Bortezomib
Bortezomib Prescribing Information
For injection: Each single-dose vial of bortezomib for injection contains 3.5 mg of bortezomib as a sterile lyophilized white to off-white powder for reconstitution and withdrawal of the appropriate individual patient dose
Use proper aseptic technique. Reconstitute
Different volumes of 0.9% sodium chloride are used to reconstitute the product for the different routes of administration. The reconstituted concentration of bortezomib for subcutaneous administration (2.5 mg per mL) is greater than the reconstituted concentration of bortezomib for intravenous administration (1 mg per mL).
For each 3.5 mg single-dose vial of bortezomib, reconstitute with the following volume of 0.9% sodium chloride based on route of administration
| Route of Administration | Bortezomib (mg per vial) | Diluent (0.9% Sodium Chloride) | Final Bortezomib Concentration (mg per mL) |
|---|---|---|---|
| Intravenous | 3.5 mg | 3.5 mL | 1 mg per mL |
| Subcutaneous | 3.5 mg | 1.4 mL | 2.5 mg per mL |
Dose must be individualized to prevent overdosage. After determining patient body surface area (BSA) in square meters, use the following equations to calculate the total volume (mL) of reconstituted bortezomib for injection to be administered:
•
| Bortezomib for Injection dose (mg/m2) × patient BSA (m2) | = Total bortezomib for injection volume (mL) to be administered |
| 1 mg per mL |
•
| Bortezomib for Injection dose (mg/m2) × patient BSA (m2) | = Total bortezomib for injection volume (mL) to be administered |
| 2.5 mg per mL |
Stickers that indicate the route of administration are provided with each bortezomib for injection vial. These stickers should be placed directly on the syringe of bortezomib for injection once bortezomib for injection is prepared to help alert practitioners of the correct route of administration for bortezomib for injection.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit. If any discoloration or particulate matter is observed, the reconstituted product should not be used.
Unopened vials of bortezomib for injection are stable until the date indicated on the package when stored in the original package protected from light.
Bortezomib for injection contains no antimicrobial preservative. Administer reconstituted bortezomib for injection within eight hours of preparation. When reconstituted as directed, bortezomib for injection may be stored at 25°C (77°F). The reconstituted material may be stored in the original vial and/or the syringe prior to administration. The product may be stored for up to eight hours in a syringe; however, total storage time for the reconstituted material must not exceed eight hours when exposed to normal indoor lighting.
Bortezomib for injection is contraindicated in patients with hypersensitivity (not including local reactions) to bortezomib, boron, or mannitol. Reactions have included anaphylactic reactions
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Table 9 describes safety data from 340 patients with previously untreated multiple myeloma who received bortezomib (1.3 mg/m2) administered intravenously in combination with melphalan (9 mg/m2) and prednisone (60 mg/m2) in a prospective randomized study.
The safety profile of bortezomib in combination with melphalan/prednisone is consistent with the known safety profiles of both bortezomib and melphalan/prednisone.
| Bortezomib, Melphalan and Prednisone | Melphalan and Prednisone | |||||
|---|---|---|---|---|---|---|
| (n=340) | (n=337) | |||||
| Body System | Total | Toxicity Grade, n (%) | Total | Toxicity Grade, n (%) | ||
| Adverse Reaction | n (%) | 3 | ≥4 | n (%) | 3 | ≥4 |
| Blood and Lymphatic System Disorders | ||||||
| Thrombocytopenia | 164 (48) | 60 (18) | 57 (17) | 140 (42) | 48 (14) | 39 (12) |
| Neutropenia | 160 (47) | 101 (30) | 33 (10) | 143 (42) | 77 (23) | 42 (12) |
| Anemia | 109 (32) | 41 (12) | 4 (1) | 156 (46) | 61 (18) | 18 (5) |
| Leukopenia | 108 (32) | 64 (19) | 8 (2) | 93 (28) | 53 (16) | 11 (3) |
| Lymphopenia | 78 (23) | 46 (14) | 17 (5) | 51 (15) | 26 (8) | 7 (2) |
| Gastrointestinal Disorders | ||||||
| Nausea | 134 (39) | 10 (3) | 0 | 70 (21) | 1 (<1) | 0 |
| Diarrhea | 119 (35) | 19 (6) | 2 (1) | 20 (6) | 1 (<1) | 0 |
| Vomiting | 87 (26) | 13 (4) | 0 | 41 (12) | 2 (1) | 0 |
| Constipation | 77 (23) | 2 (1) | 0 | 14 (4) | 0 | 0 |
| Abdominal pain upper | 34 (10) | 1 (<1) | 0 | 20 (6) | 0 | 0 |
| Nervous System Disorders | ||||||
| Peripheral neuropathyRepresents High Level Term Peripheral Neuropathies NEC | 156 (46) | 42 (12) | 2 (1) | 4 (1) | 0 | 0 |
| Neuralgia | 117 (34) | 27 (8) | 2 (1) | 1 (<1) | 0 | 0 |
| Paresthesia | 42 (12) | 6 (2) | 0 | 4 (1) | 0 | 0 |
| General Disorders and Administration Site Conditions | ||||||
| Fatigue | 85 (25) | 19 (6) | 2 (1) | 48 (14) | 4 (1) | 0 |
| Asthenia | 54 (16) | 18 (5) | 0 | 23 (7) | 3 (1) | 0 |
| Pyrexia | 53 (16) | 4 (1) | 0 | 19 (6) | 1 (<1) | 1 (<1) |
| Infections and Infestations | ||||||
| Herpes Zoster | 39 (11) | 11 (3) | 0 | 9 (3) | 4 (1) | 0 |
| Metabolism and Nutrition Disorders | ||||||
| Anorexia | 64 (19) | 6 (2) | 0 | 19 (6) | 0 | 0 |
| Skin and Subcutaneous Tissue Disorders | ||||||
| Rash | 38 (11) | 2 (1) | 0 | 7 (2) | 0 | 0 |
| Psychiatric Disorders | ||||||
| Insomnia | 35 (10) | 1 (<1) | 0 | 21 (6) | 0 | 0 |
The safety data described below and in Table 10 reflect exposure to either bortezomib (n=331) or dexamethasone (n=332) in a study of patients with relapsed multiple myeloma. Bortezomib was administered intravenously at doses of 1.3 mg/m2twice weekly for two out of three weeks (21 day cycle). After eight, 21 day cycles patients continued therapy for three, 35 day cycles on a weekly schedule. Duration of treatment was up to 11 cycles (nine months) with a median duration of six cycles (4.1 months). For inclusion in the trial, patients must have had measurable disease and one to three prior therapies. There was no upper age limit for entry. Creatinine clearance could be as low as 20 mL/min and bilirubin levels as high as 1.5 times the upper limit of normal. The overall frequency of adverse reactions was similar in men and women, and in patients <65 and ≥65 years of age. Most patients were Caucasian
Among the 331 bortezomib-treated patients, the most commonly reported (>20%) adverse reactions overall were nausea (52%), diarrhea (52%), fatigue (39%), peripheral neuropathies (35%), thrombocytopenia (33%), constipation (30%), vomiting (29%), and anorexia (21%). The most commonly reported (>20%) adverse reaction reported among the 332 patients in the dexamethasone group was fatigue (25%). Eight percent (8%) of patients in the bortezomib-treated arm experienced a Grade 4 adverse reaction; the most common reactions were thrombocytopenia (4%) and neutropenia (2%). Nine percent (9%) of dexamethasone-treated patients experienced a Grade 4 adverse reaction. All individual dexamethasone-related Grade 4 adverse reactions were less than 1%.
Serious adverse reactions are defined as any reaction that results in death, is life-threatening, requires hospitalization or prolongs a current hospitalization, results in a significant disability, or is deemed to be an important medical event. A total of 80 (24%) patients from the bortezomib treatment arm experienced a serious adverse reaction during the study, as did 83 (25%) dexamethasone-treated patients. The most commonly reported serious adverse reactions in the bortezomib treatment arm were diarrhea (3%), dehydration, herpes zoster, pyrexia, nausea, vomiting, dyspnea, and thrombocytopenia (2% each). In the dexamethasone treatment group, the most commonly reported serious adverse reactions were pneumonia (4%), hyperglycemia (3%), pyrexia, and psychotic disorder (2% each).
A total of 145 patients, including 84 (25%) of 331 patients in the bortezomib treatment group and 61 (18%) of 332 patients in the dexamethasone treatment group were discontinued from treatment due to adverse reactions. Among the 331 bortezomib-treated patients, the most commonly reported adverse reaction leading to discontinuation was peripheral neuropathy (8%). Among the 332 patients in the dexamethasone group, the most commonly reported adverse reactions leading to treatment discontinuation were psychotic disorder and hyperglycemia (2% each).
Four deaths were considered to be bortezomib-related in this relapsed multiple myeloma study: one case each of cardiogenic shock, respiratory insufficiency, congestive heart failure and cardiac arrest. Four deaths were considered dexamethasone-related: two cases of sepsis, one case of bacterial meningitis, and one case of sudden death at home.
The most common adverse reactions from the relapsed multiple myeloma study are shown in Table 10. All adverse reactions with incidence ≥10% in the bortezomib arm are included.
| Bortezomib (N=331) | Dexamethasone (N=332) | |||||
|---|---|---|---|---|---|---|
| Adverse Reactions | All | Grade 3 | Grade 4 | All | Grade 3 | Grade 4 |
| Any Adverse Reactions | 324 (98) | 193 (58) | 28 (8) | 297 (89) | 110 (33) | 29 (9) |
| Nausea | 172 (52) | 8 (2) | 0 | 31 (9) | 0 | 0 |
| Diarrhea NOS | 171 (52) | 22 (7) | 0 | 36 (11) | 2 (<1) | 0 |
| Fatigue | 130 (39) | 15 (5) | 0 | 82 (25) | 8 (2) | 0 |
| Peripheral neuropathiesRepresents High Level Term Peripheral Neuropathies NEC | 115 (35) | 23 (7) | 2 (<1) | 14 (4) | 0 | 1 (<1) |
| Thrombocytopenia | 109 (33) | 80 (24) | 12 (4) | 11 (3) | 5 (2) | 1 (<1) |
| Constipation | 99 (30) | 6 (2) | 0 | 27 (8) | 1 (<1) | 0 |
| Vomiting NOS | 96 (29) | 8 (2) | 0 | 10 (3) | 1 (<1) | 0 |
| Anorexia | 68 (21) | 8 (2) | 0 | 8 (2) | 1 (<1) | 0 |
| Pyrexia | 66 (20) | 2 (<1) | 0 | 21 (6) | 3 (<1) | 1 (<1) |
| Paresthesia | 64 (19) | 5 (2) | 0 | 24 (7) | 0 | 0 |
| Anemia NOS | 63 (19) | 20 (6) | 1 (<1) | 21 (6) | 8 (2) | 0 |
| Headache NOS | 62 (19) | 3 (<1) | 0 | 23 (7) | 1 (<1) | 0 |
| Neutropenia | 58 (18) | 37 (11) | 8 (2) | 1 (<1) | 1 (<1) | 0 |
| Rash NOS | 43 (13) | 3 (<1) | 0 | 7 (2) | 0 | 0 |
| Appetite decreased NOS | 36 (11) | 0 | 0 | 12 (4) | 0 | 0 |
| Dyspnea NOS | 35 (11) | 11 (3) | 1 (<1) | 37 (11) | 7 (2) | 1 (<1) |
| Abdominal pain NOS | 35 (11) | 5 (2) | 0 | 7 (2) | 0 | 0 |
| Weakness | 34 (10) | 10 (3) | 0 | 28 (8) | 8 (2) | 0 |
In the Phase 2 extension study of 63 patients, no new cumulative or new long-term toxicities were observed with prolonged bortezomib treatment. These patients were treated for a total of 5.3 to 23 months, including time on bortezomib in the prior bortezomib study
The safety and efficacy of bortezomib administered subcutaneously were evaluated in one Phase 3 study at the recommended dose of 1.3 mg/m2. This was a randomized, comparative study of bortezomib subcutaneous vs intravenous in 222 patients with relapsed multiple myeloma. The safety data described below and in Table 11 reflect exposure to either bortezomib subcutaneous (N=147) or bortezomib intravenous (N=74)
| Subcutaneous | Intravenous | |||||
|---|---|---|---|---|---|---|
| (N=147) | (N=74) | |||||
| Body System | Total | Toxicity Grade, n (%) | Total | Toxicity Grade, n (%) | ||
| Adverse Reaction | n (%) | 3 | ≥4 | n (%) | 3 | ≥4 |
| Blood and Lymphatic System Disorders | ||||||
| Anemia | 28 (19) | 8 (5) | 0 | 17 (23) | 3 (4) | 0 |
| Leukopenia | 26 (18) | 8 (5) | 0 | 15 (20) | 4 (5) | 1 (1) |
| Neutropenia | 34 (23) | 15 (10) | 4 (3) | 20 (27) | 10 (14) | 3 (4) |
| Thrombocytopenia | 44 (30) | 7 (5) | 5 (3) | 25 (34) | 7 (9) | 5 (7) |
| Gastrointestinal Disorders | ||||||
| Diarrhea | 28 (19) | 1 (1) | 0 | 21 (28) | 3 (4) | 0 |
| Nausea | 24 (16) | 0 | 0 | 10 (14) | 0 | 0 |
| Vomiting | 13 (9) | 3 (2) | 0 | 8 (11) | 0 | 0 |
| General Disorders and Administration Site Conditions | ||||||
| Asthenia | 10 (7) | 1 (1) | 0 | 12 (16) | 4 (5) | 0 |
| Fatigue | 11 (7) | 3 (2) | 0 | 11 (15) | 3 (4) | 0 |
| Pyrexia | 18 (12) | 0 | 0 | 6 (8) | 0 | 0 |
| Nervous System Disorders | ||||||
| Neuralgia | 34 (23) | 5 (3) | 0 | 17 (23) | 7 (9) | 0 |
| Peripheral neuropathies * | 55 (37) | 8 (5) | 1 (1) | 37 (50) | 10 (14) | 1 (1) |
Note: Safety population: 147 patients in the subcutaneous treatment group and 74 patients in the intravenous treatment group who received at least one dose of study medication
* Represents High Level Term Peripheral Neuropathies NEC
In general, safety data were similar for the subcutaneous and intravenous treatment groups. Differences were observed in the rates of some Grade ≥3 adverse reactions. Differences of ≥5% were reported in neuralgia (3% subcutaneous vs 9% intravenous), peripheral neuropathies (6% subcutaneous vs 15% intravenous), neutropenia (13% subcutaneous vs 18% intravenous), and thrombocytopenia (8% subcutaneous vs 16% intravenous).
A local reaction was reported in 6% of patients in the subcutaneous group, mostly redness. Only two (1%) patients were reported as having severe reactions, one case of pruritus and one case of redness. Local reactions led to reduction in injection concentration in one patient and drug discontinuation in one patient. Local reactions resolved in a median of six days.
Dose reductions occurred due to adverse reactions in 31% of patients in the subcutaneous treatment group compared with 43% of the intravenously-treated patients. The most common adverse reactions leading to a dose reduction included peripheral sensory neuropathy (17% in the subcutaneous treatment group compared with 31% in the intravenous treatment group); and neuralgia (11% in the subcutaneous treatment group compared with 19% in the intravenous treatment group).
The incidence of serious adverse reactions was similar for the subcutaneous treatment group (20%) and the intravenous treatment group (19%). The most commonly reported serious adverse reactions in the subcutaneous treatment arm were pneumonia and pyrexia (2% each). In the intravenous treatment group, the most commonly reported serious adverse reactions were pneumonia, diarrhea, and peripheral sensory neuropathy (3% each).
In the subcutaneous treatment group, 27 patients (18%) discontinued study treatment due to an adverse reaction compared with 17 patients (23%) in the intravenous treatment group
Two patients (1%) in the subcutaneous treatment group and one (1%) patient in the intravenous treatment group died due to an adverse reaction during treatment. In the subcutaneous group the causes of death were one case of pneumonia and one case of sudden death. In the intravenous group the cause of death was coronary artery insufficiency.
Table 12 describes safety data from 240 patients with previously untreated mantle cell lymphoma who received bortezomib (1.3 mg/m2) administered intravenously in combination with rituximab (375 mg/m2), cyclophosphamide (750 mg/m2), doxorubicin (50 mg/m2), and prednisone (100 mg/m2) (VcR-CAP) in a prospective randomized study.
Infections were reported for 31% of patients in the VcR-CAP arm and 23% of the patients in the comparator (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone [R-CHOP]) arm, including the predominant preferred term of pneumonia (VcR-CAP 8% vs R-CHOP 5%).
| VcR-CAP (n=240) | R-CHOP (n=242) | |||||
|---|---|---|---|---|---|---|
| Body System Adverse Reactions | All n (%) | Toxicity Grade 3 n (%) | Toxicity Grade ≥4 n (%) | All n (%) | Toxicity Grade 3 n (%) | Toxicity Grade ≥4 n (%) |
| Blood and Lymphatic System Disorders | ||||||
| Neutropenia | 209 (87) | 32 (13) | 168 (70) | 172 (71) | 31 (13) | 125 (52) |
| Leukopenia | 116 (48) | 34 (14) | 69 (29) | 87 (36) | 39 (16) | 27 (11) |
| Anemia | 106 (44) | 27 (11) | 4 (2) | 71 (29) | 23 (10) | 4 (2) |
| Thrombocytopenia | 172 (72) | 59 (25) | 76 (32) | 42 (17) | 9 (4) | 3 (1) |
| Febrile neutropenia | 41 (17) | 24 (10) | 12 (5) | 33 (14) | 17 (7) | 15 (6) |
| Lymphopenia | 68 (28) | 25 (10) | 36 (15) | 28 (12) | 15 (6) | 2 (1) |
| Nervous System Disorders | ||||||
| Peripheral neuropathy * | 71 (30) | 17 (7) | 1 (<1) | 65 (27) | 10 (4) | 0 |
| Hypoesthesia | 14 (6) | 3 (1) | 0 | 13 (5) | 0 | 0 |
| Paresthesia | 14 (6) | 2 (1) | 0 | 11 (5) | 0 | 0 |
| Neuralgia | 25 (10) | 9 (4) | 0 | 1 (<1) | 0 | 0 |
| General Disorders and Administration Site Conditions | ||||||
| Fatigue | 43 (18) | 11 (5) | 1 (<1) | 38 (16) | 5 (2) | 0 |
| Pyrexia | 48 (20) | 7 (3) | 0 | 23 (10) | 5 (2) | 0 |
| Asthenia | 29 (12) | 4 (2) | 1 (<1) | 18 (7) | 1 (<1) | 0 |
| Edema peripheral | 16 (7) | 1 (<1) | 0 | 13 (5) | 0 | 0 |
| Gastrointestinal Disorders | ||||||
| Nausea | 54 (23) | 1 (<1) | 0 | 28 (12) | 0 | 0 |
| Constipation | 42 (18) | 1 (<1) | 0 | 22 (9) | 2 (1) | 0 |
| Stomatitis | 20 (8) | 2 (1) | 0 | 19 (8) | 0 | 1 (<1) |
| Diarrhea | 59 (25) | 11 (5) | 0 | 11 (5) | 3 (1) | 1 (<1) |
| Vomiting | 24 (10) | 1 (<1) | 0 | 8 (3) | 0 | 0 |
| Abdominal distension | 13 (5) | 0 | 0 | 4 (2) | 0 | 0 |
| Infections and Infestations | ||||||
| Pneumonia | 20 (8) | 8 (3) | 5 (2) | 11 (5) | 5 (2) | 3 (1) |
| Skin and Subcutaneous Tissue Disorders | ||||||
| Alopecia | 31 (13) | 1 (<1) | 1 (<1) | 33 (14) | 4 (2) | 0 |
| Metabolism and Nutrition Disorders | ||||||
| Hyperglycemia | 10 (4) | 1 (<1) | 0 | 17 (7) | 10 (4) | 0 |
| Decreased appetite | 36 (15) | 2 (1) | 0 | 15 (6) | 1 (<1) | 0 |
| Vascular Disorders | ||||||
| Hypertension | 15 (6) | 1 (<1) | 0 | 3 (1) | 0 | 0 |
| Psychiatric Disorders | ||||||
| Insomnia | 16 (7) | 1 (<1) | 0 | 8 (3) | 0 | 0 |
Key: R-CHOP=rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone; VcR-CAP=Bortezomib, rituximab, cyclophosphamide, doxorubicin, and prednisone.
* Represents High Level Term Peripheral Neuropathies NEC
The incidence of herpes zoster reactivation was 4.6% in the VcR-CAP arm and 0.8% in the R-CHOP arm. Antiviral prophylaxis was mandated by protocol amendment.
The incidences of Grade ≥3 bleeding events were similar between the two arms (four patients in the VcR-CAP arm and three patients in the R-CHOP arm). All of the Grade ≥3 bleeding events resolved without sequelae in the VcR-CAP arm.
Adverse reactions leading to discontinuation occurred in 8% of patients in VcR-CAP group and 6% of patients in R-CHOP group. In the VcR-CAP group, the most commonly reported adverse reaction leading to discontinuation was peripheral sensory neuropathy (1%; three patients). The most commonly reported adverse reaction leading to discontinuation in the R-CHOP group was febrile neutropenia (<1%; two patients).
Safety data from Phase 2 and 3 studies of single agent bortezomib 1.3 mg/m2/dose twice weekly for two weeks followed by a ten day rest period in 1,163 patients with previously-treated multiple myeloma (N=1,008) and previously-treated mantle cell lymphoma (N=155) were integrated and tabulated. This analysis does not include data from the Phase 3 open-label study of bortezomib subcutaneous vs intravenous in relapsed multiple myeloma. In the integrated studies, the safety profile of bortezomib was similar in patients with multiple myeloma and mantle cell lymphoma.
In the integrated analysis, the most commonly reported (>20%) adverse reactions were nausea (49%), diarrhea (46%), asthenic conditions including fatigue (41%) and weakness (11%), peripheral neuropathies (38%), thrombocytopenia (32%), vomiting (28%), constipation (25%), and pyrexia (21%). Eleven percent (11%) of patients experienced at least one episode of ≥Grade 4 toxicity, most commonly thrombocytopenia (4%) and neutropenia (2%).
In the Phase 2 relapsed multiple myeloma clinical trials of bortezomib administered intravenously, local skin irritation was reported in 5% of patients, but extravasation of bortezomib was not associated with tissue damage.
A total of 26% of patients experienced a serious adverse reaction during the studies. The most commonly reported serious adverse reactions included diarrhea, vomiting and pyrexia (3% each), nausea, dehydration, and thrombocytopenia (2% each) and pneumonia, dyspnea, peripheral neuropathies, and herpes zoster (1% each).
Adverse reactions leading to discontinuation occurred in 22% of patients. The reasons for discontinuation included peripheral neuropathy (8%), and fatigue, thrombocytopenia, and diarrhea (2% each).
In total, 2% of the patients died and the cause of death was considered by the investigator to be possibly related to study drug: including reports of cardiac arrest, congestive heart failure, respiratory failure, renal failure, pneumonia and sepsis.
The most common adverse reactions are shown in Table 13. All adverse reactions occurring at ≥10% are included. In the absence of a randomized comparator arm, it is often not possible to distinguish between adverse events that are drug-caused and those that reflect the patient's underlying disease. Please see the discussion of specific adverse reactions that follows.
| All Patients (N=1,163) | Multiple Myeloma (N=1,008) | Mantle Cell Lymphoma (N=155) | ||||
|---|---|---|---|---|---|---|
| Adverse Reactions | All | ≥Grade 3 | All | ≥Grade 3 | All | ≥Grade 3 |
| Nausea | 567 (49) | 36 (3) | 511 (51) | 32 (3) | 56 (36) | 4 (3) |
| Diarrhea NOS | 530 (46) | 83 (7) | 470 (47) | 72 (7) | 60 (39) | 11 (7) |
| Fatigue | 477 (41) | 86 (7) | 396 (39) | 71 (7) | 81 (52) | 15 (10) |
| Peripheral neuropathiesRepresents High Level Term Peripheral Neuropathies NEC | 443 (38) | 129 (11) | 359 (36) | 110 (11) | 84 (54) | 19 (12) |
| Thrombocytopenia | 369 (32) | 295 (25) | 344 (34) | 283 (28) | 25 (16) | 12 (8) |
| Vomiting NOS | 321 (28) | 44 (4) | 286 (28) | 40 (4) | 35 (23) | 4 (3) |
| Constipation | 296 (25) | 17 (1) | 244 (24) | 14 (1) | 52 (34) | 3 (2) |
| Pyrexia | 249 (21) | 16 (1) | 233 (23) | 15 (1) | 16 (10) | 1 (<1) |
| Anorexia | 227 (20) | 19 (2) | 205 (20) | 16 (2) | 22 (14) | 3 (2) |
| Anemia NOS | 209 (18) | 65 (6) | 190 (19) | 63 (6) | 19 (12) | 2 (1) |
| Headache NOS | 175 (15) | 8 (<1) | 160 (16) | 8 (<1) | 15 (10) | 0 |
| Neutropenia | 172 (15) | 121 (10) | 164 (16) | 117 (12) | 8 (5) | 4 (3) |
| Rash NOS | 156 (13) | 8 (<1) | 120 (12) | 4 (<1) | 36 (23) | 4 (3) |
| Paresthesia | 147 (13) | 9 (<1) | 136 (13) | 8 (<1) | 11 (7) | 1 (<1) |
| Dizziness (excl vertigo) | 129 (11) | 13 (1) | 101 (10) | 9 (<1) | 28 (18) | 4 (3) |
| Weakness | 124 (11) | 31 (3) | 106 (11) | 28 (3) | 18 (12) | 3 (2) |
A total of 75% of patients experienced at least one gastrointestinal disorder. The most common gastrointestinal disorders included nausea, diarrhea, constipation, vomiting, and appetite decreased. Other gastrointestinal disorders included dyspepsia and dysgeusia. Grade 3 adverse reactions occurred in 14% of patients; ≥Grade 4 adverse reactions were ≤1%. Gastrointestinal adverse reactions were considered serious in 7% of patients. Four percent (4%) of patients discontinued due to a gastrointestinal adverse reaction. Nausea was reported more often in patients with multiple myeloma (51%) compared to patients with mantle cell lymphoma (36%).
Across the studies, bortezomib-associated thrombocytopenia was characterized by a decrease in platelet count during the dosing period (Days 1 to 11) and a return toward baseline during the ten day rest period during each treatment cycle. Overall, thrombocytopenia was reported in 32% of patients. Thrombocytopenia was Grade 3 in 22%, ≥Grade 4 in 4%, and serious in 2% of patients, and the reaction resulted in bortezomib discontinuation in 2% of patients
Overall, peripheral neuropathies occurred in 38% of patients. Peripheral neuropathy was Grade 3 for 11% of patients and ≥Grade 4 for <1% of patients. Eight percent (8%) of patients discontinued bortezomib due to peripheral neuropathy. The incidence of peripheral neuropathy was higher among patients with mantle cell lymphoma (54%) compared to patients with multiple myeloma (36%).
In the bortezomib vs dexamethasone Phase 3 relapsed multiple myeloma study, among the 62 bortezomib-treated patients who experienced ≥Grade 2 peripheral neuropathy and had dose adjustments, 48% had improved or resolved with a median of 3.8 months from first onset.
In the Phase 2 relapsed multiple myeloma studies, among the 30 patients who experienced Grade 2 peripheral neuropathy resulting in discontinuation or who experienced ≥Grade 3 peripheral neuropathy, 73% reported improvement or resolution with a median time of 47 days to improvement of one grade or more from the last dose of bortezomib.
The incidence of hypotension (postural, orthostatic and hypotension NOS) was 8% in patients treated with bortezomib. Hypotension was Grade 1 or 2 in the majority of patients and Grade 3 in 2% and ≥Grade 4 in <1%. Two percent (2%) of patients had hypotension reported as a serious adverse reaction, and 1% discontinued due to hypotension. The incidence of hypotension was similar in patients with multiple myeloma (8%) and those with mantle cell lymphoma (9%). In addition, <1% of patients experienced hypotension associated with a syncopal reaction.
Neutrophil counts decreased during the bortezomib dosing period (Days 1 to 11) and returned toward baseline during the ten day rest period during each treatment cycle. Overall, neutropenia occurred in 15% of patients and was Grade 3 in 8% of patients and ≥Grade 4 in 2%. Neutropenia was reported as a serious adverse reaction in <1% of patients and <1% of patients discontinued due to neutropenia. The incidence of neutropenia was higher in patients with multiple myeloma (16%) compared to patients with mantle cell lymphoma (5%). The incidence of ≥Grade 3 neutropenia also was higher in patients with multiple myeloma (12%) compared to patients with mantle cell lymphoma (3%).
Asthenic conditions were reported in 54% of patients. Fatigue was reported as Grade 3 in 7% and ≥Grade 4 in <1% of patients. Asthenia was reported as Grade 3 in 2% and ≥Grade 4 in < 1% of patients. Two percent (2%) of patients discontinued treatment due to fatigue and < 1% due to weakness and asthenia. Asthenic conditions were reported in 53% of patients with multiple myeloma and 59% of patients with mantle cell lymphoma.
Pyrexia (>38°C) was reported as an adverse reaction for 21% of patients. The reaction was Grade 3 in 1% and ≥Grade 4 in <1%. Pyrexia was reported as a serious adverse reaction in 3% of patients and led to bortezomib discontinuation in <1% of patients. The incidence of pyrexia was higher among patients with multiple myeloma (23%) compared to patients with mantle cell lymphoma (10%). The incidence of ≥Grade 3 pyrexia was 1% in patients with multiple myeloma and <1% in patients with mantle cell lymphoma.
Consider using antiviral prophylaxis in subjects being treated with bortezomib. In the randomized studies in previously untreated and relapsed multiple myeloma, herpes zoster reactivation was more common in subjects treated with bortezomib (ranging between 6% to 11%) than in the control groups (3% to 4%). Herpes simplex was seen in 1% to 3% in subjects treated with bortezomib and 1% to 3% in the control groups. In the previously untreated multiple myeloma study, herpes zoster virus reactivation in the bortezomib, melphalan and prednisone arm was less common in subjects receiving prophylactic antiviral therapy (3%) than in subjects who did not receive prophylactic antiviral therapy (17%).
A single-arm trial was conducted in 130 patients with relapsed multiple myeloma to determine the efficacy and safety of retreatment with intravenous bortezomib. The safety profile of patients in this trial is consistent with the known safety profile of bortezomib-treated patients with relapsed multiple myeloma as demonstrated in Table 10, Table 11, and Table 13; no cumulative toxicities were observed upon retreatment. The most common adverse drug reaction was thrombocytopenia which occurred in 52% of the patients. The incidence of ≥Grade 3 thrombocytopenia was 24%. Peripheral neuropathy occurred in 28% of patients, with the incidence of ≥Grade 3 peripheral neuropathy reported at 6%. The incidence of serious adverse reactions was 12.3%. The most commonly reported serious adverse reactions were thrombocytopenia (3.8%), diarrhea (2.3%), and herpes zoster and pneumonia (1.5% each).
Adverse reactions leading to discontinuation occurred in 13% of patients. The reasons for discontinuation included peripheral neuropathy (5%) and diarrhea (3%).
Two deaths considered to be bortezomib-related occurred within 30 days of the last bortezomib dose; one in a patient with cerebrovascular accident and one in a patient with sepsis.
The following clinically important serious adverse reactions that are not described above have been reported in clinical trials in patients treated with bortezomib administered as monotherapy or in combination with other chemotherapeutics. These studies were conducted in patients with hematological malignancies and in solid tumors.
Blood and Lymphatic System Disorders: Anemia, disseminated intravascular coagulation, febrile neutropenia, lymphopenia, leukopenia
Cardiac Disorders: Angina pectoris, atrial fibrillation aggravated, atrial flutter, bradycardia, sinus arrest, cardiac amyloidosis, complete atrioventricular block, myocardial ischemia, myocardial infarction, pericarditis, pericardial effusion,
Ear and Labyrinth Disorders: Hearing impaired, vertigo
Eye Disorders: Diplopia and blurred vision, conjunctival infection, irritation
Gastrointestinal Disorders: Abdominal pain, ascites, dysphagia, fecal impaction, gastroenteritis, gastritis hemorrhagic, hematemesis, hemorrhagic duodenitis, ileus paralytic, large intestinal obstruction, paralytic intestinal obstruction, peritonitis, small intestinal obstruction, large intestinal perforation, stomatitis, melena, pancreatitis acute, oral mucosal petechiae, gastroesophageal reflux
General Disorders and Administration Site Conditions: Chills, edema, edema peripheral, injection site erythema, neuralgia, injection site pain, irritation, malaise, phlebitis
Hepatobiliary Disorders: Cholestasis, hepatic hemorrhage, hyperbilirubinemia, portal vein thrombosis, hepatitis, liver failure
Immune System Disorders: Anaphylactic reaction, drug hypersensitivity, immune complex mediated hypersensitivity, angioedema, laryngeal edema
Infections and Infestations: Aspergillosis, bacteremia, bronchitis, urinary tract infection, herpes viral infection, listeriosis, nasopharyngitis, pneumonia, respiratory tract infection, septic shock, toxoplasmosis, oral candidiasis, sinusitis, catheter-related infection
Injury, Poisoning and Procedural Complications: Catheter-related complication, skeletal fracture, subdural hematoma
Investigations: Weight decreased
Metabolism and Nutrition Disorders: Dehydration, hypocalcemia, hyperuricemia, hypokalemia, hyperkalemia, hyponatremia, hypernatremia
Musculoskeletal and Connective Tissue Disorders: Arthralgia, back pain, bone pain, myalgia, pain in extremity
Nervous System Disorders: Ataxia, coma, dizziness, dysarthria, dysesthesia, dysautonomia, encephalopathy, cranial palsy, grand mal convulsion, headache, hemorrhagic stroke, motor dysfunction, neuralgia, spinal cord compression, paralysis, postherpetic neuralgia, transient ischemic attack
Psychiatric Disorders: Agitation, anxiety, confusion, insomnia, mental status change, psychotic disorder, suicidal ideation
Renal and Urinary Disorders: Calculus renal, bilateral hydronephrosis, bladder spasm, hematuria, hemorrhagic cystitis, urinary incontinence, urinary retention, renal failure (acute and chronic), glomerular nephritis proliferative
Respiratory, Thoracic and Mediastinal Disorders: Acute respiratory distress syndrome, aspiration pneumonia, atelectasis, chronic obstructive airways disease exacerbated, cough, dysphagia, dyspnea, dyspnea exertional, epistaxis, hemoptysis, hypoxia, lung infiltration, pleural effusion, pneumonitis, respiratory distress, pulmonary hypertension
Skin and Subcutaneous Tissue Disorders: Urticaria, face edema, rash (which may be pruritic), leukocytoclastic vasculitis, pruritus.
Vascular Disorders: Cerebrovascular accident, cerebral hemorrhage, deep venous thrombosis, hypertension, peripheral embolism, pulmonary embolism, pulmonary hypertension
Bortezomib for injection is contraindicated for intrathecal administration. Fatal events have occurred with intrathecal administration of bortezomib for injection.
The following clinically significant adverse reactions are also discussed in other sections of the labeling:
- Peripheral Neuropathy[see]
5.1 Peripheral NeuropathyBortezomib treatment causes a peripheral neuropathy that is predominantly sensory; however, cases of severe sensory and motor peripheral neuropathy have been reported. Patients with pre-existing symptoms (numbness, pain or a burning feeling in the feet or hands) and/or signs of peripheral neuropathy may experience worsening peripheral neuropathy (including ≥Grade 3) during treatment with bortezomib. Patients should be monitored for symptoms of neuropathy, such as a burning sensation, hyperesthesia, hypoesthesia, paresthesia, discomfort, neuropathic pain or weakness. In the Phase 3 relapsed multiple myeloma trial comparing bortezomib subcutaneous vs intravenous, the incidence of Grade ≥2 peripheral neuropathy was 24% for subcutaneous and 39% for intravenous. Grade ≥3 peripheral neuropathy occurred in 6% of patients in the subcutaneous treatment group, compared with 15% in the intravenous treatment group
[see Adverse Reactions (6.1)]. Starting bortezomib subcutaneously may be considered for patients with pre-existing or at high risk of peripheral neuropathy.Patients experiencing new or worsening peripheral neuropathy during bortezomib therapy may require a decrease in the dose and/or a less dose-intense schedule
[see Dosage and Administration (2.7)]. In the bortezomib vs dexamethasone Phase 3 relapsed multiple myeloma study, improvement in or resolution of peripheral neuropathy was reported in 48% of patients with ≥Grade 2 peripheral neuropathy following dose adjustment or interruption. Improvement in or resolution of peripheral neuropathy was reported in 73% of patients who discontinued due to Grade 2 neuropathy or who had ≥Grade 3 peripheral neuropathy in the Phase 2 multiple myeloma studies. The long-term outcome of peripheral neuropathy has not been studied in mantle cell lymphoma. - Hypotension[see]
5.2 HypotensionThe incidence of hypotension (postural, orthostatic, and hypotension NOS) was 8%
[see Adverse Reactions (6.1)]. These events are observed throughout therapy. Patients with a history of syncope, patients receiving medications known to be associated with hypotension, and patients who are dehydrated may be at increased risk of hypotension. Management of orthostatic/postural hypotension may include adjustment of antihypertensive medications, hydration, and administration of mineralocorticoids and/or sympathomimetics. - Cardiac Toxicity[see]
5.3 Cardiac ToxicityAcute development or exacerbation of congestive heart failure and new onset of decreased left ventricular ejection fraction have occurred during bortezomib therapy, including reports in patients with no risk factors for decreased left ventricular ejection fraction
[see Adverse Reactions (6.1)]. Patients with risk factors for, or existing heart disease should be frequently monitored. In the relapsed multiple myeloma study of bortezomib vs dexamethasone, the incidence of any treatment-related cardiac disorder was 8% and 5% in the bortezomib and dexamethasone groups, respectively. The incidence of adverse reactions suggestive of heart failure (acute pulmonary edema, pulmonary edema, cardiac failure, congestive cardiac failure, cardiogenic shock) was ≤1% for each individual reaction in the bortezomib group. In the dexamethasone group the incidence was ≤1% for cardiac failure and congestive cardiac failure; there were no reported reactions of acute pulmonary edema, pulmonary edema, or cardiogenic shock. There have been isolated cases of QT-interval prolongation in clinical studies; causality has not been established. - Pulmonary Toxicity[see]
5.4 Pulmonary ToxicityAcute Respiratory Distress Syndrome (ARDS) and acute diffuse infiltrative pulmonary disease of unknown etiology such as pneumonitis, interstitial pneumonia, lung infiltration have occurred in patients receiving bortezomib. Some of these events have been fatal.
In a clinical trial, the first two patients given high-dose cytarabine (2 g/m2per day) by continuous infusion with daunorubicin and bortezomib for relapsed acute myelogenous leukemia died of ARDS early in the course of therapy.
There have been reports of pulmonary hypertension associated with bortezomib administration in the absence of left heart failure or significant pulmonary disease.
In the event of new or worsening cardiopulmonary symptoms, consider interrupting bortezomib until a prompt and comprehensive diagnostic evaluation is conducted.
- Posterior Reversible Encephalopathy Syndrome (PRES)[see]
5.5 Posterior Reversible Encephalopathy Syndrome (PRES)Posterior Reversible Encephalopathy Syndrome (PRES; formerly termed Reversible Posterior Leukoencephalopathy Syndrome (RPLS)) has occurred in patients receiving bortezomib. PRES is a rare, reversible, neurological disorder which can present with seizure, hypertension, headache, lethargy, confusion, blindness, and other visual and neurological disturbances. Brain imaging, preferably MRI (Magnetic Resonance Imaging), is used to confirm the diagnosis. In patients developing PRES, discontinue bortezomib. The safety of reinitiating bortezomib therapy in patients previously experiencing PRES is not known.
- Gastrointestinal Toxicity[see]
5.6 Gastrointestinal ToxicityBortezomib treatment can cause nausea, diarrhea, constipation, and vomiting
[see Adverse Reactions (6.1)]sometimes requiring use of antiemetic and antidiarrheal medications. Ileus can occur. Fluid and electrolyte replacement should be administered to prevent dehydration. Interrupt bortezomib for severe symptoms. - Thrombocytopenia/Neutropenia[see]
5.7 Thrombocytopenia/NeutropeniaBortezomib is associated with thrombocytopenia and neutropenia that follow a cyclical pattern with nadirs occurring following the last dose of each cycle and typically recovering prior to initiation of the subsequent cycle. The cyclical pattern of platelet and neutrophil decreases and recovery remain consistent in the studies of multiple myeloma and mantle cell lymphoma, with no evidence of cumulative thrombocytopenia or neutropenia in the treatment regimens studied.
Monitor complete blood counts (CBC) frequently during treatment with bortezomib. Measure platelet counts prior to each dose of bortezomib. Adjust dose/schedule for thrombocytopenia
[see Dosage and Administration (2.6)]. Gastrointestinal and intracerebral hemorrhage has occurred during thrombocytopenia in association with bortezomib. Support with transfusions and supportive care, according to published guidelines.In the single agent, relapsed multiple myeloma study of bortezomib vs dexamethasone, the mean platelet count nadir measured was approximately 40% of baseline. The severity of thrombocytopenia related to pretreatment platelet count is shown in Table 8. The incidence of bleeding (≥Grade 3) was 2% on the bortezomib arm and was <1% in the dexamethasone arm.
Table 8: Severity of Thrombocytopenia Related to Pretreatment Platelet Count in the Relapsed Multiple Myeloma Study of Bortezomib vs Dexamethasone Pretreatment Platelet CountA baseline platelet count of 50,000/µL was required for study eligibility Number of Patients
(N=331)Data were missing at baseline for one patientNumber (%) of Patients with Platelet Count <10,000/µL Number (%) of Patients with Platelet Count 10,000 to 25,000/µL ≥75,000/µL 309 8 (3%) 36 (12%) ≥50,000/µL to <75,000/µL 14 2 (14%) 11 (79%) ≥10,000/µL to <50,000/µL 7 1 (14%) 5 (71%) In the combination study of bortezomib with rituximab, cyclophosphamide, doxorubicin and prednisone (VcR-CAP) in previously untreated mantle cell lymphoma patients, the incidence of thrombocytopenia (≥Grade 4) was 32% vs 1% for the rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) arm as shown in Table 12. The incidence of bleeding events (≥Grade 3) was 1.7% in the VcR-CAP arm (four patients) and was 1.2% in the R-CHOP arm (three patients).
Platelet transfusions were given to 23% of the patients in the VcR-CAP arm and 3% of the patients in the R-CHOP arm.
The incidence of neutropenia (≥Grade 4) was 70% in the VcR-CAP arm and was 52% in the R-CHOP arm. The incidence of febrile neutropenia (≥Grade 4) was 5% in the VcR-CAP arm and was 6% in the R-CHOP arm. Myeloid growth factor support was provided at a rate of 78% in the VcR-CAP arm and 61% in the R-CHOP arm.
- Tumor Lysis Syndrome[see]
5.8 Tumor Lysis SyndromeTumor lysis syndrome has been reported with bortezomib therapy. Patients at risk of tumor lysis syndrome are those with high tumor burden prior to treatment. Monitor patients closely and take appropriate precautions.
- Hepatic Toxicity[see]
5.9 Hepatic ToxicityCases of acute liver failure have been reported in patients receiving multiple concomitant medications and with serious underlying medical conditions. Other reported hepatic reactions include hepatitis, increases in liver enzymes, and hyperbilirubinemia. Interrupt bortezomib therapy to assess reversibility. There is limited rechallenge information in these patients.
- Thrombotic Microangiopathy[see]
5.10 Thrombotic MicroangiopathyCases, sometimes fatal, of thrombotic microangiopathy, including thrombotic thrombocytopenic purpura/hemolytic uremic syndrome (TTP/HUS), have been reported in the postmarketing setting in patients who received bortezomib. Monitor for signs and symptoms of TTP/HUS. If the diagnosis is suspected, stop bortezomib and evaluate. If the diagnosis of TTP/HUS is excluded, consider restarting bortezomib. The safety of reinitiating bortezomib therapy in patients previously experiencing TTP/HUS is not known.
Bortezomib for injection, a proteasome inhibitor, contains bortezomib which is an antineoplastic agent. Bortezomib is a modified dipeptidyl boronic acid. The chemical name for bortezomib, the monomeric boronic acid, is [(1R)-3-methyl-1-[[(2S)-1-oxo-3-phenyl-2-[(pyrazinylcarbonyl) amino]propyl]amino]butyl] boronic acid.
Bortezomib has the following chemical structure:
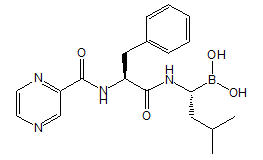
The molecular weight is 384.24. The molecular formula is C19H25BN4O4. The solubility of bortezomib, as the monomeric boronic acid, in water is 3.3 to 3.8 mg/mL in a pH range of 2 to 6.5.
Bortezomib for injection is available for intravenous injection or subcutaneous use. Each single-dose vial contains 3.5 mg of bortezomib as a sterile lyophilized powder. It also contains the inactive ingredient: 35 mg mannitol, USP. The product is provided as a mannitol boronic ester which, in reconstituted form, consists of the mannitol ester in equilibrium with its hydrolysis product, the monomeric boronic acid. The drug substance exists in its cyclic anhydride form as a trimeric boroxine.
Bortezomib for injection is supplied as individually cartoned 8 mL vials containing 3.5 mg of bortezomib as a white to off-white cake or powder.
NDC 0143-9098-01
3.5 mg single-dose vial
Bortezomib is a reversible inhibitor of the chymotrypsin-like activity of the 26S proteasome in mammalian cells. The 26S proteasome is a large protein complex that degrades ubiquitinated proteins. The ubiquitin-proteasome pathway plays an essential role in regulating the intracellular concentration of specific proteins, thereby maintaining homeostasis within cells. Inhibition of the 26S proteasome prevents this targeted proteolysis, which can affect multiple signaling cascades within the cell. This disruption of normal homeostatic mechanisms can lead to cell death. Experiments have demonstrated that bortezomib is cytotoxic to a variety of cancer cell types