Brisdelle Prescribing Information
SSRIs increased the risk of suicidal thoughts and behavior in pediatric and young adult patients in short-term trials for the treatment of major depressive disorder (MDD) and other psychiatric disorders - BRISDELLE is not approved for use in any psychiatric condition or in pediatric and young adult patients
Monitor all BRISDELLE-treated patients for any emergence of suicidal thoughts and behaviors, especially during the initial few months of BRISDELLE therapy. Counsel family members to monitor for changes in behavior and to alert the health care provider if such changes occur. Consider discontinuing BRISDELLE in patients who experience emergent suicidal thoughts or behaviors or symptoms that might be precursors to suicidal thoughts or behaviors, especially if these symptoms are severe, abrupt in onset, or were not part of the patient’s presenting symptoms.
BRISDELLE is indicated for the treatment of moderate to severe vasomotor symptoms (VMS) associated with menopause.
BRISDELLE is not indicated for the treatment of any psychiatric condition. BRISDELLE has a lower recommended paroxetine dosage than that used to treat major depressive disorder, obsessive compulsive disorder, panic disorder, generalized anxiety disorder, social anxiety disorder, and post-traumatic stress disorder. The safety and effectiveness of the lower BRISDELLE dosage has not been established for any psychiatric condition. Patients who require paroxetine for treatment of a psychiatric condition should discontinue BRISDELLE and initiate a paroxetine-containing product that is indicated for such use.
BRISDELLE is a selective serotonin reuptake inhibitor (SSRI) indicated for the treatment of moderate to severe vasomotor symptoms associated with menopause (VMS)
Safety and effectiveness of BRISDELLE in pediatric patients have not been established; BRISDELLE is not indicated in the pediatric population.
BRISDELLE is indicated for the treatment of moderate to severe vasomotor symptoms (VMS) associated with menopause.
BRISDELLE is not indicated for the treatment of any psychiatric condition. BRISDELLE has a lower recommended paroxetine dosage than that used to treat major depressive disorder, obsessive compulsive disorder, panic disorder, generalized anxiety disorder, social anxiety disorder, and post-traumatic stress disorder. The safety and effectiveness of the lower BRISDELLE dosage has not been established for any psychiatric condition. Patients who require paroxetine for treatment of a psychiatric condition should discontinue BRISDELLE and initiate a paroxetine-containing product that is indicated for such use.
BRISDELLE is available as 7.5 mg pink capsules printed with black edible ink with “BRISDELLE” and “7.5 mg” on the capsule. Each capsule contains 9.69 mg of paroxetine mesylate equivalent to 7.5 mg paroxetine base.
BRISDELLE is contraindicated in patients:
- Taking, or within 14 days of stopping, MAOIs (including the MAOIs linezolid and intravenous methylene blue) because of an increased risk of serotonin syndrome [see Warnings and Precautions (.), Drug Interaction (
5.2 Serotonin SyndromeBRISDELLE can precipitate serotonin syndrome, a potentially life-threatening condition. The risk is increased with concomitant use of other serotonergic agents (including triptans, tricyclic antidepressants, fentanyl, tramadol, meperidine, methadone, lithium, tryptophan, buspirone, amphetamines, and St. John’s Wort) and with drugs that impair metabolism of serotonin, i.e., monoamine oxidase inhibitors (MAOIs)
[see Contraindications , Drug Interactions ]. Serotonin syndrome can also occur when BRISDELLE is used alone.Serotonin syndrome symptoms may include mental status changes (e.g., agitation, hallucinations, delirium, and coma), autonomic instability (e.g., tachycardia, labile blood pressure, dizziness, diaphoresis, flushing, hyperthermia), neuromuscular symptoms (e.g., tremor, rigidity, myoclonus, hyperreflexia, incoordination), and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea).
The concomitant use of BRISDELLE with MAOIs is contraindicated. Do not start BRISDELLE in a patient who is being treated with MAOIs such as linezolid or intravenous methylene blue. All reports with methylene blue that provided information on the route of administration involved intravenous administration in the dosage range of 1 mg/kg to 8 mg/kg. No reports involved the administration of methylene blue by other routes (such as oral or local tissue injection) or at lower dosages. If it is necessary to initiate treatment with an MAOI such as linezolid or intravenous methylene blue in a patient taking BRISDELLE, The patient should be taken off BRISDELLE before initiating treatment with the MAOI
[see Contraindications ]. If concomitant use of BRISDELLE with other serotonergic drugs (besides MAOIs) is clinically warranted, consider the increased risk of serotonin syndrome and carefully observe the patient, particularly during BRISDELLE initiation[see Contraindications Drug Interactions ].Monitor all patients taking BRISDELLE for the emergence of serotonin syndrome. Discontinue BRISDELLE and any concomitant serotonergic agents immediately if the above events occur and initiate supportive symptomatic treatment.
)]7 DRUG INTERACTIONSParoxetine is a strong CYP2D6 inhibitor. Co-administration of BRISDELLE can alter concentrations of other drugs that are metabolized by CYP2D6. Consider potential drug interactions prior to and during therapy . See Full Prescribing Information for a list of clinically significant drug interactions
7.1 Potential for BRISDELLE to Affect Other DrugsParoxetine is a strong CYP2D6 inhibitor. Clinical drug interaction studies have been performed with substrates of CYP2D6 and show that paroxetine can inhibit the metabolism of drugs metabolized by CYP2D6
[see Clinical Pharmacology ]. Table 2 contains examples of drugs with a metabolism that may be affected by concomitant use with BRISDELLE.Table 2 Effects of Paroxetine on Other DrugsConcomitant Drug NameEffect of Paroxetine on Other DrugsClinical RecommendationsThioridazine Increased plasma concentrations of thioridazine
Potential QTc prolongationConcomitant use of thioridazine and BRISDELLE is contraindicated. Pimozide Increased plasma concentrations of pimozide.
Potential QTc prolongationConcomitant use of pimozide and BRISDELLE is contraindicated. Tamoxifen Reduced plasma concentrations of active tamoxifen metabolite Consider avoiding concomitant use of tamoxifen and BRISDELLE. Tricyclic Antidepressants (TCA) (e.g., Desipramine) Increased plasma concentrations and elimination half-life Plasma TCA concentrations may need to be monitored and the TCA dosage may need to be reduced if a TCA is used concomitantly with BRISDELLE. Monitor tolerability. Risperidone Increased plasma concentrations of risperidone A lower risperidone dosage may be necessary (see the risperidone Prescribing Information for). Monitor tolerability. Atomoxetine Increased exposure of atomoxetine A lower atomoxetine dosage of may be necessary (see atomoxetine Prescribing Information for). Monitor tolerability. Drugs Highly Bound to Plasma Protein (e.g., Warfarin) Increased free plasma concentrations The warfarin dosage may need to be reduced. Monitor tolerability and the International Normalized Ratio. Digoxin Decreased plasma concentrations
of digoxinThe digoxin dosage of may need to be increased. Monitor digoxin concentrations and clinical effect. Theophylline Increased plasma concentrations
of theophyllineThe theophylline dosage of may need to be decreased. Monitor theophylline concentrations and tolerability. Use caution with concomitant use of BRISDELLE with other drugs that are metabolized by CYP2D6, including nortriptyline, amitriptyline, imipramine, desipramine, fluoxetine, phenothiazines, risperidone, and Type 1C antiarrhythmics (e.g., propafenone, flecainide, and encainide).
7.2 Potential for Other Drugs to Affect BRISDELLEThe metabolism and pharmacokinetics of paroxetine may be affected by the induction and inhibition of drug metabolizing enzymes such as CYP2D6. Table 3 contains a list of drugs that may affect the pharmacokinetics of BRISDELLE when administered concomitantly
[see Clinical Pharmacology ].Table 3 Effects of Other Drugs on ParoxetineConcomitant
Drug NameEffect of Concomitant Drug on ParoxetineClinical RecommendationsPhenobarbital Decreased paroxetine exposure Phenytoin Decreased paroxetine exposure Fosamprenavir/Ritonavir Decreased plasma concentration
of paroxetineMonitor clinical effect of BRISDELLE.
No BRISDELLE dosage adjustment is needed.Cimetidine Increased plasma concentration
of paroxetineUse caution if BRISDELLE is used concomitantly with other drugs that inhibit CYP2D6 (e.g., quinidine).
7.3 Other Potentially Significant Drug InteractionsMonoamine Oxidase InhibitorsSerious adverse reactions such as serotonin syndrome have been reported in patients treated with a SSRI and a concomitant monoamine oxidase inhibitor (MAOI), in patients started on an SSRI who recently received an MAOI and in patients started on an MAOI who recently received an SSRI. Therefore, concomitant use of MAOIs with BRISDELLE or use of BRISDELLE and an MAOI within 14 days of each other is contraindicated
[see Dosage and Administration , Contraindications and Warnings and Precautions ].Serotonergic DrugsIf concomitant use of BRISDELLE with other serotonergic drugs (e.g., other SNRIs, SSRIs, triptans, tricyclic antidepressants, opioids, lithium, tryptophan, buspirone, amphetamines, and St. John’s Wort) is clinically warranted, consider the increased risk of serotonin syndrome and carefully observe the patient, particularly during treatment initiation
[see Warnings and Precautions ].An interaction between paroxetine and tryptophan may occur when they are co-administered. Adverse reactions, consisting primarily of headache, nausea, sweating, and dizziness, have been reported when tryptophan was administered to patients taking paroxetine. Consequently, concomitant use of BRISDELLE with tryptophan is not recommended.
Drugs that Interfere with Hemostasis (e.g., NSAIDs, Aspirin, and Warfarin)Altered anticoagulant effects, including increased bleeding, have been reported when SSRIs are concomitantly administered with NSAIDs, aspirin, warfarin. or other drugs that affect coagulation. There may be a pharmacodynamic interaction between paroxetine and warfarin that causes an increased bleeding diathesis despite unaltered prothrombin time. Carefully monitor patients receiving warfarin therapy when BRISDELLE is initiated or discontinued
[see Warnings and Precautions ]. - Taking thioridazine because of risk of QT prolongation [see Warnings and Precautions (.), Drug Interaction (
5.3 Potential Impact on Tamoxifen EfficacySome studies have shown that the efficacy of tamoxifen, as measured by the risk of breast cancer relapse/mortality, may be reduced when concomitantly administered with paroxetine as a result of paroxetine’s irreversible inhibition of CYP2D6 and lower tamoxifen blood levels
[see Drug Interactions ]. However, other studies have failed to demonstrate such a risk.When tamoxifen is used for the treatment or prevention of breast cancer, weigh the likely benefit of BRISDELLE for treating moderate to severe VMS associated with menopause vs. the risk of possible decreased tamoxifen effectiveness, and consider avoiding the concomitant use of BRISDELLE.
)]7 DRUG INTERACTIONSParoxetine is a strong CYP2D6 inhibitor. Co-administration of BRISDELLE can alter concentrations of other drugs that are metabolized by CYP2D6. Consider potential drug interactions prior to and during therapy . See Full Prescribing Information for a list of clinically significant drug interactions
7.1 Potential for BRISDELLE to Affect Other DrugsParoxetine is a strong CYP2D6 inhibitor. Clinical drug interaction studies have been performed with substrates of CYP2D6 and show that paroxetine can inhibit the metabolism of drugs metabolized by CYP2D6
[see Clinical Pharmacology ]. Table 2 contains examples of drugs with a metabolism that may be affected by concomitant use with BRISDELLE.Table 2 Effects of Paroxetine on Other DrugsConcomitant Drug NameEffect of Paroxetine on Other DrugsClinical RecommendationsThioridazine Increased plasma concentrations of thioridazine
Potential QTc prolongationConcomitant use of thioridazine and BRISDELLE is contraindicated. Pimozide Increased plasma concentrations of pimozide.
Potential QTc prolongationConcomitant use of pimozide and BRISDELLE is contraindicated. Tamoxifen Reduced plasma concentrations of active tamoxifen metabolite Consider avoiding concomitant use of tamoxifen and BRISDELLE. Tricyclic Antidepressants (TCA) (e.g., Desipramine) Increased plasma concentrations and elimination half-life Plasma TCA concentrations may need to be monitored and the TCA dosage may need to be reduced if a TCA is used concomitantly with BRISDELLE. Monitor tolerability. Risperidone Increased plasma concentrations of risperidone A lower risperidone dosage may be necessary (see the risperidone Prescribing Information for). Monitor tolerability. Atomoxetine Increased exposure of atomoxetine A lower atomoxetine dosage of may be necessary (see atomoxetine Prescribing Information for). Monitor tolerability. Drugs Highly Bound to Plasma Protein (e.g., Warfarin) Increased free plasma concentrations The warfarin dosage may need to be reduced. Monitor tolerability and the International Normalized Ratio. Digoxin Decreased plasma concentrations
of digoxinThe digoxin dosage of may need to be increased. Monitor digoxin concentrations and clinical effect. Theophylline Increased plasma concentrations
of theophyllineThe theophylline dosage of may need to be decreased. Monitor theophylline concentrations and tolerability. Use caution with concomitant use of BRISDELLE with other drugs that are metabolized by CYP2D6, including nortriptyline, amitriptyline, imipramine, desipramine, fluoxetine, phenothiazines, risperidone, and Type 1C antiarrhythmics (e.g., propafenone, flecainide, and encainide).
7.2 Potential for Other Drugs to Affect BRISDELLEThe metabolism and pharmacokinetics of paroxetine may be affected by the induction and inhibition of drug metabolizing enzymes such as CYP2D6. Table 3 contains a list of drugs that may affect the pharmacokinetics of BRISDELLE when administered concomitantly
[see Clinical Pharmacology ].Table 3 Effects of Other Drugs on ParoxetineConcomitant
Drug NameEffect of Concomitant Drug on ParoxetineClinical RecommendationsPhenobarbital Decreased paroxetine exposure Phenytoin Decreased paroxetine exposure Fosamprenavir/Ritonavir Decreased plasma concentration
of paroxetineMonitor clinical effect of BRISDELLE.
No BRISDELLE dosage adjustment is needed.Cimetidine Increased plasma concentration
of paroxetineUse caution if BRISDELLE is used concomitantly with other drugs that inhibit CYP2D6 (e.g., quinidine).
7.3 Other Potentially Significant Drug InteractionsMonoamine Oxidase InhibitorsSerious adverse reactions such as serotonin syndrome have been reported in patients treated with a SSRI and a concomitant monoamine oxidase inhibitor (MAOI), in patients started on an SSRI who recently received an MAOI and in patients started on an MAOI who recently received an SSRI. Therefore, concomitant use of MAOIs with BRISDELLE or use of BRISDELLE and an MAOI within 14 days of each other is contraindicated
[see Dosage and Administration , Contraindications and Warnings and Precautions ].Serotonergic DrugsIf concomitant use of BRISDELLE with other serotonergic drugs (e.g., other SNRIs, SSRIs, triptans, tricyclic antidepressants, opioids, lithium, tryptophan, buspirone, amphetamines, and St. John’s Wort) is clinically warranted, consider the increased risk of serotonin syndrome and carefully observe the patient, particularly during treatment initiation
[see Warnings and Precautions ].An interaction between paroxetine and tryptophan may occur when they are co-administered. Adverse reactions, consisting primarily of headache, nausea, sweating, and dizziness, have been reported when tryptophan was administered to patients taking paroxetine. Consequently, concomitant use of BRISDELLE with tryptophan is not recommended.
Drugs that Interfere with Hemostasis (e.g., NSAIDs, Aspirin, and Warfarin)Altered anticoagulant effects, including increased bleeding, have been reported when SSRIs are concomitantly administered with NSAIDs, aspirin, warfarin. or other drugs that affect coagulation. There may be a pharmacodynamic interaction between paroxetine and warfarin that causes an increased bleeding diathesis despite unaltered prothrombin time. Carefully monitor patients receiving warfarin therapy when BRISDELLE is initiated or discontinued
[see Warnings and Precautions ]. - Taking pimozide because of risk of QT prolongation [see Warnings and Precautions (.), Drug Interaction (
5.3 Potential Impact on Tamoxifen EfficacySome studies have shown that the efficacy of tamoxifen, as measured by the risk of breast cancer relapse/mortality, may be reduced when concomitantly administered with paroxetine as a result of paroxetine’s irreversible inhibition of CYP2D6 and lower tamoxifen blood levels
[see Drug Interactions ]. However, other studies have failed to demonstrate such a risk.When tamoxifen is used for the treatment or prevention of breast cancer, weigh the likely benefit of BRISDELLE for treating moderate to severe VMS associated with menopause vs. the risk of possible decreased tamoxifen effectiveness, and consider avoiding the concomitant use of BRISDELLE.
)]7 DRUG INTERACTIONSParoxetine is a strong CYP2D6 inhibitor. Co-administration of BRISDELLE can alter concentrations of other drugs that are metabolized by CYP2D6. Consider potential drug interactions prior to and during therapy . See Full Prescribing Information for a list of clinically significant drug interactions
7.1 Potential for BRISDELLE to Affect Other DrugsParoxetine is a strong CYP2D6 inhibitor. Clinical drug interaction studies have been performed with substrates of CYP2D6 and show that paroxetine can inhibit the metabolism of drugs metabolized by CYP2D6
[see Clinical Pharmacology ]. Table 2 contains examples of drugs with a metabolism that may be affected by concomitant use with BRISDELLE.Table 2 Effects of Paroxetine on Other DrugsConcomitant Drug NameEffect of Paroxetine on Other DrugsClinical RecommendationsThioridazine Increased plasma concentrations of thioridazine
Potential QTc prolongationConcomitant use of thioridazine and BRISDELLE is contraindicated. Pimozide Increased plasma concentrations of pimozide.
Potential QTc prolongationConcomitant use of pimozide and BRISDELLE is contraindicated. Tamoxifen Reduced plasma concentrations of active tamoxifen metabolite Consider avoiding concomitant use of tamoxifen and BRISDELLE. Tricyclic Antidepressants (TCA) (e.g., Desipramine) Increased plasma concentrations and elimination half-life Plasma TCA concentrations may need to be monitored and the TCA dosage may need to be reduced if a TCA is used concomitantly with BRISDELLE. Monitor tolerability. Risperidone Increased plasma concentrations of risperidone A lower risperidone dosage may be necessary (see the risperidone Prescribing Information for). Monitor tolerability. Atomoxetine Increased exposure of atomoxetine A lower atomoxetine dosage of may be necessary (see atomoxetine Prescribing Information for). Monitor tolerability. Drugs Highly Bound to Plasma Protein (e.g., Warfarin) Increased free plasma concentrations The warfarin dosage may need to be reduced. Monitor tolerability and the International Normalized Ratio. Digoxin Decreased plasma concentrations
of digoxinThe digoxin dosage of may need to be increased. Monitor digoxin concentrations and clinical effect. Theophylline Increased plasma concentrations
of theophyllineThe theophylline dosage of may need to be decreased. Monitor theophylline concentrations and tolerability. Use caution with concomitant use of BRISDELLE with other drugs that are metabolized by CYP2D6, including nortriptyline, amitriptyline, imipramine, desipramine, fluoxetine, phenothiazines, risperidone, and Type 1C antiarrhythmics (e.g., propafenone, flecainide, and encainide).
7.2 Potential for Other Drugs to Affect BRISDELLEThe metabolism and pharmacokinetics of paroxetine may be affected by the induction and inhibition of drug metabolizing enzymes such as CYP2D6. Table 3 contains a list of drugs that may affect the pharmacokinetics of BRISDELLE when administered concomitantly
[see Clinical Pharmacology ].Table 3 Effects of Other Drugs on ParoxetineConcomitant
Drug NameEffect of Concomitant Drug on ParoxetineClinical RecommendationsPhenobarbital Decreased paroxetine exposure Phenytoin Decreased paroxetine exposure Fosamprenavir/Ritonavir Decreased plasma concentration
of paroxetineMonitor clinical effect of BRISDELLE.
No BRISDELLE dosage adjustment is needed.Cimetidine Increased plasma concentration
of paroxetineUse caution if BRISDELLE is used concomitantly with other drugs that inhibit CYP2D6 (e.g., quinidine).
7.3 Other Potentially Significant Drug InteractionsMonoamine Oxidase InhibitorsSerious adverse reactions such as serotonin syndrome have been reported in patients treated with a SSRI and a concomitant monoamine oxidase inhibitor (MAOI), in patients started on an SSRI who recently received an MAOI and in patients started on an MAOI who recently received an SSRI. Therefore, concomitant use of MAOIs with BRISDELLE or use of BRISDELLE and an MAOI within 14 days of each other is contraindicated
[see Dosage and Administration , Contraindications and Warnings and Precautions ].Serotonergic DrugsIf concomitant use of BRISDELLE with other serotonergic drugs (e.g., other SNRIs, SSRIs, triptans, tricyclic antidepressants, opioids, lithium, tryptophan, buspirone, amphetamines, and St. John’s Wort) is clinically warranted, consider the increased risk of serotonin syndrome and carefully observe the patient, particularly during treatment initiation
[see Warnings and Precautions ].An interaction between paroxetine and tryptophan may occur when they are co-administered. Adverse reactions, consisting primarily of headache, nausea, sweating, and dizziness, have been reported when tryptophan was administered to patients taking paroxetine. Consequently, concomitant use of BRISDELLE with tryptophan is not recommended.
Drugs that Interfere with Hemostasis (e.g., NSAIDs, Aspirin, and Warfarin)Altered anticoagulant effects, including increased bleeding, have been reported when SSRIs are concomitantly administered with NSAIDs, aspirin, warfarin. or other drugs that affect coagulation. There may be a pharmacodynamic interaction between paroxetine and warfarin that causes an increased bleeding diathesis despite unaltered prothrombin time. Carefully monitor patients receiving warfarin therapy when BRISDELLE is initiated or discontinued
[see Warnings and Precautions ]. - With known hypersensitivity (e.g., anaphylaxis, angioedema, Stevens-Johnson syndrome) to paroxetine or to any of the inactive ingredients in BRISDELLE [see Adverse Reactions ()]
6.2 Postmarketing ExperienceThe following adverse reactions have been identified during post-approval use of this and other paroxetine products. Because some of these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a
causal relationship to drug exposure.Blood and Lymphatic System Disorders:Idiopathic thrombocytopenic purpura, Events related to impaired hematopoiesis (including aplastic anemia, pancytopenia, bone marrow aplasia, agranulocytosis).Cardiac Disorders:Atrial fibrillation, Pulmonary edema, Ventricular fibrillation, Ventricular tachycardia (including torsades de pointes).Gastrointestinal Disorders:Pancreatitis, Pancreatitis hemorrhagic, Vomiting.General Disorders and Administration Site Conditions: Death, Drug withdrawal syndrome, Malaise.Hepatobiliary Disorders:Drug-induced liver injury, Hepatic failure, Jaundice.Immune System Disorders:Anaphylaxis, Angioedema, Toxic epidermal necrolysis.Investigations:Elevated liver tests (the most severe cases were deaths due to liver necrosis, and grossly elevated transaminases associated with severe liver dysfunction).Metabolism and Nutrition Disorders:Diabetes mellitus inadequate control, Type 2 diabetes mellitus.Nervous System Disorders:Neuroleptic malignant syndrome, Paresthesia, Somnolence, Tremor, Anosmia, HyposmiaPsychiatric Disorders:Aggression, Agitation, Anxiety, Confusional state, Depression, Disorientation, Homicidal ideation, Insomnia, Restlessness.Respiratory, Thoracic and Mediastinal Disorders:Pulmonary hypertension.Skin and Subcutaneous Tissue Disorders:Hyperhidrosis, Stevens-Johnson syndrome, Drug reaction with eosinophilia and systemic symptoms (DRESS). - Who are or become pregnant because menopausal VMS does not occur during pregnancy and BRISDELLE may cause fetal harm [see Use in Specific Populations (.)]
8.1 PregnancyRisk SummaryBRISDELLE is contraindicated in pregnant females and not indicated for use in pre-menopausal females. Based on epidemiologic and animal studies, paroxetine can cause fetal harm. Based on data from published observational studies, exposure to SSRIs, particularly in the month before delivery, has been associated with a less than 2-fold increase in the risk of postpartum hemorrhage
[see Warnings and Precautions ].Paroxetine is associated with a less than 2-fold increase in cardiovascular malformations when administered to a pregnant female during the first trimester. While individual epidemiological studies on the association between paroxetine use and cardiovascular malformations have reported inconsistent findings, some meta-analyses of epidemiological studies have identified an increased risk of cardiovascular malformations
(see Data). There are risks of persistent pulmonary hypertension of the newborn (PPHN)(see Data)and/or poor neonatal adaptation with exposure to selective serotonin reuptake inhibitors (SSRIs), including BRISDELLE, during pregnancy.No evidence of treatment related malformations was observed in animal reproduction studies, when paroxetine was administered during the period of organogenesis at doses up to 50 mg/kg/day in rats and 6 mg/kg/day in rabbits. These doses are approximately 64 times (rat) and less than 16 times (rabbit) the maximum recommended human dose (MRHD) of BRISDELLE (7.5 mg) on an mg/m2basis. When paroxetine was administered to female rats during the last trimester of gestation and continued through lactation, there was an increase in the number of pup deaths during the first four days of lactation. This effect occurred at a dose of 1 mg/kg/day which is 1.3 times the MRHD on an mg/m2basis
(see Data).DataHuman Data:Published epidemiological studies on the association between first trimester paroxetine use and cardiovascular malformations have reported inconsistent results; however, meta-analyses of population-based cohort studies published between 1996-2017 indicate a less than 2-fold increased risk for overall cardiovascular malformations. Specific cardiac malformations identified in two meta-analyses include approximately 2 to 2.5-fold increased risk for right ventricular outflow tract defects. One meta-analysis also identified an increased risk (less than 2-fold) for bulbus cordis anomalies and anomalies of cardiac septal closure, and an increased risk for atrial septal defects (pooled OR 2.38, 95% CI 1.14-4.97). Important limitations of the studies included in these meta-analyses include potential confounding by indication, depression severity, and potential exposure misclassification.Exposure to SSRIs, particularly later in pregnancy, may have an increased risk for PPHN. PPHN occurs in 1-2 per 1000 live births in the general population and is associated with substantial neonatal morbidity and mortality.
Animal Data:Reproduction studies were performed at doses up to 50 mg/kg/day in rats and 6 mg/kg/day in rabbits administered during organogenesis. These doses are approximately 64 times (rat) and less than 16 times (rabbit) MRHD of BRISDELLE (7.5 mg) on an mg/m2basis. These studies have revealed no evidence of malformations. However, in rats, there was an increase in pup deaths during the first 4 days of lactation when dosing occurred during the last trimester of gestation and continued throughout lactation. This effect occurred at a dose of 1 mg/kg/day which is 1.3 times the MRHD on an mg/m2basis. The no effect dose for rat pup mortality was not determined. The cause of these deaths is not known.
The following serious adverse reactions are discussed elsewhere in labeling:
- Suicidality [see Warnings and Precautions ()]
5.1 Suicidal Thoughts and Behaviors in Adolescents and Young AdultsSSRIs increased the risk of suicidal thoughts and behavior in pediatric and young adult patients in short-term trials for the treatment of major depressive disorder (MDD) and other psychiatric disorders - BRISDELLE is not approved for use in any psychiatric condition or in pediatric and young adult patients
[see Indications and Usage and Use in Specific Populations ]. There is limited information regarding suicidal thoughts and behaviors in females who use BRISDELLE for treatment of moderate to severe VMS associated with menopause. The BRISDELLE trials excluded females with a presence or history of previous psychiatric disorders.Monitor all BRISDELLE-treated patients for any emergence of suicidal thoughts and behaviors, especially during the initial few months of BRISDELLE therapy. Counsel family members to monitor for changes in behavior and to alert the health care provider if such changes occur. Consider discontinuing BRISDELLE in patients who experience emergent suicidal thoughts or behaviors or symptoms that might be precursors to suicidal thoughts or behaviors, especially if these symptoms are severe, abrupt in onset, or were not part of the patient’s presenting symptoms.
- Serotonin syndrome [see Warnings and Precautions ()]
5.2 Serotonin SyndromeBRISDELLE can precipitate serotonin syndrome, a potentially life-threatening condition. The risk is increased with concomitant use of other serotonergic agents (including triptans, tricyclic antidepressants, fentanyl, tramadol, meperidine, methadone, lithium, tryptophan, buspirone, amphetamines, and St. John’s Wort) and with drugs that impair metabolism of serotonin, i.e., monoamine oxidase inhibitors (MAOIs)
[see Contraindications , Drug Interactions ]. Serotonin syndrome can also occur when BRISDELLE is used alone.Serotonin syndrome symptoms may include mental status changes (e.g., agitation, hallucinations, delirium, and coma), autonomic instability (e.g., tachycardia, labile blood pressure, dizziness, diaphoresis, flushing, hyperthermia), neuromuscular symptoms (e.g., tremor, rigidity, myoclonus, hyperreflexia, incoordination), and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea).
The concomitant use of BRISDELLE with MAOIs is contraindicated. Do not start BRISDELLE in a patient who is being treated with MAOIs such as linezolid or intravenous methylene blue. All reports with methylene blue that provided information on the route of administration involved intravenous administration in the dosage range of 1 mg/kg to 8 mg/kg. No reports involved the administration of methylene blue by other routes (such as oral or local tissue injection) or at lower dosages. If it is necessary to initiate treatment with an MAOI such as linezolid or intravenous methylene blue in a patient taking BRISDELLE, The patient should be taken off BRISDELLE before initiating treatment with the MAOI
[see Contraindications ]. If concomitant use of BRISDELLE with other serotonergic drugs (besides MAOIs) is clinically warranted, consider the increased risk of serotonin syndrome and carefully observe the patient, particularly during BRISDELLE initiation[see Contraindications Drug Interactions ].Monitor all patients taking BRISDELLE for the emergence of serotonin syndrome. Discontinue BRISDELLE and any concomitant serotonergic agents immediately if the above events occur and initiate supportive symptomatic treatment.
- Abnormal bleeding [see Warnings and Precautions ()]
5.4 Increased Risk of BleedingSSRIs, including BRISDELLE, increased the risk of bleeding events. Concomitant use of aspirin, nonsteroidal anti-inflammatory drugs (NSAIDs), other antiplatelet drugs, warfarin, and other anticoagulants may add to this risk
[see Drug Interactions ]. Case reports and epidemiological studies (case-control and cohort design) have demonstrated an association between use of drugs that interfere with serotonin reuptake and the occurrence of gastrointestinal bleeding. Bleeding events related to SSRIs have ranged from ecchymosis, hematoma, epistaxis, and petechiae to life-threatening hemorrhages.Inform patients about the risk of bleeding associated with the concomitant use of BRISDELLE and antiplatelet agents or anticoagulants
[see Drug Interactions ]. For patients taking warfarin, carefully monitor the international normalized ratio. - Angle-Closure Glaucoma [see Warnings and Precautions ()]
5.5 Angle-Closure GlaucomaThe pupillary dilation that occurs following use of SSRIs, including BRISDELLE, may trigger an angle closure attack in a patient with anatomically narrow angles who does not have a patent iridectomy. Cases of angle-closure glaucoma associated with use of paroxetine have been reported. Avoid use of SSRIs, including BRISDELLE, in patients with untreated anatomically narrow angles.
- Hyponatremia [see Warnings and Precautions ()]
5.6 HyponatremiaHyponatremia may occur as a result of treatment with SSRIs, including BRISDELLE. Cases with serum sodium lower than 110 mmol/L have been reported in patients using SSRIs. Geriatric patients, patients taking diuretics, and those who are volume-depleted may be at greater risk of developing hyponatremia with SSRIs
[see Use in Specific Populations ]. Signs and symptoms of hyponatremia include headache, difficulty concentrating, memory impairment, confusion, weakness, and unsteadiness, which can lead to falls. Signs and symptoms associated with more severe and/or acute cases have included hallucination, syncope, seizure, coma, respiratory arrest, and death. In many cases, the hyponatremia appears to be the result of the syndrome of inappropriate antidiuretic hormone secretion (SIADH).In patients with symptomatic hyponatremia, discontinue BRISDELLE and institute appropriate medical intervention.
- Bone Fracture [see Warnings and Precautions ()]
5.7 Bone FractureEpidemiological studies on bone fracture risk following exposure to SSRIs have reported an association between SSRI treatment and fractures. It is unknown to what extent fracture risk is directly attributable to SSRI treatment. If a BRISDELLE-treated patient presents with unexplained bone pain, point tenderness, swelling, or bruising, consider the possibility of a fragility fracture.
- Mania/Hypomania [see Warnings and Precautions ()]
5.8 Screening Patients for Bipolar Disorder and Monitoring for Mania/HypomaniaBRISDELLE is only indicated for the treatment of moderate to severe VMS and is not approved for use in treating either depression or bipolar depression. However, prior to initiating treatment with BRISDELLE, all patients should be adequately screened to determine if they are at risk for bipolar disorder; such screening should include a detailed psychiatric history, including a family history of suicide, bipolar disorder, and depression. It is generally believed (though not established in controlled trials) that use of an antidepressant alone may increase the likelihood of precipitation of a mixed/manic episode in patients at risk for bipolar disorder.
- Seizure [see Warnings and Precautions ()]
5.9 SeizuresIn clinical studies of another paroxetine product, seizures occurred in 0.1% of paroxetine-treated patients.
Use BRISDELLE cautiously in patients with a history of seizures or with conditions that potentially lower the seizure threshold. Discontinue BRISDELLE in any patient who develops seizures.
BRISDELLE (paroxetine) is an orally administered selective serotonin reuptake inhibitor (SSRI) for the treatment of moderate to severe VMS associated with menopause. It is identified chemically as (-)-
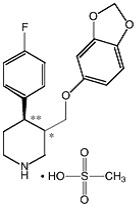
The mesylate salt of paroxetine is an odorless, off-white powder, having a melting point range of 147° to 150°C and a solubility of more than 1 g/mL in water.
Each pink capsule contains 9.69 mg of paroxetine mesylate equivalent to 7.5 mg paroxetine base.
Inactive ingredients consist of: dibasic calcium phosphate, sodium starch glycolate, magnesium stearate, gelatin, titanium dioxide, FD&C Yellow #6, FD&C Red #3, FD&C Red #40, shellac, and black iron oxide.