Bosentan - Bosentan tablet, Coated Prescribing Information
Because of the risks of hepatotoxicity and birth defects, bosentan is available only through a restricted program called the Bosentan REMS Program. As a component of the bosentan REMS, prescribers, patients, and pharmacies must enroll in the program
Required components of the bosentan REMS are:
- Healthcare professionals who prescribe bosentan must review the prescriber educational materials, enroll in the Bosentan REMS Program and comply with its requirements.
- Healthcare professionals must (1) review serum aminotransferases (ALT/AST) and bilirubin, and agree to order and monitor these tests monthly; and (2) for females of reproductive potential, confirm that the patient is not pregnant, and agree to order and monitor pregnancy tests monthly.
- To receive bosentan, all patients must understand the risks and benefits, and complete a patient enrollment form.
- Pharmacies that dispense bosentan must enroll in the program and agree to comply with the Bosentan REMS Program requirements.
Further information about Bosentan and the Bosentan REMS Program is available at www.BosentanREMSProgram.com or 1-866-359-2612.
ALT or AST > 3 x ULN were observed in 11% of bosentan-treated patients (n = 658) compared to 2% of placebo-treated patients (n = 280). Three-fold increases were seen in 12% of 95 pulmonary arterial hypertension (PAH) patients on 125 mg twice daily and 14% of 70 PAH patients on 250 mg twice daily. Eight-fold increases were seen in 2% of PAH patients on 125 mg twice daily and 7% of PAH patients on 250 mg twice daily. Bilirubin increases to ≥ 3 x ULN were associated with aminotransferase increases in 2 of 658 (0.3%) of patients treated with bosentan. The combination of hepatocellular injury (increases in aminotransferases of > 3 x ULN) and increases in total bilirubin (≥ 2x ULN) is a marker for potential serious hepatotoxicity.
Elevations of AST or ALT associated with bosentan are dose-dependent, occur both early and late in treatment, usually progress slowly, are typically asymptomatic, and usually have been reversible after treatment interruption or cessation. Aminotransferase elevations also may reverse spontaneously while continuing treatment with bosentan.
Liver aminotransferase levels must be measured prior to initiation of treatment and then monthly and therapy adjusted accordingly
Avoid initiation of bosentan in patients with elevated aminotransferases (> 3 x ULN) prior to drug initiation because monitoring hepatotoxicity in these patients may be more difficult
In WHO Functional Class II patients, consider whether the benefits of bosentan are sufficient to offset the risk of hepatotoxicity, which may preclude future use as their disease progresses.
- Measure liver aminotransferases prior to initiation of treatment and then monthly (,2.1 Required Monitoring
Healthcare professionals who prescribe bosentan must enroll in the Bosentan REMS Program and must comply with the required monitoring to minimize the risks associated with bosentan
[see Warnings and Precautions ].Obtain a pregnancy test in females of reproductive potential prior to bosentan treatment, monthly during treatment and one month after stopping bosentan. Initiate treatment with bosentan in females of reproductive potential only after a negative pregnancy test
[see Boxed Warning,Contraindications , Warnings and Precautions,Use in Specific Populations ].Measure liver aminotransferase levels prior to initiation of treatment and then monthly
[see Warnings and Precautions ].).5.1 HepatotoxicityALT or AST > 3 x ULN were observed in 11% of bosentan-treated patients (n = 658) compared to 2% of placebo-treated patients (n = 280). Three-fold increases were seen in 12% of 95 pulmonary arterial hypertension (PAH) patients on 125 mg twice daily and 14% of 70 PAH patients on 250 mg twice daily. Eight-fold increases were seen in 2% of PAH patients on 125 mg twice daily and 7% of PAH patients on 250 mg twice daily. Bilirubin increases to ≥ 3 x ULN were associated with aminotransferase increases in 2 of 658 (0.3%) of patients treated with bosentan. The combination of hepatocellular injury (increases in aminotransferases of > 3 x ULN) and increases in total bilirubin (≥ 2x ULN) is a marker for potential serious hepatotoxicity.
Elevations of AST or ALT associated with bosentan are dose-dependent, occur both early and late in treatment, usually progress slowly, are typically asymptomatic, and usually have been reversible after treatment interruption or cessation. Aminotransferase elevations also may reverse spontaneously while continuing treatment with bosentan.
Liver aminotransferase levels must be measured prior to initiation of treatment and then monthly and therapy adjusted accordingly
[see Dosage and Administration ]. Discontinue bosentan if liver aminotransferase elevations are accompanied by clinical symptoms of hepatotoxicity (such as nausea, vomiting, fever, abdominal pain, jaundice, or unusual lethargy or fatigue) or increases in bilirubin ≥ 2 x ULN.Avoid initiation of bosentan in patients with elevated aminotransferases (> 3 x ULN) prior to drug initiation because monitoring hepatotoxicity in these patients may be more difficult
[see Boxed Warning, Dosage and Administration , Use in Specific Populations ].In WHO Functional Class II patients, consider whether the benefits of bosentan are sufficient to offset the risk of hepatotoxicity, which may preclude future use as their disease progresses.
- Discontinue bosentan if aminotransferase elevations are accompanied by signs or symptoms of liver dysfunction or injury or increases in bilirubin ≥ 2 x ULN (,2.4 Dosage Adjustments for Aminotransferase Elevations
If aminotransferase levels increase, adjust monitoring and treatment plan according to Table 2.
Discontinue bosentan if liver aminotransferase elevations are accompanied by clinical symptoms of hepatotoxicity (such as nausea, vomiting, fever, abdominal pain, jaundice, or unusual lethargy or fatigue) or bilirubin ≥ 2 x Upper Limit of Normal (ULN). There is no experience with the reintroduction of bosentan in these circumstances.
Table 2 Dosage Adjustment and Monitoring in Patients Developing Aminotransferase Elevations > 3 x ULN ALT/AST levelsTreatment and monitoring recommendations> 3 and ≤ 5 x ULN Confirm by another aminotransferase test; if confirmed,
-in adult patients > 12 years and > 40 kg,reduce the daily dose to 62.5 mg twice daily or interrupt treatment, and monitor aminotransferase levels at least every 2 weeks. If the aminotransferase levels return to pretreatment values, treatment may continue or be reintroduced at 62.5 mg twice daily, with reassessment of aminotransferase levels within 3 days.- in patients > 12 years and < 40 kg,interrupt treatment with no prior dose reduction. If the aminotransferase levels return to pretreatment values, reintroduce at the dose used prior to treatment interruption, with reassessment of aminotransferase levels within 3 days.> 5 and ≤ 8 x ULN Confirm by another aminotransferase test; if confirmed, stop treatment and monitor aminotransferase levels at least every 2 weeks. Once the aminotransferase levels return to pretreatment values,
-in adult patients > 12 years and >40 kg,consider reintroduction of treatment at 62.5 mg twice daily, with reassessment of aminotransferase levels within 3 days.> 8 x ULN Stop treatment permanently. There is no experience with reintroduction of bosentan in these circumstances. ).5.1 HepatotoxicityALT or AST > 3 x ULN were observed in 11% of bosentan-treated patients (n = 658) compared to 2% of placebo-treated patients (n = 280). Three-fold increases were seen in 12% of 95 pulmonary arterial hypertension (PAH) patients on 125 mg twice daily and 14% of 70 PAH patients on 250 mg twice daily. Eight-fold increases were seen in 2% of PAH patients on 125 mg twice daily and 7% of PAH patients on 250 mg twice daily. Bilirubin increases to ≥ 3 x ULN were associated with aminotransferase increases in 2 of 658 (0.3%) of patients treated with bosentan. The combination of hepatocellular injury (increases in aminotransferases of > 3 x ULN) and increases in total bilirubin (≥ 2x ULN) is a marker for potential serious hepatotoxicity.
Elevations of AST or ALT associated with bosentan are dose-dependent, occur both early and late in treatment, usually progress slowly, are typically asymptomatic, and usually have been reversible after treatment interruption or cessation. Aminotransferase elevations also may reverse spontaneously while continuing treatment with bosentan.
Liver aminotransferase levels must be measured prior to initiation of treatment and then monthly and therapy adjusted accordingly
[see Dosage and Administration ]. Discontinue bosentan if liver aminotransferase elevations are accompanied by clinical symptoms of hepatotoxicity (such as nausea, vomiting, fever, abdominal pain, jaundice, or unusual lethargy or fatigue) or increases in bilirubin ≥ 2 x ULN.Avoid initiation of bosentan in patients with elevated aminotransferases (> 3 x ULN) prior to drug initiation because monitoring hepatotoxicity in these patients may be more difficult
[see Boxed Warning, Dosage and Administration , Use in Specific Populations ].In WHO Functional Class II patients, consider whether the benefits of bosentan are sufficient to offset the risk of hepatotoxicity, which may preclude future use as their disease progresses.
Based on data from animal reproduction studies, bosentan may cause fetal harm when administered to a pregnant female and is contraindicated in females who are pregnant. Advise females of reproductive potential of the potential risk to a fetus. Obtain a pregnancy test prior to bosentan treatment, monthly during treatment and for one month after stopping treatment. Advise females of reproductive potential to use two reliable forms of contraception during treatment with bosentan and for at least one month after the last dose
Bosentan is only available for females through a restricted program under REMS
Based on data from animal reproduction studies, bosentan may cause fetal harm, including birth defects and fetal death, when administered to a pregnant female and is contraindicated during pregnancy
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Bosentan was teratogenic in rats given oral doses two times the MRHD (on a mg/m2basis). In an embryo-fetal toxicity study in rats, bosentan showed dose-dependent teratogenic effects, including malformations of the head, mouth, face and large blood vessels. Bosentan increased stillbirths and pup mortality at oral doses 2 and 10 times the MRHD (on a mg/m2basis). Although birth defects were not observed in rabbits given oral doses of up to the equivalent of 10.5 g/day in a 70 kg person, plasma concentrations of bosentan in rabbits were lower than those reached in the rat. The similarity of malformations induced by bosentan and those observed in endothelin-1 knockout mice and in animals treated with other endothelin receptor antagonists indicates that embryo-fetal toxicity is a class effect of these drugs
- Must exclude pregnancy before and during treatment (,2.1 Required Monitoring
Healthcare professionals who prescribe bosentan must enroll in the Bosentan REMS Program and must comply with the required monitoring to minimize the risks associated with bosentan
[see Warnings and Precautions ].Obtain a pregnancy test in females of reproductive potential prior to bosentan treatment, monthly during treatment and one month after stopping bosentan. Initiate treatment with bosentan in females of reproductive potential only after a negative pregnancy test
[see Boxed Warning,Contraindications , Warnings and Precautions,Use in Specific Populations ].Measure liver aminotransferase levels prior to initiation of treatment and then monthly
[see Warnings and Precautions ].,4.1 PregnancyUse of bosentan is contraindicated in females who are or may become pregnant. To prevent pregnancy, females of reproductive potential must use two reliable forms of contraception during treatment and for one month after stopping bosentan.[see Boxed Warning, Warnings and Precautions , Drug Interactions , Use in Specific Populations ].).8.1 PregnancyRisk SummaryBased on data from animal reproduction studies, bosentan may cause fetal harm, including birth defects and fetal death, when administered to a pregnant female and is contraindicated during pregnancy
[see Contraindications ]. There are limited data on bosentan use in pregnant women. In animal reproduction studies, oral administration of bosentan to pregnant rats at 2times the maximum recommended human dose (MRHD) on a mg/m2basis caused teratogenic effects in rats, including malformations of the head, mouth, face, and large blood vessels[see Animal Data]. Advise pregnant women of the potential risk to a fetus.The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
DataAnimal DataBosentan was teratogenic in rats given oral doses two times the MRHD (on a mg/m2basis). In an embryo-fetal toxicity study in rats, bosentan showed dose-dependent teratogenic effects, including malformations of the head, mouth, face and large blood vessels. Bosentan increased stillbirths and pup mortality at oral doses 2 and 10 times the MRHD (on a mg/m2basis). Although birth defects were not observed in rabbits given oral doses of up to the equivalent of 10.5 g/day in a 70 kg person, plasma concentrations of bosentan in rabbits were lower than those reached in the rat. The similarity of malformations induced by bosentan and those observed in endothelin-1 knockout mice and in animals treated with other endothelin receptor antagonists indicates that embryo-fetal toxicity is a class effect of these drugs
. - To prevent pregnancy, females of reproductive potential must use two reliable forms of contraception during treatment and for one month after stopping bosentan (,4.1 PregnancyUse of bosentan is contraindicated in females who are or may become pregnant. To prevent pregnancy, females of reproductive potential must use two reliable forms of contraception during treatment and for one month after stopping bosentan.[see Boxed Warning, Warnings and Precautions , Drug Interactions , Use in Specific Populations ].,5.2 Embryo-fetal Toxicity
Based on data from animal reproduction studies, bosentan may cause fetal harm when administered to a pregnant female and is contraindicated in females who are pregnant. Advise females of reproductive potential of the potential risk to a fetus. Obtain a pregnancy test prior to bosentan treatment, monthly during treatment and for one month after stopping treatment. Advise females of reproductive potential to use two reliable forms of contraception during treatment with bosentan and for at least one month after the last dose
[see Dosage and Administration , Use in Specific Populations ].Bosentan is only available for females through a restricted program under REMS
[see Warnings and Precautions ].).8.1 PregnancyRisk SummaryBased on data from animal reproduction studies, bosentan may cause fetal harm, including birth defects and fetal death, when administered to a pregnant female and is contraindicated during pregnancy
[see Contraindications ]. There are limited data on bosentan use in pregnant women. In animal reproduction studies, oral administration of bosentan to pregnant rats at 2times the maximum recommended human dose (MRHD) on a mg/m2basis caused teratogenic effects in rats, including malformations of the head, mouth, face, and large blood vessels[see Animal Data]. Advise pregnant women of the potential risk to a fetus.The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
DataAnimal DataBosentan was teratogenic in rats given oral doses two times the MRHD (on a mg/m2basis). In an embryo-fetal toxicity study in rats, bosentan showed dose-dependent teratogenic effects, including malformations of the head, mouth, face and large blood vessels. Bosentan increased stillbirths and pup mortality at oral doses 2 and 10 times the MRHD (on a mg/m2basis). Although birth defects were not observed in rabbits given oral doses of up to the equivalent of 10.5 g/day in a 70 kg person, plasma concentrations of bosentan in rabbits were lower than those reached in the rat. The similarity of malformations induced by bosentan and those observed in endothelin-1 knockout mice and in animals treated with other endothelin receptor antagonists indicates that embryo-fetal toxicity is a class effect of these drugs
.
Bosentan tablets are indicated for the treatment of pulmonary arterial hypertension (PAH) (WHO Group 1):
- in adults to improve exercise ability and to decrease clinical worsening. Studies establishing effectiveness included predominantly patients with WHO Functional Class II-IV symptoms and etiologies of idiopathic or heritable PAH (60%), PAH associated with connective tissue diseases (21%), and PAH associated with congenital heart disease with left-to-right shunts (18%) [see Clinical Studies (.)]14.1 Pulmonary Arterial HypertensionWHO Functional Class III-IV
Two randomized, double-blind, multi-center, placebo-controlled trials were conducted in 32 and 213 patients. The larger study (BREATHE-1) compared 2 doses (125 mg twice daily and 250 mg twice daily) of bosentan with placebo. The smaller study (Study 351) compared 125 mg twice daily with placebo. Patients had severe (WHO functional Class III–IV) PAH: idiopathic or heritable PAH (72%) or PAH associated with scleroderma or other connective tissue diseases (21%), or to autoimmune diseases (7%). There were no patients with PAH associated with other conditions such as HIV disease or recurrent pulmonary emboli.
In both studies, bosentan or placebo was added to patients' current therapy, which could have included a combination of digoxin, anticoagulants, diuretics, and vasodilators (e.g.calcium channel blockers, ACE inhibitors), but not epoprostenol. Bosentan was given at a dose of 62.5 mg twice daily for 4 weeks and then at 125 mg twice daily or 250 mg twice daily for either 12 (BREATHE-1) or 8 (Study 351) additional weeks. The primary study endpoint was 6-minute walk distance. In addition, symptoms and functional status were assessed. Hemodynamic measurements were made at 12 weeks in Study 351.
The mean age was about 49 years. About 80% of patients were female, and about 80% were Caucasian. Patients had been diagnosed with pulmonary hypertension for a mean of 2.4 years.
Submaximal Exercise AbilityResults of the 6-minute walk distance at 3 months (Study 351) or 4 months (BREATHE-1) are shown in Table 4.
Table 4 Effects of Bosentan on 6-minute Walk Distance Distance in meters: mean ± standard deviation. Changes are to week 16 for BREATHE-1 and to week 12 for Study 351.(a)p=0.01; by Wilcoxon;(b)p=0.0001; by Wilcoxon;(c)p=0.02; by Student's t-test.
BREATHE-1Study 351Bosentan 125 mg twice daily(n = 74)Bosentan 250 mg twice daily(n = 70)Placebo(n = 69)Bosentan 125 mg twice daily(n = 21)Placebo(n = 11)Baseline326 ± 73 333 ± 75 344 ± 76 360 ± 86 355 ± 82 End point353 ± 115 379 ± 101 336 ± 129 431 ± 66 350 ± 147 Change from baseline27 ± 75 46 ± 62 -8 ± 96 70 ± 56 -6 ± 121 Placebo – subtracted35(a) 54(b) 76(c) In both trials, treatment with bosentan resulted in a significant increase in exercise ability. The improvement in walk distance was apparent after 1 month of treatment (with 62.5 mg twice daily) and fully developed by about 2 months of treatment (Figure 5). It was maintained for up to 7 months of double-blind treatment. Walking distance was somewhat greater with 250 mg twice daily, but the potential for increased hepatotoxicity causes this dose not to be recommended
[see Dosage and Administration ]. There were no apparent differences in treatment effects on walk distance among subgroups analyzed by demographic factors, baseline disease severity, or disease etiology, but the studies had little power to detect such differences.Figure 5Mean Change in 6-min Walk Distance (BREATHE-1)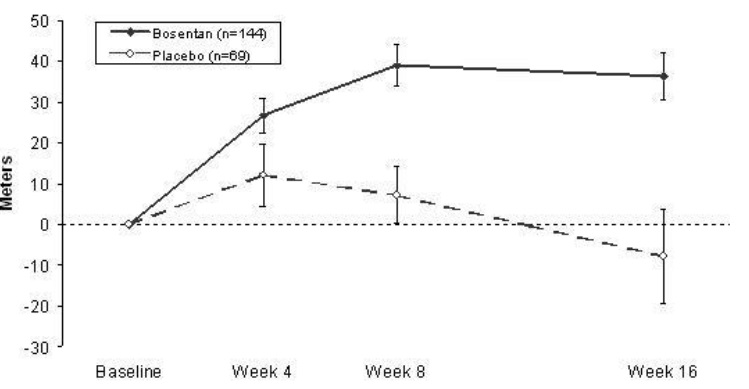 Change from baseline in 6-minute walking distance from start of therapy to week 16 in the placebo and combined bosentan(125 mg twice daily and 250 mg twice daily) groups. Values are expressed as mean ± standard error of the mean.Hemodynamic Changes
Change from baseline in 6-minute walking distance from start of therapy to week 16 in the placebo and combined bosentan(125 mg twice daily and 250 mg twice daily) groups. Values are expressed as mean ± standard error of the mean.Hemodynamic ChangesInvasive hemodynamic parameters were assessed in Study 351. Treatment with bosentan led to a significant increase in cardiac index (CI) associated with a significant reduction in pulmonary artery pressure (PAP), pulmonary vascular resistance (PVR), and mean right atrial pressure (RAP) (Table 5).
The relationship between hemodynamic effects and improvements in 6-minute walk distance is unknown.
Table 5: Change from Baseline to Week 12: Hemodynamic Parameters Values shown are means ± SD
(a)p ≤ 0.001;(b)p < 0.02
Bosentan 125 mg twice dailyPlaceboCI (L/min/m2)
Baseline
Absolute Change
Treatment Effectn=20
2.35±0.73
0.50±0.461.02(a) n=10
2.48±1.03
-0.52±0.48Mean PAP (mmHg)
Baseline
Absolute Change
Treatment Effectn=20
53.7±13.4
-1.6±5.1-6.7(b) n=10
55.7±10.5
5.1±8.8PVR (dyn•sec•cm-5)
Baseline
Absolute Change
Treatment Effectn=19
896±425
-223±245-415(a) n=10
942±430
191±235Mean RAP (mmHg)
Baseline
Absolute Change
Treatment Effectn=19
9.7±5.6
-1.3±4.1-6.2(a) n=10
9.9±4.1
4.9±4.6Symptoms and Functional StatusSymptoms of PAH were assessed by Borg dyspnea score, WHO functional class, and rate of "clinical worsening." Clinical worsening was assessed as the sum of death, hospitalizations for PAH, discontinuation of therapy because of PAH, and need for epoprostenol. There was a significant reduction in dyspnea during walk tests (Borg dyspnea score), and significant improvement in WHO functional class in bosentan-treated patients. There was a significant reduction in the rate of clinical worsening (Table 6 and Figure 6). Figure 6 shows the log-rank test reflecting clinical worsening over 28 weeks.
Table 6 Incidence of Clinical Worsening, Intent To Treat Population Note: Patients may have had more than one reason for clinical worsening.
(a)p=0.0015 vs placebo by log-rank test. There was no relevant difference between the 125 mg and 250 mg twice daily groups.
(b)p=0.033 vs placebo by Fisher's exact test.
(c)Receipt of epoprostenol was always a consequence of clinical worsening.
BREATHE-1Study 351Bosentan 125/250 mg twice daily(n = 144)Placebo(n = 69)Bosentan125 mgtwice daily(n = 21)Placebo(n = 11)Patients with clinical worsening[n (%)]9 (6%)(a) 14 (20%) 0 (0%)(b) 3 (27%) Death1 (1%) 2 (3%) 0 (0%) 0 (0%) Hospitalization for PAH6 (4%) 9 (13%) 0 (0%) 3 (27%) Discontinuation due to worsening of PAH5 (3%) 6 (9%) 0 (0%) 3 (27%) Receipt of epoprostenol(c)4 (3%) 3 (4%) 0 (0%) 3 (27%) Figure 6.Time to Clinical Worsening (BREATHE-1)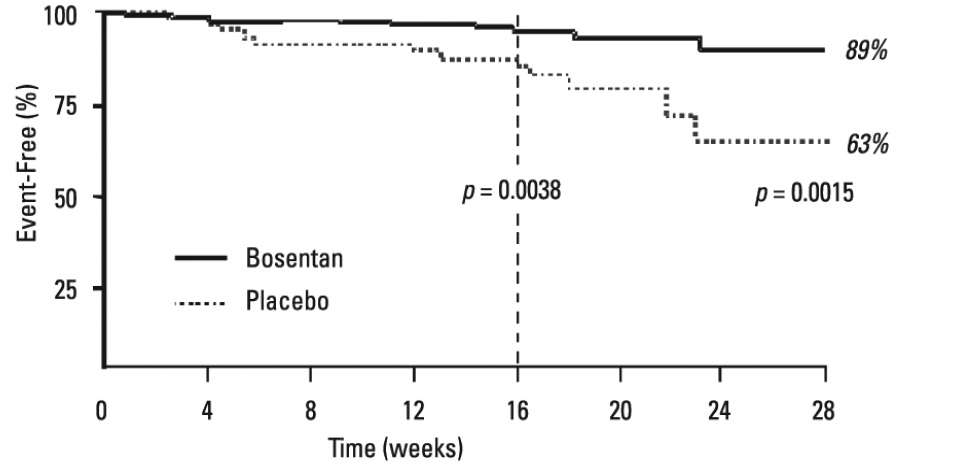 Time from randomization to clinical worsening with Kaplan-Meier estimate of the proportions of failures in BREATHE-1. All patients (n=144 in the bosentan group and n=69 in the placebo group) participated in the first 16 weeks of the study. A subset of this population (n=35 in the bosentan group and 13 in the placebo group) continued double-blind therapy for up to 28 weeks.WHO Functional Class II
Time from randomization to clinical worsening with Kaplan-Meier estimate of the proportions of failures in BREATHE-1. All patients (n=144 in the bosentan group and n=69 in the placebo group) participated in the first 16 weeks of the study. A subset of this population (n=35 in the bosentan group and 13 in the placebo group) continued double-blind therapy for up to 28 weeks.WHO Functional Class IIIn a randomized, double-blind, multicenter, placebo-controlled trial, 185 mildly symptomatic PAH patients with WHO Functional Class II (mean baseline 6-minute walk distance of 443 meters) received bosentan 62.5 mg twice daily for 4 weeks followed by 125 mg twice daily (n = 93), or placebo (n = 92) for 6 months. Enrolled patients were treatment-naïve (n = 156) or on a stable dose of sildenafil (n = 29). The coprimary endpoints were change from baseline to month 6 in PVR and 6-minute walk distance. Time to clinical worsening (assessed as the sum of death, hospitalization due to PAH complications, or symptomatic progression of PAH), Borg dyspnea index, change in WHO functional class and hemodynamics were assessed as secondary endpoints.
Compared with placebo, bosentan treatment was associated with a reduced incidence of worsening of at least one functional class (3% bosentan vs 13% placebo, p = 0.03), and improvement in hemodynamic variables (PVR, mPAP, TPR, cardiac index, and SVO2; p < 0.05). The
+19 m mean (+14 m median) increase in 6-minute walk distance with bosentanvsplacebo was not significant (p = 0.08). There was a significant delay in time to clinical worsening (first seen primarily as symptomatic progression of PAH) with bosentan compared with placebo (hazard ratio 0.2, p = 0.01). Findings were consistent in strata with or without treatment with sildenafil at baseline.Long-term Treatment of PAHLong-term follow-up of patients with Class III and IV PAH who were treated with bosentan in open-label extensions of trials (N=235) showed that 93% and 84% of patients were still alive at 1 and 2 years, respectively, after the start of treatment.
These uncontrolled observations do not allow comparison with a group not given bosentan and cannot be used to determine the long-term effect of bosentan on mortality.
Pulmonary Arterial Hypertension in Adults related to Congenital Heart Disease with Left-to- Right ShuntsA small study (N=54) and its open-label extension (N=37) of up to 40 weeks in adult patients with Eisenmenger physiology demonstrated effects of bosentan on exercise and safety that were similar to those seen in other trials in patients with PAH (WHO Group 1).
- Patients older than 12 years of age: initiate at 62.5 mg orally twice daily; for patients weighing greater than 40 kg, increase to 125 mg orally twice daily after 4 weeks ().2.2 Recommended Dosage
Administer bosentan orally following the dosing recommendations in Table 1. Doses above 125 mg twice daily did not appear to confer additional benefit sufficient to offset the increased risk of hepatotoxicity.
Table 1Dosing RecommendationsInitial 4 weeks Maintenance (after 4 weeks) Patients > 12 years of age and > 40 kg 62.5 mg twice daily 125 mg twice daily Patients > 12 years of age and < 40 kg 62.5 mg twice daily 62.5 mg twice daily - Reduce the dose and closely monitor patients developing aminotransferase elevations more than 3 X Upper Limit of Normal (ULN) ().2.1 Required Monitoring
Healthcare professionals who prescribe bosentan must enroll in the Bosentan REMS Program and must comply with the required monitoring to minimize the risks associated with bosentan
[see Warnings and Precautions ].Obtain a pregnancy test in females of reproductive potential prior to bosentan treatment, monthly during treatment and one month after stopping bosentan. Initiate treatment with bosentan in females of reproductive potential only after a negative pregnancy test
[see Boxed Warning,Contraindications , Warnings and Precautions,Use in Specific Populations ].Measure liver aminotransferase levels prior to initiation of treatment and then monthly
[see Warnings and Precautions ].
- Film-coated Tablet: 62.5 mg and 125 mg ()
3 DOSAGE FORMS AND STRENGTHS- Film-coated Tablet: 62.5 mg and 125 mg
62.5 mg and 125 mg film-coated, tablets for oral administration.
62.5 mg tablets: Peach to light pink colored, coated, round, biconvex, tablets debossed with '446' on one side and plain on other side.
125 mg tablets: Peach to light pink colored, coated, oval, biconvex, tablets debossed with '447' on one side and plain on other side.
- Nursing mothers: Choose breastfeeding or bosentan ().
8.2 LactationRisk SummaryData from a case report describe the presence of bosentan in human milk. There is insufficient information about the effects of bosentan on the breastfed infant and no information on the effects of bosentan on milk production. Because of the potential for serious adverse reactions, such as fluid retention and hepatotoxicity, in breastfed infants from bosentan, advise women not to breastfeed during treatment with bosentan.
- Pregnancy ()4.1 PregnancyUse of bosentan is contraindicated in females who are or may become pregnant. To prevent pregnancy, females of reproductive potential must use two reliable forms of contraception during treatment and for one month after stopping bosentan.[see Boxed Warning, Warnings and Precautions , Drug Interactions , Use in Specific Populations ].
- Use with Cyclosporine A ()4.2 Use with Cyclosporine A
Coadministration of cyclosporine A and bosentan resulted in markedly increased plasma concentrations of bosentan. Therefore, concomitant use of bosentan and cyclosporine A is contraindicated.
[see Cytochrome P450 Drug Interactions , Description ].