Blisovi Fe 1.5/30 Prescribing Information
Blisovi Fe 1.5/30 is indicated for the prevention of pregnancy in women who elect to use oral contraceptives as a method of contraception.
Oral contraceptives are highly effective. Table I lists the typical accidental pregnancy rates for users of combination oral contraceptives and other methods of contraception. The efficacy of these contraceptive methods, except sterilization, depends upon the reliability with which they are used. Correct and consistent use of methods can result in lower failure rates.
Adapted from RA Hatcher et al, Reference 7. | ||
% of Women Experiencing an Unintended Pregnancy in the First Year of Continuous Use | ||
Method | Lowest Expected The authors' best guess of the percentage of women expected to experience an accidental pregnancy among couples who initiate a method (not necessarily for the first time) and who use it consistently and correctly during the first year if they do not stop for any other reason. | Typical This term represents "typical" couples who initiate use of a method (not necessarily for the first time), who experience an accidental pregnancy during the first year if they do not stop use for any other reason. |
| (No contraception) | (85) | (85) |
| Oral contraceptives | 3 | |
| Combined | 0.1 | N/AN/A--Data not available |
| Progestin only | 0.5 | N/A |
| Diaphragm with spermicidal cream or jelly | 6 | 20 |
| Spermicides alone (foam, creams, gels, vaginal suppositories, and vaginal film) | 6 | 26 |
| Vaginal Sponge | ||
| Nulliparous | 9 | 20 |
| Parous | 20 | 40 |
| Implant | 0.05 | 0.05 |
| Injection:depot medroxyprogesterone acetate | 0.3 | 0.3 |
| IUD | ||
| Progesterone T | 1.5 | 2.0 |
| Copper T 380A | 0.6 | 0.8 |
| LNg 20 | 0.1 | 0.1 |
| Condom without spermicides | ||
| Female | 5 | 21 |
| Male | 3 | 14 |
| Cervical Cap with spermicidal Cream of jelly | ||
| Nulliparous | 9 | 20 |
| Parous | 26 | 40 |
| Periodic abstinence (all methods) | 1 to 9 | 25 |
| Withdrawal | 4 | 19 |
| Female sterilization | 0.5 | 0.5 |
| Male sterilization | 0.10 | 0.15 |
The blister has been designed to make oral contraceptive dosing as easy and as convenient as possible. The tablets are arranged in four rows of seven tablets each, with the days of the week appearing on the blister above the first row of tablets.
The possibility of ovulation and conception prior to initiation of use should be considered.
Oral contraceptives are contraindicated in women who currently have the following conditions:
- Thrombophlebitis or thromboembolic disorders
- A past history of deep vein thrombophlebitis or thromboembolic disorders
- Cerebral vascular or coronary artery disease
- Current diagnosis of, or history of, breast cancer, which may be hormone sensitive
- Carcinoma of the endometrium or other known or suspected estrogen-dependent neoplasia
- Undiagnosed abnormal genital bleeding
- Cholestatic jaundice of pregnancy or jaundice with prior pill use
- Hepatic adenomas or carcinomas
- Are receiving Hepatitis C drug combinations containing ombitasvir/paritaprevir/ritonavir, with or without dasabuvir, due to the potential for ALT elevations (see WARNINGS,.RISK OF LIVER ENZYME ELEVATIONS WITH CONCOMITANT HEPATITIS C TREATMENT)
An increased risk of the following serious adverse reactions has been associated with the use of oral contraceptives
- Thrombophlebitis
- Arterial thromboembolism
- Pulmonary embolism
- Myocardial infarction
- Cerebral hemorrhage
- Cerebral thrombosis
- Hypertension
- Gallbladder disease
- Hepatic adenomas or benign liver tumors
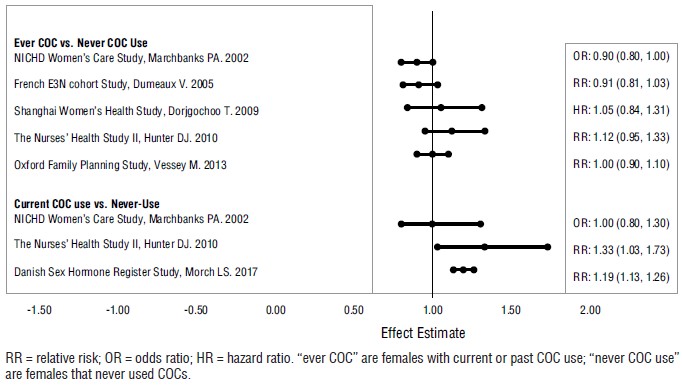
Five studies that compared breast cancer risk between ever-users (current or past use) of COCs and never-users of COCs reported no association between ever use of COCs and breast cancer risk, with effect estimates ranging from 0.90 to 1.12 (Figure 1) (70-74).
Three studies compared breast cancer risk between current or recent COC users (<6 months since last use) and never users of COCs (Figure 1) (70, 73, 75). One of these studies reported no association between breast cancer risk and COC use. The other two studies found an increased relative risk of 1.19 to 1.33 with current or recent use. Both of these studies found an increased risk of breast cancer with current use of longer duration, with relative risks ranging from 1.03 with less than one year of COC use to approximately 1.4 with more than 8 to 10 years of COC use.
There is evidence of an association between the following conditions and the use of oral contraceptives, although additional confirmatory studies are needed:
- Mesenteric thrombosis
- Retinal thrombosis
The following adverse reactions have been reported in patients receiving oral contraceptives and are believed to be drug-related:
- Nausea
- Vomiting
- Gastrointestinal symptoms (such as abdominal cramps and bloating)
- Breakthrough bleeding
- Spotting
- Change in menstrual flow
- Amenorrhea
- Temporary infertility after discontinuation of treatment
- Edema
- Melasma which may persist
- Breast changes: tenderness, enlargement, secretion
- Change in weight (increase or decrease)
- Change in cervical erosion and secretion
- Diminution in lactation when given immediately postpartum
- Cholestatic jaundice
- Migraine
- Rash (allergic)
- Depression
- Reduced tolerance to carbohydrates
- Vaginal candidiasis
- Change in corneal curvature (steepening)
- Intolerance to contact lenses
The following adverse reactions have been reported in users of oral contraceptives and the association has been neither confirmed nor refuted:
- Pre-menstrual syndrome
- Cataracts
- Changes in appetite
- Cystitis-like syndrome
- Headache
- Nervousness
- Dizziness
- Hirsutism
- Loss of scalp hair
- Erythema multiforme
- Erythema nodosum
- Hemorrhagic eruption
- Vaginitis
- Porphyria
- Impaired renal function
- Hemolytic uremic syndrome
- Budd-Chiari syndrome
- Acne
- Changes in libido
- Colitis
Blisovi Fe 1.5/30 is progestogen-estrogen combination.
Blisovi Fe 1.5/30 provides a continuous dosage regimen consisting of 21 oral contraceptive tablets and seven ferrous fumarate tablets. The ferrous fumarate tablets are present to facilitate ease of drug administration via a 28-day regimen, are non-hormonal, and do not serve any therapeutic purpose.
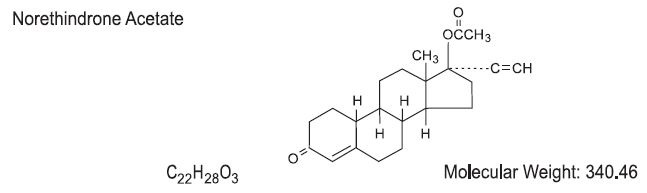
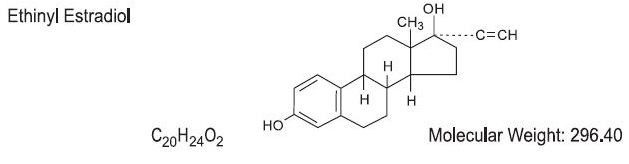
Each pink tablet contains norethindrone acetate (17α-ethinyl-19-nortestosterone acetate), 1.5 mg; ethinyl estradiol (17α-ethinyl-1,3,5(10)-estratriene-3,17β-diol), 30 mcg.
Each pink tablet contains the following inactive ingredients: acacia, confectioner's sugar, corn starch, FD & C red no. 40, lactose monohydrate, magnesium stearate and talc.
Each brown placebo tablet contains ferrous fumarate, magnesium stearate, microcrystalline cellulose, povidone, sodium starch glycolate, and sucrose.
The ferrous fumarate tablets do not serve any therapeutic purpose.