, and Titanium Dioxide. The 10 mg/6.25mg tablet also contains Colloidal Silicon Dioxide. The 5 mg/6.25 mg tablet also contains Colloidal Silicon Dioxide, and Red and Yellow Iron Oxide. The 2.5 mg/6.25 mg tablet also contains Crospovidone, Pregelatinized Starch, and Yellow Iron Oxide.
Bisoprolol fumarate and HCTZ have been used individually and in combination for the treatment of hypertension. The antihypertensive effects of these agents are additive; HCTZ 6.25 mg significantly increases the antihypertensive effect of bisoprolol fumarate. The incidence of hypokalemia with the bisoprolol fumarate and HCTZ 6.25 mg combination (B/H) is significantly lower than with HCTZ 25 mg. In clinical trials of bisoprolol fumarate and hydrochlorothiazide tablets, mean changes in serum potassium for patients treated with bisoprolol fumarate and hydrochlorothiazide tablets 2.5/6.25 mg, 5/6.25 mg or 10/6.25 mg or placebo were less than ± 0.1 mEq/L. Mean changes in serum potassium for patients treated with any dose of bisoprolol in combination with HCTZ 25 mg ranged from -0.1 to -0.3 mEq/L.
Bisoprolol fumarate is a beta1-selective (cardioselective) adrenoceptor blocking agent without significant membrane stabilizing or intrinsic sympathomimetic activities in its therapeutic dose range. At higher doses (≥20 mg) bisoprolol fumarate also inhibits beta2-adrenoreceptors located in bronchial and vascular musculature. To retain relative selectivity, it is important to use the lowest effective dose.
Hydrochlorothiazide is a benzothiadiazine diuretic. Thiazides affect renal tubular mechanisms of electrolyte reabsorption and increase excretion of sodium and chloride in approximately equivalent amounts. Natriuresis causes a secondary loss of potassium.